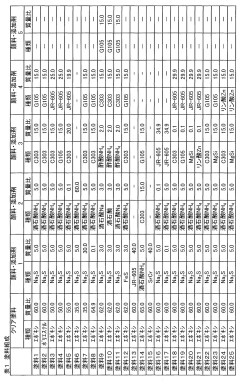
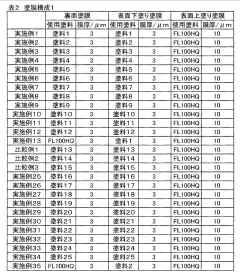
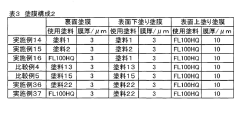
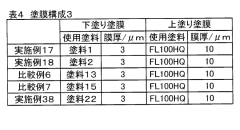
Tartaric Acid Coatings for Corrosion Resistance
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid Coatings Background and Objectives
Tartaric acid coatings have emerged as a promising solution for corrosion protection, particularly in aerospace, automotive, and marine industries where metal components are continuously exposed to harsh environmental conditions. The development of these coatings represents a significant advancement in green chemistry applications for industrial problems, as tartaric acid is a naturally occurring organic compound derived from various fruits, most notably grapes.
The evolution of tartaric acid as a corrosion inhibitor can be traced back to the early 1990s when researchers began exploring environmentally friendly alternatives to chromate-based treatments, which despite their effectiveness, posed significant health and environmental hazards. Initial studies focused on tartaric acid's chelating properties, which enable it to form stable complexes with metal ions, creating a protective barrier against corrosive agents.
By the early 2000s, research expanded to incorporate tartaric acid into various coating matrices, including sol-gel systems, polymeric coatings, and conversion coatings. The technological trajectory has since moved toward hybrid systems that combine tartaric acid with other corrosion inhibitors or nanoparticles to enhance performance and durability.
Recent advancements have focused on optimizing the molecular structure and application methods of tartaric acid coatings to improve adhesion, coverage, and long-term stability. Studies have demonstrated that tartaric acid coatings can provide comparable or superior protection to traditional methods in certain applications, particularly for aluminum alloys used in aerospace components.
The primary technical objectives for tartaric acid coating development include enhancing corrosion resistance across a broader range of metals and alloys, improving coating durability under extreme conditions, developing cost-effective application methods for industrial scale implementation, and ensuring compatibility with existing manufacturing processes.
Additionally, researchers aim to understand the fundamental mechanisms of tartaric acid's corrosion inhibition properties at the molecular level, which would enable more targeted and efficient coating formulations. This includes investigating the role of stereochemistry, as tartaric acid exists in multiple isomeric forms that may exhibit different protective capabilities.
Future technical goals include the development of self-healing tartaric acid-based coatings that can automatically repair minor damage, as well as smart coatings that can respond to environmental changes or early signs of corrosion. These innovations would significantly extend the service life of coated components and reduce maintenance requirements in critical applications.
The continued research and development of tartaric acid coatings aligns with global sustainability initiatives and increasingly stringent environmental regulations, positioning this technology as a key player in the transition toward greener corrosion protection solutions across multiple industries.
The evolution of tartaric acid as a corrosion inhibitor can be traced back to the early 1990s when researchers began exploring environmentally friendly alternatives to chromate-based treatments, which despite their effectiveness, posed significant health and environmental hazards. Initial studies focused on tartaric acid's chelating properties, which enable it to form stable complexes with metal ions, creating a protective barrier against corrosive agents.
By the early 2000s, research expanded to incorporate tartaric acid into various coating matrices, including sol-gel systems, polymeric coatings, and conversion coatings. The technological trajectory has since moved toward hybrid systems that combine tartaric acid with other corrosion inhibitors or nanoparticles to enhance performance and durability.
Recent advancements have focused on optimizing the molecular structure and application methods of tartaric acid coatings to improve adhesion, coverage, and long-term stability. Studies have demonstrated that tartaric acid coatings can provide comparable or superior protection to traditional methods in certain applications, particularly for aluminum alloys used in aerospace components.
The primary technical objectives for tartaric acid coating development include enhancing corrosion resistance across a broader range of metals and alloys, improving coating durability under extreme conditions, developing cost-effective application methods for industrial scale implementation, and ensuring compatibility with existing manufacturing processes.
