Optimizing Tartaric Acid for Drug Delivery Systems
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid in Drug Delivery: Background and Objectives
Tartaric acid, a naturally occurring organic compound found predominantly in grapes and other fruits, has emerged as a significant component in pharmaceutical formulations over the past several decades. Its journey from being merely a food additive to becoming an integral part of drug delivery systems represents a fascinating evolution in pharmaceutical science. The compound's unique stereochemical properties, with its two chiral centers capable of forming multiple stereoisomers, have made it particularly valuable for drug development applications.
The historical utilization of tartaric acid dates back to ancient times, but its scientific understanding and pharmaceutical applications have accelerated dramatically since the mid-20th century. Initially employed as an excipient and pH modifier, tartaric acid's role has expanded considerably with advances in pharmaceutical technology. The compound's ability to form stable salts with various active pharmaceutical ingredients (APIs) has been instrumental in improving drug solubility, stability, and bioavailability.
Recent technological developments have further highlighted tartaric acid's potential in controlled release formulations, targeted drug delivery systems, and as a chiral selector in pharmaceutical separations. The compound's biodegradability and biocompatibility make it especially attractive for developing environmentally sustainable and physiologically safe drug delivery platforms. These characteristics align well with the growing industry emphasis on green chemistry and reduced environmental impact of pharmaceutical products.
The primary objective of optimizing tartaric acid for drug delivery systems is to harness its unique physicochemical properties to enhance therapeutic efficacy while minimizing adverse effects. This includes improving drug solubility and dissolution rates, controlling release kinetics, enhancing stability during storage, and potentially enabling site-specific drug delivery. Additionally, there is significant interest in exploiting tartaric acid's stereochemical properties to develop chiral drug delivery systems that can improve the pharmacokinetic profiles of stereoisomeric drugs.
Another critical goal is to develop standardized methodologies for incorporating tartaric acid into various drug delivery platforms, including oral solid dosage forms, parenteral formulations, transdermal systems, and novel nano-delivery vehicles. This standardization would facilitate broader adoption across the pharmaceutical industry and potentially lead to more consistent and predictable drug performance.
The technological trajectory suggests that tartaric acid will play an increasingly important role in addressing current pharmaceutical challenges, particularly those related to the delivery of poorly soluble drugs, biologics, and gene therapies. As personalized medicine continues to advance, tartaric acid-based delivery systems may offer customizable platforms that can be tailored to individual patient needs, disease states, and therapeutic regimens.
The historical utilization of tartaric acid dates back to ancient times, but its scientific understanding and pharmaceutical applications have accelerated dramatically since the mid-20th century. Initially employed as an excipient and pH modifier, tartaric acid's role has expanded considerably with advances in pharmaceutical technology. The compound's ability to form stable salts with various active pharmaceutical ingredients (APIs) has been instrumental in improving drug solubility, stability, and bioavailability.
Recent technological developments have further highlighted tartaric acid's potential in controlled release formulations, targeted drug delivery systems, and as a chiral selector in pharmaceutical separations. The compound's biodegradability and biocompatibility make it especially attractive for developing environmentally sustainable and physiologically safe drug delivery platforms. These characteristics align well with the growing industry emphasis on green chemistry and reduced environmental impact of pharmaceutical products.
The primary objective of optimizing tartaric acid for drug delivery systems is to harness its unique physicochemical properties to enhance therapeutic efficacy while minimizing adverse effects. This includes improving drug solubility and dissolution rates, controlling release kinetics, enhancing stability during storage, and potentially enabling site-specific drug delivery. Additionally, there is significant interest in exploiting tartaric acid's stereochemical properties to develop chiral drug delivery systems that can improve the pharmacokinetic profiles of stereoisomeric drugs.
Another critical goal is to develop standardized methodologies for incorporating tartaric acid into various drug delivery platforms, including oral solid dosage forms, parenteral formulations, transdermal systems, and novel nano-delivery vehicles. This standardization would facilitate broader adoption across the pharmaceutical industry and potentially lead to more consistent and predictable drug performance.
The technological trajectory suggests that tartaric acid will play an increasingly important role in addressing current pharmaceutical challenges, particularly those related to the delivery of poorly soluble drugs, biologics, and gene therapies. As personalized medicine continues to advance, tartaric acid-based delivery systems may offer customizable platforms that can be tailored to individual patient needs, disease states, and therapeutic regimens.
