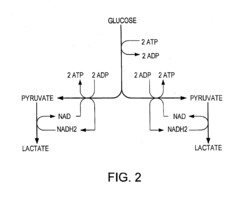
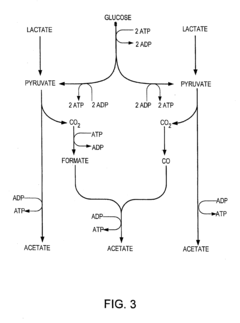
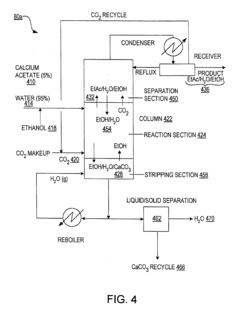
Tartaric Acid vs Acetic Acid in Fermentation Processes
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fermentation Acids Background and Objectives
Fermentation processes have been integral to human civilization for millennia, with evidence of fermented beverages dating back to 7000 BCE. The evolution of fermentation technology has progressed from traditional empirical methods to today's precisely controlled industrial processes, representing a significant advancement in biotechnology and food science. Understanding the role of organic acids in these processes is fundamental to optimizing fermentation outcomes across various industries.
Tartaric acid and acetic acid represent two distinct yet crucial organic acids in fermentation contexts. Tartaric acid, naturally occurring in many fruits, particularly grapes, plays a significant role in wine production by influencing pH stability, microbial control, and sensory characteristics. Its presence affects the growth of beneficial yeasts while inhibiting spoilage organisms, contributing to the preservation and quality of fermented products.
Acetic acid, conversely, is both a product and mediator of fermentation processes. While it represents the desired end product in vinegar production, it often indicates spoilage in wine and beer fermentations. The balance between these acids significantly impacts product stability, flavor profile, and shelf life across various fermented goods.
The technological evolution in fermentation acid management has accelerated in recent decades. From traditional methods relying on natural acid presence to modern approaches involving precise acid addition, pH monitoring systems, and genetically modified microorganisms with altered acid metabolism, the field continues to advance rapidly. These developments aim to enhance product consistency, quality, and production efficiency.
Our technical research objectives focus on comparing the functional roles, biochemical interactions, and industrial applications of tartaric and acetic acids in controlled fermentation environments. We seek to establish optimal concentration parameters for each acid across different fermentation processes, identify synergistic or antagonistic relationships between these acids, and develop predictive models for acid behavior under varying fermentation conditions.
Additionally, we aim to explore novel applications for these acids in emerging fermentation markets, including plant-based protein fermentation, biofuel production, and pharmaceutical fermentation processes. The research will also investigate sustainable sourcing methods for these acids, addressing growing industry concerns regarding environmental impact and production costs.
By comprehensively understanding the comparative advantages and limitations of tartaric and acetic acids in fermentation processes, this research endeavors to provide actionable insights for process optimization, product innovation, and quality enhancement across the fermentation industry landscape.
Tartaric acid and acetic acid represent two distinct yet crucial organic acids in fermentation contexts. Tartaric acid, naturally occurring in many fruits, particularly grapes, plays a significant role in wine production by influencing pH stability, microbial control, and sensory characteristics. Its presence affects the growth of beneficial yeasts while inhibiting spoilage organisms, contributing to the preservation and quality of fermented products.
Acetic acid, conversely, is both a product and mediator of fermentation processes. While it represents the desired end product in vinegar production, it often indicates spoilage in wine and beer fermentations. The balance between these acids significantly impacts product stability, flavor profile, and shelf life across various fermented goods.
The technological evolution in fermentation acid management has accelerated in recent decades. From traditional methods relying on natural acid presence to modern approaches involving precise acid addition, pH monitoring systems, and genetically modified microorganisms with altered acid metabolism, the field continues to advance rapidly. These developments aim to enhance product consistency, quality, and production efficiency.
Our technical research objectives focus on comparing the functional roles, biochemical interactions, and industrial applications of tartaric and acetic acids in controlled fermentation environments. We seek to establish optimal concentration parameters for each acid across different fermentation processes, identify synergistic or antagonistic relationships between these acids, and develop predictive models for acid behavior under varying fermentation conditions.
Additionally, we aim to explore novel applications for these acids in emerging fermentation markets, including plant-based protein fermentation, biofuel production, and pharmaceutical fermentation processes. The research will also investigate sustainable sourcing methods for these acids, addressing growing industry concerns regarding environmental impact and production costs.
By comprehensively understanding the comparative advantages and limitations of tartaric and acetic acids in fermentation processes, this research endeavors to provide actionable insights for process optimization, product innovation, and quality enhancement across the fermentation industry landscape.