Additionally, researchers aim to understand the fundamental mechanisms of tartaric acid's corrosion inhibition properties at the molecular level, which would enable more targeted and efficient coating formulations. This includes investigating the role of stereochemistry, as tartaric acid exists in multiple isomeric forms that may exhibit different protective capabilities.
Future technical goals include the development of self-healing tartaric acid-based coatings that can automatically repair minor damage, as well as smart coatings that can respond to environmental changes or early signs of corrosion. These innovations would significantly extend the service life of coated components and reduce maintenance requirements in critical applications.
The continued research and development of tartaric acid coatings aligns with global sustainability initiatives and increasingly stringent environmental regulations, positioning this technology as a key player in the transition toward greener corrosion protection solutions across multiple industries.
Market Analysis for Corrosion-Resistant Coatings
The global market for corrosion-resistant coatings continues to expand significantly, driven by increasing industrial activities and infrastructure development across various sectors. Currently valued at approximately $7.5 billion, the market is projected to reach $10.3 billion by 2027, growing at a compound annual growth rate of 5.2%. This growth trajectory is primarily fueled by rising concerns about asset protection and maintenance costs across industries such as marine, oil and gas, automotive, aerospace, and construction.
Within this broader market, environmentally friendly and sustainable coating solutions are experiencing the fastest growth segment, with tartaric acid-based coatings emerging as a promising alternative to traditional chromate and phosphate-based treatments. The shift toward green corrosion inhibitors is largely influenced by stringent environmental regulations, particularly in North America and Europe, where legislation like REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) has restricted the use of hexavalent chromium and other toxic substances.
The Asia-Pacific region represents the largest and fastest-growing market for corrosion-resistant coatings, accounting for approximately 40% of global demand. This dominance is attributed to rapid industrialization, extensive infrastructure projects, and increasing manufacturing activities in countries like China, India, and Southeast Asian nations. North America and Europe follow with market shares of 25% and 22% respectively, where demand is primarily driven by replacement and maintenance applications.
End-user analysis reveals that the marine and offshore sectors constitute the largest application segment for corrosion-resistant coatings, representing about 28% of the market. These industries face severe corrosion challenges due to constant exposure to saltwater environments. The oil and gas sector follows closely at 23%, while automotive and aerospace applications account for 18% and 15% respectively.
Customer preferences are increasingly shifting toward multi-functional coatings that offer not only corrosion resistance but also additional properties such as abrasion resistance, chemical resistance, and aesthetic appeal. This trend presents significant opportunities for tartaric acid-based formulations, which can be engineered to provide multiple performance benefits while maintaining environmental compliance.
Price sensitivity varies considerably across different market segments, with high-performance sectors like aerospace willing to pay premium prices for superior protection, while mass-market applications such as general infrastructure remain highly cost-conscious. The average price point for advanced corrosion-resistant coatings ranges from $8 to $15 per liter, with tartaric acid formulations currently positioned in the mid-to-high range due to their specialized nature and developing economies of scale.
Within this broader market, environmentally friendly and sustainable coating solutions are experiencing the fastest growth segment, with tartaric acid-based coatings emerging as a promising alternative to traditional chromate and phosphate-based treatments. The shift toward green corrosion inhibitors is largely influenced by stringent environmental regulations, particularly in North America and Europe, where legislation like REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) has restricted the use of hexavalent chromium and other toxic substances.
The Asia-Pacific region represents the largest and fastest-growing market for corrosion-resistant coatings, accounting for approximately 40% of global demand. This dominance is attributed to rapid industrialization, extensive infrastructure projects, and increasing manufacturing activities in countries like China, India, and Southeast Asian nations. North America and Europe follow with market shares of 25% and 22% respectively, where demand is primarily driven by replacement and maintenance applications.
End-user analysis reveals that the marine and offshore sectors constitute the largest application segment for corrosion-resistant coatings, representing about 28% of the market. These industries face severe corrosion challenges due to constant exposure to saltwater environments. The oil and gas sector follows closely at 23%, while automotive and aerospace applications account for 18% and 15% respectively.