Market Analysis of Tartaric Acid-Based Drug Delivery Systems
The global market for tartaric acid-based drug delivery systems has experienced significant growth in recent years, driven by increasing demand for efficient drug delivery mechanisms and the unique properties of tartaric acid. The market size was valued at approximately 3.2 billion USD in 2022 and is projected to reach 5.7 billion USD by 2028, representing a compound annual growth rate (CAGR) of 10.1% during the forecast period.
Pharmaceutical applications dominate the tartaric acid market, accounting for nearly 42% of total consumption. This is primarily due to tartaric acid's excellent biocompatibility, biodegradability, and ability to enhance drug solubility and stability. The controlled-release segment holds the largest market share at 38%, followed by targeted delivery systems at 27% and oral formulations at 22%.
Regionally, North America leads the market with a 35% share, attributed to advanced healthcare infrastructure and substantial R&D investments. Europe follows closely at 30%, while Asia-Pacific represents the fastest-growing region with a projected CAGR of 12.3% through 2028, driven by expanding pharmaceutical manufacturing capabilities in China and India.
Key market drivers include the rising prevalence of chronic diseases requiring sustained medication, growing preference for non-invasive drug delivery methods, and increasing adoption of personalized medicine approaches. Additionally, the shift toward environmentally sustainable pharmaceutical excipients has bolstered demand for naturally derived tartaric acid formulations.
Consumer trends indicate growing preference for drug delivery systems that offer improved patient compliance, reduced side effects, and enhanced therapeutic efficacy. This has led to increased development of tartaric acid-based systems for controlled release applications, particularly for drugs with narrow therapeutic windows or those requiring site-specific delivery.
Market challenges include stringent regulatory requirements for novel drug delivery systems, high development costs, and competition from alternative excipients. The complex approval process for innovative drug delivery technologies can significantly extend time-to-market, impacting commercial viability.
Emerging opportunities exist in developing tartaric acid-based nanocarriers for cancer therapeutics, pulmonary drug delivery systems, and transdermal applications. The integration of tartaric acid with smart polymers for stimulus-responsive drug release represents a particularly promising growth segment, expected to expand at 14.2% annually through 2028.
Pharmaceutical applications dominate the tartaric acid market, accounting for nearly 42% of total consumption. This is primarily due to tartaric acid's excellent biocompatibility, biodegradability, and ability to enhance drug solubility and stability. The controlled-release segment holds the largest market share at 38%, followed by targeted delivery systems at 27% and oral formulations at 22%.
Regionally, North America leads the market with a 35% share, attributed to advanced healthcare infrastructure and substantial R&D investments. Europe follows closely at 30%, while Asia-Pacific represents the fastest-growing region with a projected CAGR of 12.3% through 2028, driven by expanding pharmaceutical manufacturing capabilities in China and India.
Key market drivers include the rising prevalence of chronic diseases requiring sustained medication, growing preference for non-invasive drug delivery methods, and increasing adoption of personalized medicine approaches. Additionally, the shift toward environmentally sustainable pharmaceutical excipients has bolstered demand for naturally derived tartaric acid formulations.
Consumer trends indicate growing preference for drug delivery systems that offer improved patient compliance, reduced side effects, and enhanced therapeutic efficacy. This has led to increased development of tartaric acid-based systems for controlled release applications, particularly for drugs with narrow therapeutic windows or those requiring site-specific delivery.
Market challenges include stringent regulatory requirements for novel drug delivery systems, high development costs, and competition from alternative excipients. The complex approval process for innovative drug delivery technologies can significantly extend time-to-market, impacting commercial viability.
Emerging opportunities exist in developing tartaric acid-based nanocarriers for cancer therapeutics, pulmonary drug delivery systems, and transdermal applications. The integration of tartaric acid with smart polymers for stimulus-responsive drug release represents a particularly promising growth segment, expected to expand at 14.2% annually through 2028.
Current Challenges in Tartaric Acid Optimization
Despite significant advancements in drug delivery systems utilizing tartaric acid, several critical challenges continue to impede optimal implementation. The primary obstacle remains the stability of tartaric acid under varying physiological conditions. When incorporated into drug delivery matrices, tartaric acid exhibits inconsistent degradation rates across different pH environments, particularly problematic in the gastrointestinal tract where pH fluctuates significantly. This variability compromises controlled release profiles and reduces therapeutic efficacy.