Market Analysis of Acid Applications in Fermentation Industries
The global market for acids in fermentation processes has witnessed significant growth over the past decade, with tartaric acid and acetic acid emerging as key players in various industrial applications. The fermentation industry, valued at approximately $149.4 billion in 2022, is projected to reach $255.6 billion by 2030, growing at a CAGR of 6.9%. Within this expanding market, acids play crucial roles as pH regulators, flavor enhancers, and preservatives.
Tartaric acid commands a premium market position due to its natural origin and application in high-value fermentation processes. The global tartaric acid market was valued at $425 million in 2021, with the wine industry consuming nearly 45% of the total production. European countries, particularly Italy, France, and Spain, represent the largest markets for tartaric acid, corresponding with their dominant positions in wine production.
Acetic acid, conversely, holds a substantially larger market share, valued at $8.3 billion in 2022, with fermentation applications accounting for approximately 18% of total consumption. The food and beverage sector represents the largest end-user segment for acetic acid in fermentation, followed by pharmaceutical and industrial biotechnology applications.
Regional analysis reveals distinct consumption patterns, with North America and Europe showing preference for tartaric acid in premium fermentation applications, while Asia-Pacific markets demonstrate stronger growth in acetic acid consumption, particularly in emerging economies like China and India where food preservation and industrial fermentation are rapidly expanding sectors.
Price dynamics between these acids show significant variation, with tartaric acid commanding prices between $3.50-5.00 per kilogram, while acetic acid is considerably more economical at $0.50-0.80 per kilogram. This price differential significantly influences adoption patterns across different market segments and applications.
Market forecasts indicate that tartaric acid will maintain steady growth at 4.2% CAGR through 2028, driven by increasing demand for natural and clean-label ingredients in fermented products. Meanwhile, acetic acid in fermentation applications is projected to grow at 5.7% CAGR, benefiting from expanding industrial biotechnology applications and cost advantages in large-scale operations.
Consumer trends are increasingly favoring natural fermentation aids, benefiting tartaric acid's market position despite its higher cost. Sustainability considerations are also reshaping the market, with manufacturers exploring bio-based production methods for both acids to reduce environmental impact and meet evolving regulatory requirements.
Tartaric acid commands a premium market position due to its natural origin and application in high-value fermentation processes. The global tartaric acid market was valued at $425 million in 2021, with the wine industry consuming nearly 45% of the total production. European countries, particularly Italy, France, and Spain, represent the largest markets for tartaric acid, corresponding with their dominant positions in wine production.
Acetic acid, conversely, holds a substantially larger market share, valued at $8.3 billion in 2022, with fermentation applications accounting for approximately 18% of total consumption. The food and beverage sector represents the largest end-user segment for acetic acid in fermentation, followed by pharmaceutical and industrial biotechnology applications.
Regional analysis reveals distinct consumption patterns, with North America and Europe showing preference for tartaric acid in premium fermentation applications, while Asia-Pacific markets demonstrate stronger growth in acetic acid consumption, particularly in emerging economies like China and India where food preservation and industrial fermentation are rapidly expanding sectors.
Price dynamics between these acids show significant variation, with tartaric acid commanding prices between $3.50-5.00 per kilogram, while acetic acid is considerably more economical at $0.50-0.80 per kilogram. This price differential significantly influences adoption patterns across different market segments and applications.
Market forecasts indicate that tartaric acid will maintain steady growth at 4.2% CAGR through 2028, driven by increasing demand for natural and clean-label ingredients in fermented products. Meanwhile, acetic acid in fermentation applications is projected to grow at 5.7% CAGR, benefiting from expanding industrial biotechnology applications and cost advantages in large-scale operations.
Consumer trends are increasingly favoring natural fermentation aids, benefiting tartaric acid's market position despite its higher cost. Sustainability considerations are also reshaping the market, with manufacturers exploring bio-based production methods for both acids to reduce environmental impact and meet evolving regulatory requirements.
Current Challenges in Acid Selection for Fermentation
The selection of appropriate acids in fermentation processes presents significant challenges for manufacturers across food, beverage, and pharmaceutical industries. Currently, the industry faces a complex decision matrix when choosing between tartaric acid and acetic acid, with each presenting distinct advantages and limitations depending on application contexts.
A primary challenge lies in achieving consistent pH control throughout the fermentation process. While tartaric acid offers superior buffering capacity in wine fermentation, maintaining stable pH levels with acetic acid requires more frequent monitoring and adjustment, particularly in large-scale operations. This creates operational inefficiencies and increases production costs for manufacturers utilizing acetic acid in certain applications.
Microbial compatibility represents another critical consideration. Recent research indicates that certain yeast strains exhibit reduced viability when exposed to higher concentrations of acetic acid, whereas tartaric acid demonstrates better tolerance profiles across a wider range of microorganisms. However, the specific mechanisms of acid-microbe interactions remain incompletely characterized, complicating selection decisions for novel fermentation applications.