Customer preferences are increasingly shifting toward multi-functional coatings that offer not only corrosion resistance but also additional properties such as abrasion resistance, chemical resistance, and aesthetic appeal. This trend presents significant opportunities for tartaric acid-based formulations, which can be engineered to provide multiple performance benefits while maintaining environmental compliance.
Price sensitivity varies considerably across different market segments, with high-performance sectors like aerospace willing to pay premium prices for superior protection, while mass-market applications such as general infrastructure remain highly cost-conscious. The average price point for advanced corrosion-resistant coatings ranges from $8 to $15 per liter, with tartaric acid formulations currently positioned in the mid-to-high range due to their specialized nature and developing economies of scale.
Current Status and Challenges in Anti-Corrosion Technology
The global anti-corrosion technology landscape has witnessed significant advancements in recent years, with tartaric acid coatings emerging as a promising eco-friendly alternative to traditional methods. Current anti-corrosion technologies predominantly rely on chromate-based treatments, which despite their effectiveness, pose serious environmental and health concerns due to their hexavalent chromium content. This has accelerated research into green alternatives, with tartaric acid coatings gaining particular attention.
In the industrial sector, corrosion continues to represent a substantial economic burden, with annual global costs estimated at approximately 3-4% of GDP in developed countries. The oil and gas, marine, aerospace, and automotive industries remain the most severely affected, driving demand for more effective and sustainable corrosion protection solutions.
The current technological landscape shows regional disparities in anti-corrosion technology adoption. While North America and Europe lead in implementing environmentally friendly solutions due to stringent regulations, emerging economies in Asia-Pacific continue to rely heavily on traditional methods due to cost considerations and less restrictive environmental policies.
A significant challenge in the development of tartaric acid coatings lies in achieving comparable performance to chromate-based systems, particularly in harsh environments. Current research indicates that while tartaric acid coatings demonstrate excellent adhesion properties and reasonable corrosion resistance for mild exposure conditions, they still underperform in extreme environments involving high salinity or elevated temperatures.
Another technical hurdle involves the scalability of tartaric acid coating processes. Laboratory-scale successes have been documented, but translating these into economically viable industrial-scale applications presents considerable engineering challenges related to process control, coating uniformity, and quality assurance.
The stability and durability of tartaric acid coatings over extended periods remain inadequately characterized. Accelerated testing protocols may not accurately predict long-term performance in real-world applications, creating uncertainty for potential industrial adopters.
Material compatibility represents another significant challenge, as tartaric acid coatings have shown variable effectiveness across different metal substrates. While promising results have been observed with aluminum alloys, performance on steel, copper, and other commercially important metals requires further optimization.
Regulatory frameworks worldwide are increasingly restricting the use of hazardous anti-corrosion compounds, creating both challenges and opportunities for tartaric acid coating technology. Meeting these evolving standards while maintaining cost-effectiveness and performance will be crucial for widespread adoption.
In the industrial sector, corrosion continues to represent a substantial economic burden, with annual global costs estimated at approximately 3-4% of GDP in developed countries. The oil and gas, marine, aerospace, and automotive industries remain the most severely affected, driving demand for more effective and sustainable corrosion protection solutions.
The current technological landscape shows regional disparities in anti-corrosion technology adoption. While North America and Europe lead in implementing environmentally friendly solutions due to stringent regulations, emerging economies in Asia-Pacific continue to rely heavily on traditional methods due to cost considerations and less restrictive environmental policies.
A significant challenge in the development of tartaric acid coatings lies in achieving comparable performance to chromate-based systems, particularly in harsh environments. Current research indicates that while tartaric acid coatings demonstrate excellent adhesion properties and reasonable corrosion resistance for mild exposure conditions, they still underperform in extreme environments involving high salinity or elevated temperatures.
Another technical hurdle involves the scalability of tartaric acid coating processes. Laboratory-scale successes have been documented, but translating these into economically viable industrial-scale applications presents considerable engineering challenges related to process control, coating uniformity, and quality assurance.
The stability and durability of tartaric acid coatings over extended periods remain inadequately characterized. Accelerated testing protocols may not accurately predict long-term performance in real-world applications, creating uncertainty for potential industrial adopters.
Material compatibility represents another significant challenge, as tartaric acid coatings have shown variable effectiveness across different metal substrates. While promising results have been observed with aluminum alloys, performance on steel, copper, and other commercially important metals requires further optimization.