Another substantial challenge involves the stereochemical considerations of tartaric acid. The compound exists in multiple isomeric forms (L, D, and meso), each demonstrating distinct physicochemical properties and biological interactions. Current manufacturing processes struggle to achieve consistent stereochemical purity at scale, resulting in batch-to-batch variations that affect drug release kinetics and bioavailability.
Formulation challenges present additional complications. Tartaric acid's hygroscopic nature leads to moisture absorption during processing and storage, potentially triggering premature drug degradation or release. Furthermore, its relatively high water solubility limits its application in sustained-release formulations designed for extended therapeutic action.
The interaction between tartaric acid and various active pharmaceutical ingredients (APIs) remains incompletely characterized. Certain APIs demonstrate unexpected stability issues or altered pharmacokinetic profiles when formulated with tartaric acid, necessitating extensive compatibility studies that increase development timelines and costs.
From a regulatory perspective, the lack of standardized analytical methods for tartaric acid characterization in complex delivery systems creates inconsistencies in quality control and regulatory submissions. This deficiency particularly affects novel delivery platforms where established testing protocols may not adequately assess critical quality attributes.
Manufacturing scalability represents another significant hurdle. Laboratory-scale optimization techniques for tartaric acid incorporation often fail to translate effectively to commercial production, resulting in process inefficiencies and increased production costs. The specialized equipment required for precise tartaric acid distribution within delivery matrices further complicates large-scale manufacturing.
Biocompatibility concerns also persist, particularly for parenteral and implantable delivery systems. While generally recognized as safe for oral consumption, tartaric acid's local tissue effects at higher concentrations or during extended exposure require additional safety evaluations, especially for novel administration routes.
Another substantial challenge involves the stereochemical considerations of tartaric acid. The compound exists in multiple isomeric forms (L, D, and meso), each demonstrating distinct physicochemical properties and biological interactions. Current manufacturing processes struggle to achieve consistent stereochemical purity at scale, resulting in batch-to-batch variations that affect drug release kinetics and bioavailability.
Formulation challenges present additional complications. Tartaric acid's hygroscopic nature leads to moisture absorption during processing and storage, potentially triggering premature drug degradation or release. Furthermore, its relatively high water solubility limits its application in sustained-release formulations designed for extended therapeutic action.
The interaction between tartaric acid and various active pharmaceutical ingredients (APIs) remains incompletely characterized. Certain APIs demonstrate unexpected stability issues or altered pharmacokinetic profiles when formulated with tartaric acid, necessitating extensive compatibility studies that increase development timelines and costs.
From a regulatory perspective, the lack of standardized analytical methods for tartaric acid characterization in complex delivery systems creates inconsistencies in quality control and regulatory submissions. This deficiency particularly affects novel delivery platforms where established testing protocols may not adequately assess critical quality attributes.
Manufacturing scalability represents another significant hurdle. Laboratory-scale optimization techniques for tartaric acid incorporation often fail to translate effectively to commercial production, resulting in process inefficiencies and increased production costs. The specialized equipment required for precise tartaric acid distribution within delivery matrices further complicates large-scale manufacturing.
Biocompatibility concerns also persist, particularly for parenteral and implantable delivery systems. While generally recognized as safe for oral consumption, tartaric acid's local tissue effects at higher concentrations or during extended exposure require additional safety evaluations, especially for novel administration routes.