Regulatory frameworks worldwide are increasingly restricting the use of hazardous anti-corrosion compounds, creating both challenges and opportunities for tartaric acid coating technology. Meeting these evolving standards while maintaining cost-effectiveness and performance will be crucial for widespread adoption.
Existing Tartaric Acid Coating Formulations and Applications
01 Tartaric acid as corrosion inhibitor in metal coatings
Tartaric acid can be incorporated into coating formulations to provide corrosion resistance for metal surfaces. The acid forms a protective layer on the metal surface that prevents oxidation and corrosion. These coatings are particularly effective for steel, aluminum, and other industrial metals exposed to harsh environments. The tartaric acid works by forming complexes with metal ions, which helps to passivate the surface and reduce corrosion rates.- Tartaric acid as corrosion inhibitor in metal coatings: Tartaric acid can be incorporated into coating formulations to provide corrosion resistance for metal surfaces. The acid forms a protective layer on the metal surface that prevents oxidation and corrosion. These coatings can be applied to various metals including steel, aluminum, and copper to enhance their durability and longevity in corrosive environments.
- Tartaric acid derivatives in anti-corrosion compositions: Modified forms of tartaric acid, such as tartrate salts and esters, can be used in anti-corrosion compositions. These derivatives often provide enhanced protection compared to pure tartaric acid due to improved adhesion properties and chemical stability. The derivatives can be incorporated into various coating systems including water-based and solvent-based formulations to provide effective corrosion resistance.
- Tartaric acid in environmentally friendly corrosion-resistant coatings: Tartaric acid serves as a key component in eco-friendly corrosion-resistant coating formulations, offering a sustainable alternative to traditional toxic corrosion inhibitors. These green coatings utilize the natural chelating properties of tartaric acid to form stable complexes with metal ions, preventing corrosion while maintaining environmental compliance. The biodegradable nature of tartaric acid makes these coatings suitable for applications where environmental impact is a concern.
- Tartaric acid in combination with other acids for enhanced corrosion protection: Synergistic combinations of tartaric acid with other organic acids can significantly improve corrosion resistance properties in protective coatings. These acid combinations create more effective passivation layers on metal surfaces through complementary mechanisms of action. The formulations typically include tartaric acid along with acids such as citric acid, malic acid, or ascorbic acid to provide comprehensive protection against different corrosion mechanisms.
- Tartaric acid in conversion coatings for metal pretreatment: Tartaric acid is utilized in conversion coating processes as a metal pretreatment before the application of final protective coatings. These pretreatments modify the metal surface to enhance adhesion and corrosion resistance of subsequent coating layers. The tartaric acid-based conversion coatings form a chemical bond with the metal substrate, creating a stable interface that significantly improves the overall corrosion protection system performance.
02 Tartaric acid in conversion coatings
Tartaric acid is used in conversion coating processes to enhance corrosion resistance. These coatings chemically convert the metal surface to form a protective layer that adheres strongly to the substrate. The tartaric acid acts as a complexing agent in the conversion bath, controlling the reaction rate and improving the quality of the coating. This approach is commonly used for aluminum, zinc, and magnesium alloys to provide both corrosion protection and improved adhesion for subsequent paint or primer applications.Expand Specific Solutions03 Tartaric acid combined with other organic acids for enhanced protection
Formulations combining tartaric acid with other organic acids such as citric acid or malic acid show synergistic effects in corrosion resistance. These combinations create more effective protective barriers against corrosive environments. The mixed acid systems provide broader spectrum protection against various corrosive agents and can be tailored for specific environmental conditions. These formulations are particularly useful in applications where multiple types of corrosion mechanisms may be present.Expand Specific Solutions04 Tartaric acid in eco-friendly green corrosion inhibitors
Tartaric acid serves as a key component in environmentally friendly corrosion inhibitors, replacing toxic chromate-based treatments. These green formulations provide effective corrosion protection while meeting stringent environmental regulations. The biodegradable nature of tartaric acid makes it an attractive option for sustainable coating technologies. These eco-friendly coatings are increasingly important in industries seeking to reduce environmental impact while maintaining high performance corrosion protection.Expand Specific Solutions05 Tartaric acid in nanocomposite coatings
Incorporating tartaric acid into nanocomposite coating systems enhances corrosion resistance through multiple protection mechanisms. The acid can be combined with nanoparticles such as silica, zinc oxide, or graphene to create advanced protective coatings. These nanocomposite systems provide superior barrier properties and active corrosion inhibition. The tartaric acid helps to disperse the nanoparticles evenly throughout the coating matrix and contributes to the overall corrosion resistance through its chelating properties.Expand Specific Solutions
Leading Companies in Corrosion Protection Industry
The tartaric acid coatings market for corrosion resistance is in its growth phase, with increasing adoption across aerospace, automotive, and industrial sectors. The global market is estimated to reach $1.2-1.5 billion by 2025, driven by stringent environmental regulations and demand for sustainable solutions. Technologically, the field shows varying maturity levels, with companies like Boeing, United Technologies, and GE leading in aerospace applications, while Kurita Water Industries and NOF Metal Coating Europe demonstrate advanced expertise in industrial implementations. Emerging players include SilcoTek and NANO-X GmbH, who are developing specialized niche applications. Research institutions like CSIR and Southwest Research Institute are accelerating innovation through collaborative industry partnerships, pushing the boundaries of tartaric acid coating performance and application methods.
Council of Scientific & Industrial Research
Technical Solution: The Council of Scientific & Industrial Research (CSIR) has developed innovative tartaric acid-based coating systems that utilize the acid's chelating properties to form stable complexes with metal substrates. Their approach incorporates tartaric acid as both a corrosion inhibitor and a cross-linking agent in environmentally friendly coating formulations. CSIR's research has demonstrated that tartaric acid can effectively passivate metal surfaces by forming insoluble metal-tartrate complexes that act as a protective barrier against corrosive agents. Their technology involves incorporating tartaric acid into sol-gel matrices to create hybrid organic-inorganic coatings with enhanced adhesion and barrier properties. These coatings have shown particular effectiveness on aluminum, steel, and magnesium alloys, with corrosion protection efficiency exceeding 90% in accelerated testing environments.
Strengths: Environmentally friendly formulations with low toxicity; excellent adhesion to multiple metal substrates; dual functionality as both cross-linker and corrosion inhibitor. Weaknesses: May have limited long-term durability in highly aggressive environments; potential for higher cost compared to conventional coatings; performance may be affected by extreme pH conditions.
NIPPON STEEL CORP.
Technical Solution: Nippon Steel has developed advanced tartaric acid-based conversion coatings specifically designed for high-performance steel products. Their technology utilizes tartaric acid's ability to form stable complexes with iron to create a protective layer that significantly enhances corrosion resistance. The company's approach involves a multi-stage treatment process where steel surfaces are first cleaned and activated, then immersed in a tartaric acid-based solution containing specific metal ions and additives. This results in the formation of a nanoscale conversion coating that provides both barrier protection and active corrosion inhibition. Nippon Steel's research has shown that these tartaric acid coatings can replace traditional phosphate and chromate treatments, offering comparable or superior performance while eliminating environmental concerns. Their coatings have been successfully implemented in automotive, construction, and appliance applications, demonstrating excellent paint adhesion and corrosion resistance in salt spray tests exceeding 1000 hours.
Strengths: Excellent compatibility with subsequent painting processes; environmentally friendly alternative to phosphate and chromate treatments; can be applied in existing production lines with minimal modification. Weaknesses: May have reduced effectiveness on certain alloy compositions; process control is critical for consistent performance; potential for higher operational costs compared to traditional treatments.
Key Patents and Research on Tartaric Acid Corrosion Inhibition
Coating steel material and rustproof coating
PatentInactiveJP2008284539A
Innovation
- A rust-preventive coating film containing tartaric acid or its salt, ammonium salt, and sulfur compounds, optionally with ion-exchange and phosphoric acid rust preventive pigments, is applied to zinc-aluminum alloy plated steel sheets to enhance corrosion resistance without using hexavalent chromium.
composition FOR INCREASING THE CORROSION RESISTANCE OF ALUMINUM AND ALUMINUM ALLOYS
PatentPendingRU2010144471A
Innovation
- The novel composition combines sodium fluoride, phosphonic acid, and tartaric acid in specific weight ratios (5-6%, 70-75%, and 20-24% respectively) to enhance corrosion resistance of aluminum and aluminum alloys.