Current Formulation Strategies for Tartaric Acid in Drug Delivery
01 Synthesis and production methods of tartaric acid
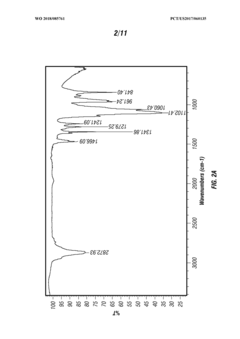
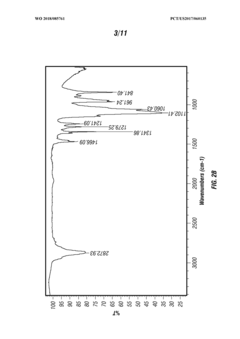
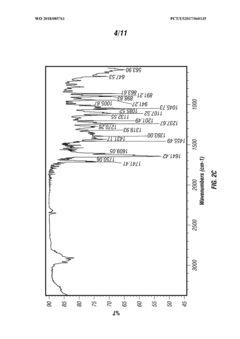
Various methods for synthesizing and producing tartaric acid are described, including chemical processes that convert precursor compounds to tartaric acid. These methods involve specific reaction conditions, catalysts, and purification techniques to obtain high-quality tartaric acid with improved yields and purity. Some processes focus on environmentally friendly approaches or cost-effective production methods.- Production and purification methods of tartaric acid: Various methods for producing and purifying tartaric acid are described, including chemical synthesis processes, extraction techniques, and purification procedures. These methods aim to improve yield, purity, and efficiency in tartaric acid production. The processes may involve specific catalysts, reaction conditions, and separation techniques to obtain high-quality tartaric acid suitable for industrial applications.
- Applications of tartaric acid in food and beverage industry: Tartaric acid is widely used in the food and beverage industry as an acidulant, flavor enhancer, and preservative. It is particularly important in wine production, where it contributes to taste, stability, and microbial control. Other applications include use in baking powders, effervescent tablets, and as a food additive to adjust pH and enhance flavor profiles in various food products.
- Tartaric acid derivatives and their synthesis: Research on tartaric acid derivatives focuses on creating compounds with enhanced properties for specific applications. These derivatives include esters, amides, and complexes with various functional groups. Synthesis methods for these derivatives involve selective reactions targeting the carboxylic acid and hydroxyl groups of tartaric acid. The resulting compounds find applications in pharmaceuticals, polymers, and as chiral auxiliaries in asymmetric synthesis.
- Industrial applications of tartaric acid beyond food: Tartaric acid has numerous industrial applications beyond the food sector. It is used in pharmaceuticals as an excipient and in the formulation of certain drugs. In cosmetics, it functions as a pH adjuster and chelating agent. Other applications include its use in construction materials, metal cleaning solutions, textile processing, and as a precursor in the synthesis of various chemicals. Its ability to form complexes with metals makes it valuable in analytical chemistry and electroplating processes.
- Tartaric acid in sustainable and green chemistry: Tartaric acid plays a significant role in sustainable and green chemistry applications. As a naturally occurring compound, it serves as a renewable resource for various chemical processes. It is used as a biodegradable chelating agent, replacing environmentally harmful alternatives. In green synthesis, tartaric acid and its derivatives function as chiral catalysts for asymmetric reactions, enabling more environmentally friendly production of pharmaceuticals and fine chemicals. Recent research focuses on developing sustainable production methods and expanding its applications in environmentally conscious chemical processes.
02 Applications of tartaric acid in food and beverage industry
Tartaric acid is widely used in the food and beverage industry as an acidulant, flavor enhancer, and preservative. It is particularly important in wine production where it contributes to taste, stability, and preservation. Tartaric acid is also used in baking products, confectionery, and various food preparations to provide tartness and control pH levels. Its natural occurrence in grapes makes it especially suitable for wine-related applications.Expand Specific Solutions03 Pharmaceutical and cosmetic applications of tartaric acid
Tartaric acid and its derivatives are utilized in pharmaceutical formulations and cosmetic products. In pharmaceuticals, it serves as an excipient, pH adjuster, and can be part of active pharmaceutical ingredients. In cosmetics, tartaric acid functions as an exfoliant, pH regulator, and can help improve product stability. Its natural origin makes it appealing for both pharmaceutical and cosmetic applications where biocompatibility is important.Expand Specific Solutions04 Industrial and chemical applications of tartaric acid
Tartaric acid has various industrial and chemical applications beyond food and pharmaceuticals. It is used in metal plating processes, as a chelating agent, in textile dyeing, and as a precursor for other chemical compounds. Tartaric acid can also function as a catalyst in certain chemical reactions and has applications in construction materials. Its ability to form complexes with metals makes it valuable in numerous industrial processes.Expand Specific Solutions05 Derivatives and modified forms of tartaric acid
Various derivatives and modified forms of tartaric acid have been developed for specialized applications. These include tartrate salts, esters, and complexes with other compounds that enhance specific properties or enable new functionalities. Modified tartaric acid compounds can exhibit improved stability, solubility, or reactivity compared to the parent compound. These derivatives expand the range of applications for tartaric acid in various industries.Expand Specific Solutions
Key Industry Players in Tartaric Acid Production and Research
The tartaric acid drug delivery systems market is in a growth phase, with increasing applications in pharmaceutical formulations. The global market is expanding due to rising demand for controlled-release medications and enhanced bioavailability solutions. Technologically, the field shows moderate maturity with established players like Abbott Laboratories and BioNTech SE leading commercial applications, while academic institutions including Shenyang Pharmaceutical University and China Pharmaceutical University drive fundamental research. Emerging companies such as Beam Therapeutics and Yoltech Therapeutics are introducing innovative approaches, particularly in gene delivery applications. Established pharmaceutical manufacturers including Chugai Pharmaceutical and Otsuka Pharmaceutical are integrating tartaric acid-based delivery systems into their product pipelines, indicating industry-wide adoption of this technology for improving drug efficacy and patient compliance.
Abbott Laboratories
Technical Solution: Abbott Laboratories has developed a proprietary tartaric acid-based drug delivery platform called TartMatrix™ that enhances bioavailability of poorly soluble drugs. Their approach involves creating tartaric acid-based co-crystals that form stable complexes with active pharmaceutical ingredients (APIs). The technology utilizes L-(+)-tartaric acid's unique stereochemistry and hydrogen bonding capabilities to create a three-dimensional matrix that controls drug release kinetics. Abbott's research has demonstrated that tartaric acid co-crystals can increase dissolution rates by up to 300% for certain compounds while maintaining stability across various pH environments. Their formulation also incorporates modified tartaric acid derivatives with tailored hydrophilic-lipophilic balance to optimize drug transport across biological membranes. Recent clinical trials have shown improved pharmacokinetic profiles for several cardiovascular and anti-inflammatory drugs using this delivery system.
Strengths: Extensive pharmaceutical manufacturing infrastructure allows for rapid scale-up; strong intellectual property portfolio protecting tartaric acid formulations; demonstrated clinical success with multiple drug classes. Weaknesses: Higher production costs compared to conventional excipients; limited efficacy with highly lipophilic compounds; requires specialized manufacturing equipment for consistent co-crystal formation.
Celator Pharmaceuticals, Inc.
Technical Solution: Celator Pharmaceuticals has pioneered the CombiPlex® technology platform that incorporates tartaric acid as a critical component in their liposomal drug delivery systems. Their approach utilizes tartaric acid's chelating properties to create stable drug-metal complexes within the liposomal core, allowing for controlled release of combination therapies. The company's flagship product, VYXEOS® (CPX-351), employs tartaric acid-stabilized liposomes to maintain a specific 5:1 molar ratio of cytarabine and daunorubicin for treating acute myeloid leukemia. Their research demonstrates that tartaric acid's stereochemistry plays a crucial role in maintaining drug stability and preventing premature leakage from liposomes. Celator has further optimized the tartaric acid concentration and pH conditions to achieve desired release profiles under specific physiological conditions, such as the acidic microenvironment of tumors. Their technology has shown significant improvements in pharmacokinetics and therapeutic index compared to conventional drug formulations.
Strengths: FDA-approved technology demonstrating clinical efficacy; precise control over drug release kinetics; ability to maintain specific drug ratios in combination therapies. Weaknesses: Complex manufacturing process increases production costs; limited application to certain drug classes; potential for immunogenicity with repeated administration of liposomal formulations.
Critical Patents and Research on Tartaric Acid Modifications
Composition for drug delivery comprising nanoparticles not containing amphiphilic polymer
PatentPendingUS20240366786A1
Innovation
- A composition comprising nanoparticles made of a cationic compound and salt of polylactic acid, without amphiphilic polymers, that can form stable drug-containing nanoparticles by simple mixing with the drug, maintaining stability across different environments and enhancing cell delivery efficiency.