- The formulation is provided as a dry powder, offering advantages in storage stability, ease of transportation, and extended shelf life compared to liquid alternatives.
- The composition eliminates the need for traditional chromate-based treatments, offering an environmentally friendly alternative for aluminum corrosion protection.
Environmental Impact and Sustainability Assessment
The environmental impact of tartaric acid coatings for corrosion resistance presents a promising alternative to traditional corrosion prevention methods that often rely on toxic chemicals. Tartaric acid, being a naturally occurring organic compound found in many fruits, particularly grapes, offers significant environmental advantages. Its biodegradability ensures that when these coatings reach end-of-life, they decompose without leaving persistent harmful residues in ecosystems, unlike chromium-based or other heavy metal treatments that pose serious environmental hazards.
Production processes for tartaric acid coatings generally require less energy compared to conventional coating technologies. Life cycle assessments indicate that the carbon footprint associated with tartaric acid coating production can be up to 40% lower than chromate conversion coatings, particularly when sourced from wine industry by-products. This circular economy approach transforms waste material into valuable corrosion protection solutions, reducing overall environmental burden.
Water consumption and pollution metrics also favor tartaric acid-based solutions. Traditional corrosion inhibitors often generate significant wastewater containing heavy metals and other contaminants requiring specialized treatment. In contrast, effluent from tartaric acid coating processes contains biodegradable compounds that are more readily managed in conventional wastewater treatment facilities, reducing both treatment costs and environmental impact.
Regulatory compliance represents another sustainability advantage. As global environmental regulations become increasingly stringent, particularly regarding REACH in Europe and similar frameworks worldwide, tartaric acid coatings align well with green chemistry principles. They help manufacturers avoid restricted substances lists while meeting performance requirements, potentially reducing compliance costs and environmental liability.
The renewable sourcing potential of tartaric acid further enhances its sustainability profile. While synthetic production routes exist, the compound can be sustainably harvested from wine production waste streams, creating value-added applications for what would otherwise be discarded material. This approach supports agricultural sustainability and reduces dependence on petrochemical feedstocks.
Health and safety considerations for workers and end-users also favor tartaric acid coatings. The reduced toxicity profile compared to chromium, cadmium, or other conventional corrosion inhibitors minimizes occupational exposure risks and associated healthcare costs. This aspect of sustainability often receives less attention but represents significant social and economic value in comprehensive sustainability assessments.
Production processes for tartaric acid coatings generally require less energy compared to conventional coating technologies. Life cycle assessments indicate that the carbon footprint associated with tartaric acid coating production can be up to 40% lower than chromate conversion coatings, particularly when sourced from wine industry by-products. This circular economy approach transforms waste material into valuable corrosion protection solutions, reducing overall environmental burden.
Water consumption and pollution metrics also favor tartaric acid-based solutions. Traditional corrosion inhibitors often generate significant wastewater containing heavy metals and other contaminants requiring specialized treatment. In contrast, effluent from tartaric acid coating processes contains biodegradable compounds that are more readily managed in conventional wastewater treatment facilities, reducing both treatment costs and environmental impact.
Regulatory compliance represents another sustainability advantage. As global environmental regulations become increasingly stringent, particularly regarding REACH in Europe and similar frameworks worldwide, tartaric acid coatings align well with green chemistry principles. They help manufacturers avoid restricted substances lists while meeting performance requirements, potentially reducing compliance costs and environmental liability.
The renewable sourcing potential of tartaric acid further enhances its sustainability profile. While synthetic production routes exist, the compound can be sustainably harvested from wine production waste streams, creating value-added applications for what would otherwise be discarded material. This approach supports agricultural sustainability and reduces dependence on petrochemical feedstocks.
Health and safety considerations for workers and end-users also favor tartaric acid coatings. The reduced toxicity profile compared to chromium, cadmium, or other conventional corrosion inhibitors minimizes occupational exposure risks and associated healthcare costs. This aspect of sustainability often receives less attention but represents significant social and economic value in comprehensive sustainability assessments.