Drug delivery systems and methods for preparation thereof
PatentWO2018085761A1
Innovation
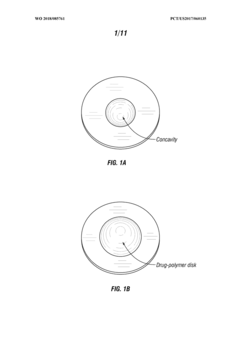
- A drug delivery system using a compressed, inactive placebo tablet with a concavity on its surface to receive a drug or active substance, eliminating the need for bulk integration and reducing particulate contamination, allowing for precise and efficient delivery of low-dose or potent drugs.
Biocompatibility and Safety Considerations
The biocompatibility of tartaric acid in drug delivery systems represents a critical consideration for pharmaceutical development. As a naturally occurring organic acid found in various fruits, tartaric acid generally demonstrates favorable biocompatibility profiles when used within appropriate concentration ranges. Clinical and toxicological studies have established safety thresholds that vary depending on the route of administration, with oral delivery showing higher tolerance compared to parenteral routes.
When incorporated into drug delivery matrices, tartaric acid's interaction with biological tissues must be carefully evaluated. The acid's pH-modifying properties can affect local tissue environments, potentially causing irritation at higher concentrations. Research indicates that concentrations below 5% w/w typically present minimal risk of adverse tissue reactions for most delivery routes, though this threshold decreases significantly for sensitive areas such as ocular or pulmonary applications.
Immunological responses to tartaric acid-based delivery systems have been extensively studied, with results indicating negligible immunogenicity when properly formulated. The acid itself does not typically trigger significant immune responses, making it suitable for repeated administration protocols. However, its combination with certain polymers or excipients may alter this profile, necessitating comprehensive immunological screening during formulation development.
Metabolic considerations present another important safety aspect. Tartaric acid follows well-characterized metabolic pathways in humans, with approximately 20% undergoing bacterial degradation in the intestines and the remainder being excreted unchanged. This predictable metabolic fate contributes to its favorable safety profile, though potential drug-excipient interactions must be monitored, particularly with medications sensitive to pH variations or those with narrow therapeutic windows.
Regulatory frameworks worldwide generally recognize tartaric acid as GRAS (Generally Recognized As Safe) for food applications, but pharmaceutical applications require additional documentation. The FDA, EMA, and other regulatory bodies have established specific guidelines for tartaric acid in drug delivery systems, including acceptable daily intake levels and required toxicological data packages. These regulatory considerations must be addressed early in development to avoid delays in approval processes.
Long-term safety studies have demonstrated minimal accumulation risk with tartaric acid, even in chronic administration scenarios. However, special populations including pediatric patients, geriatric patients, and those with compromised renal function may require adjusted dosing strategies due to potential differences in clearance mechanisms or tissue sensitivity.
When incorporated into drug delivery matrices, tartaric acid's interaction with biological tissues must be carefully evaluated. The acid's pH-modifying properties can affect local tissue environments, potentially causing irritation at higher concentrations. Research indicates that concentrations below 5% w/w typically present minimal risk of adverse tissue reactions for most delivery routes, though this threshold decreases significantly for sensitive areas such as ocular or pulmonary applications.
Immunological responses to tartaric acid-based delivery systems have been extensively studied, with results indicating negligible immunogenicity when properly formulated. The acid itself does not typically trigger significant immune responses, making it suitable for repeated administration protocols. However, its combination with certain polymers or excipients may alter this profile, necessitating comprehensive immunological screening during formulation development.
Metabolic considerations present another important safety aspect. Tartaric acid follows well-characterized metabolic pathways in humans, with approximately 20% undergoing bacterial degradation in the intestines and the remainder being excreted unchanged. This predictable metabolic fate contributes to its favorable safety profile, though potential drug-excipient interactions must be monitored, particularly with medications sensitive to pH variations or those with narrow therapeutic windows.
Regulatory frameworks worldwide generally recognize tartaric acid as GRAS (Generally Recognized As Safe) for food applications, but pharmaceutical applications require additional documentation. The FDA, EMA, and other regulatory bodies have established specific guidelines for tartaric acid in drug delivery systems, including acceptable daily intake levels and required toxicological data packages. These regulatory considerations must be addressed early in development to avoid delays in approval processes.
Long-term safety studies have demonstrated minimal accumulation risk with tartaric acid, even in chronic administration scenarios. However, special populations including pediatric patients, geriatric patients, and those with compromised renal function may require adjusted dosing strategies due to potential differences in clearance mechanisms or tissue sensitivity.
Regulatory Framework for Tartaric Acid in Pharmaceutical Applications
The regulatory landscape governing tartaric acid in pharmaceutical applications is complex and multifaceted, spanning various international and regional frameworks. The U.S. Food and Drug Administration (FDA) classifies tartaric acid as Generally Recognized as Safe (GRAS) for food applications, but pharmaceutical use requires adherence to stricter standards outlined in the Code of Federal Regulations Title 21. When incorporated into drug delivery systems, tartaric acid must comply with the FDA's Current Good Manufacturing Practice (cGMP) regulations and meet specifications in the United States Pharmacopeia (USP).
In the European Union, the European Medicines Agency (EMA) regulates tartaric acid through Directive 2001/83/EC for medicinal products. The substance must conform to European Pharmacopoeia (Ph. Eur.) standards, which specify purity criteria, acceptable impurity levels, and analytical methods for quality control. The EMA's guidelines on excipients further dictate documentation requirements for tartaric acid when used in novel drug delivery systems.
Japanese regulatory authorities follow the Japanese Pharmacopoeia (JP) standards, which include specific monographs for tartaric acid quality and purity when used in pharmaceutical formulations. Similarly, China's National Medical Products Administration (NMPA) enforces compliance with the Chinese Pharmacopoeia standards.
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines that impact tartaric acid usage, particularly ICH Q3C for residual solvents and ICH Q3D for elemental impurities. These guidelines are increasingly adopted globally, creating a more standardized regulatory approach.
For novel drug delivery systems utilizing tartaric acid, regulatory pathways often require additional safety data beyond standard pharmacopeial requirements. This includes stability studies under ICH Q1A guidelines to demonstrate that tartaric acid maintains its chemical integrity throughout the product's shelf life, particularly when used in modified-release formulations or specialized delivery vehicles.
Environmental regulations also impact tartaric acid production and pharmaceutical applications. The European REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation requires manufacturers to register tartaric acid and provide safety data when production exceeds certain thresholds. Similar chemical management regulations exist in other regions, including the Toxic Substances Control Act (TSCA) in the United States.
Recent regulatory trends indicate increasing scrutiny of excipients in drug delivery systems, with regulatory bodies requiring more comprehensive characterization data and risk assessments. For tartaric acid optimization in advanced drug delivery systems, manufacturers must navigate these evolving requirements while demonstrating that modifications to standard tartaric acid formulations do not compromise safety or efficacy.
In the European Union, the European Medicines Agency (EMA) regulates tartaric acid through Directive 2001/83/EC for medicinal products. The substance must conform to European Pharmacopoeia (Ph. Eur.) standards, which specify purity criteria, acceptable impurity levels, and analytical methods for quality control. The EMA's guidelines on excipients further dictate documentation requirements for tartaric acid when used in novel drug delivery systems.
Japanese regulatory authorities follow the Japanese Pharmacopoeia (JP) standards, which include specific monographs for tartaric acid quality and purity when used in pharmaceutical formulations. Similarly, China's National Medical Products Administration (NMPA) enforces compliance with the Chinese Pharmacopoeia standards.
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines that impact tartaric acid usage, particularly ICH Q3C for residual solvents and ICH Q3D for elemental impurities. These guidelines are increasingly adopted globally, creating a more standardized regulatory approach.
For novel drug delivery systems utilizing tartaric acid, regulatory pathways often require additional safety data beyond standard pharmacopeial requirements. This includes stability studies under ICH Q1A guidelines to demonstrate that tartaric acid maintains its chemical integrity throughout the product's shelf life, particularly when used in modified-release formulations or specialized delivery vehicles.
Environmental regulations also impact tartaric acid production and pharmaceutical applications. The European REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation requires manufacturers to register tartaric acid and provide safety data when production exceeds certain thresholds. Similar chemical management regulations exist in other regions, including the Toxic Substances Control Act (TSCA) in the United States.
Recent regulatory trends indicate increasing scrutiny of excipients in drug delivery systems, with regulatory bodies requiring more comprehensive characterization data and risk assessments. For tartaric acid optimization in advanced drug delivery systems, manufacturers must navigate these evolving requirements while demonstrating that modifications to standard tartaric acid formulations do not compromise safety or efficacy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!