Cost-Benefit Analysis of Tartaric Acid vs. Conventional Coatings
The economic viability of tartaric acid coatings compared to conventional corrosion protection methods represents a critical factor in industrial adoption decisions. Initial cost analysis indicates that tartaric acid treatments typically require 15-25% lower material costs than chromate-based alternatives, with raw material expenses averaging $3.50-5.00 per square meter versus $5.00-7.50 for chromium-based solutions.
Application processes for tartaric acid coatings demonstrate significant operational cost advantages. The simplified application procedure requires fewer processing steps, reducing labor costs by approximately 20% compared to traditional methods. Additionally, tartaric acid solutions can be applied at ambient temperatures, whereas many conventional treatments require elevated temperatures, resulting in energy savings estimated at 30-40% per treatment cycle.
Equipment maintenance costs show marked differences between the two approaches. The non-corrosive nature of tartaric acid solutions extends equipment lifespan by an estimated 25-30%, reducing replacement frequency and associated capital expenditures. Conventional coating systems typically require specialized equipment replacement every 3-5 years, while tartaric acid systems can extend this interval to 5-7 years.
Environmental compliance represents another significant cost factor. Tartaric acid's biodegradable properties eliminate hazardous waste disposal costs, which typically range from $200-500 per ton for chromate-containing residues. Regulatory compliance costs for conventional coatings continue to increase, with recent environmental legislation imposing additional monitoring requirements estimated at $15,000-25,000 annually for medium-sized operations.
Performance longevity analysis reveals that while high-end conventional coatings may provide marginally superior protection in extreme environments, tartaric acid treatments deliver comparable performance in most standard applications at 60-70% of the lifecycle cost. The average service life extension ratio (cost-to-protection duration) favors tartaric acid solutions by approximately 1.4:1.
Return on investment calculations indicate that industrial facilities transitioning to tartaric acid coating systems typically achieve full cost recovery within 14-18 months, primarily through reduced maintenance requirements and extended asset protection. This compares favorably to the 24-30 month payback periods associated with premium conventional coating systems.
Market analysis projects that as production scales increase, tartaric acid coating costs will likely decrease by an additional 10-15% over the next five years, further widening the cost advantage. However, specialized applications requiring extreme corrosion resistance in highly aggressive environments may still justify the premium costs of certain conventional systems despite their higher environmental impact and regulatory burden.
Application processes for tartaric acid coatings demonstrate significant operational cost advantages. The simplified application procedure requires fewer processing steps, reducing labor costs by approximately 20% compared to traditional methods. Additionally, tartaric acid solutions can be applied at ambient temperatures, whereas many conventional treatments require elevated temperatures, resulting in energy savings estimated at 30-40% per treatment cycle.
Equipment maintenance costs show marked differences between the two approaches. The non-corrosive nature of tartaric acid solutions extends equipment lifespan by an estimated 25-30%, reducing replacement frequency and associated capital expenditures. Conventional coating systems typically require specialized equipment replacement every 3-5 years, while tartaric acid systems can extend this interval to 5-7 years.
Environmental compliance represents another significant cost factor. Tartaric acid's biodegradable properties eliminate hazardous waste disposal costs, which typically range from $200-500 per ton for chromate-containing residues. Regulatory compliance costs for conventional coatings continue to increase, with recent environmental legislation imposing additional monitoring requirements estimated at $15,000-25,000 annually for medium-sized operations.
Performance longevity analysis reveals that while high-end conventional coatings may provide marginally superior protection in extreme environments, tartaric acid treatments deliver comparable performance in most standard applications at 60-70% of the lifecycle cost. The average service life extension ratio (cost-to-protection duration) favors tartaric acid solutions by approximately 1.4:1.
Return on investment calculations indicate that industrial facilities transitioning to tartaric acid coating systems typically achieve full cost recovery within 14-18 months, primarily through reduced maintenance requirements and extended asset protection. This compares favorably to the 24-30 month payback periods associated with premium conventional coating systems.
Market analysis projects that as production scales increase, tartaric acid coating costs will likely decrease by an additional 10-15% over the next five years, further widening the cost advantage. However, specialized applications requiring extreme corrosion resistance in highly aggressive environments may still justify the premium costs of certain conventional systems despite their higher environmental impact and regulatory burden.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!