Flavor profile management continues to challenge producers, particularly in beverage fermentation. Tartaric acid contributes a clean, sharp acidity preferred in wine production but may introduce unwanted astringency at higher concentrations. Conversely, acetic acid imparts distinctive vinegar notes that can either enhance or detract from product quality depending on the desired outcome. Balancing these sensory impacts against functional requirements represents an ongoing industry challenge.
Cost considerations further complicate acid selection. Tartaric acid typically commands a premium price point compared to acetic acid, creating economic pressure to utilize the latter despite potential performance trade-offs. This cost differential has widened in recent years due to supply chain disruptions affecting tartaric acid production regions, forcing manufacturers to reassess formulation strategies.
Regulatory compliance adds another layer of complexity. While both acids hold GRAS (Generally Recognized As Safe) status, regional variations in permitted usage levels and labeling requirements create compliance challenges for global producers. Additionally, consumer preference for "natural" ingredients has increased scrutiny on acid sourcing, with synthetic versus naturally-derived distinctions becoming increasingly important market differentiators.
Technical limitations in current analytical methods also hinder optimal acid selection. Real-time monitoring of acid concentration and dissociation during fermentation remains challenging, particularly in complex media with multiple interfering compounds. This analytical gap complicates process optimization and quality control efforts across the fermentation industry.
A primary challenge lies in achieving consistent pH control throughout the fermentation process. While tartaric acid offers superior buffering capacity in wine fermentation, maintaining stable pH levels with acetic acid requires more frequent monitoring and adjustment, particularly in large-scale operations. This creates operational inefficiencies and increases production costs for manufacturers utilizing acetic acid in certain applications.
Microbial compatibility represents another critical consideration. Recent research indicates that certain yeast strains exhibit reduced viability when exposed to higher concentrations of acetic acid, whereas tartaric acid demonstrates better tolerance profiles across a wider range of microorganisms. However, the specific mechanisms of acid-microbe interactions remain incompletely characterized, complicating selection decisions for novel fermentation applications.
Flavor profile management continues to challenge producers, particularly in beverage fermentation. Tartaric acid contributes a clean, sharp acidity preferred in wine production but may introduce unwanted astringency at higher concentrations. Conversely, acetic acid imparts distinctive vinegar notes that can either enhance or detract from product quality depending on the desired outcome. Balancing these sensory impacts against functional requirements represents an ongoing industry challenge.
Cost considerations further complicate acid selection. Tartaric acid typically commands a premium price point compared to acetic acid, creating economic pressure to utilize the latter despite potential performance trade-offs. This cost differential has widened in recent years due to supply chain disruptions affecting tartaric acid production regions, forcing manufacturers to reassess formulation strategies.
Regulatory compliance adds another layer of complexity. While both acids hold GRAS (Generally Recognized As Safe) status, regional variations in permitted usage levels and labeling requirements create compliance challenges for global producers. Additionally, consumer preference for "natural" ingredients has increased scrutiny on acid sourcing, with synthetic versus naturally-derived distinctions becoming increasingly important market differentiators.
Technical limitations in current analytical methods also hinder optimal acid selection. Real-time monitoring of acid concentration and dissociation during fermentation remains challenging, particularly in complex media with multiple interfering compounds. This analytical gap complicates process optimization and quality control efforts across the fermentation industry.
Comparative Analysis of Tartaric vs Acetic Acid Solutions
01 Optimization of fermentation conditions for tartaric acid production
Various fermentation conditions can be optimized to enhance the efficiency of tartaric acid production. These include controlling temperature, pH levels, oxygen supply, and fermentation time. Specific microorganisms can be selected or modified to improve tartaric acid yield. Additionally, the use of specific substrates and nutrient compositions can significantly impact the efficiency of the fermentation process.- Optimization of fermentation conditions for tartaric acid production: Various fermentation conditions can be optimized to enhance the efficiency of tartaric acid production. These include controlling temperature, pH levels, oxygen supply, and fermentation time. Proper control of these parameters can significantly improve the yield and quality of tartaric acid produced through microbial fermentation processes. Additionally, the selection of appropriate microbial strains and substrate concentrations plays a crucial role in maximizing production efficiency.
- Acetic acid fermentation process improvements: Innovations in acetic acid fermentation focus on enhancing production efficiency through improved bioreactor designs, continuous fermentation systems, and optimized culture conditions. These improvements include specialized equipment for controlling oxygen transfer rates, temperature regulation systems, and methods for continuous product removal to prevent inhibition. Advanced fermentation techniques can significantly increase acetic acid yield and reduce production time while maintaining product quality.
- Co-fermentation of tartaric and acetic acids: Co-fermentation processes involving both tartaric and acetic acid production can create synergistic effects that improve overall efficiency. These processes often utilize specialized microbial consortia or engineered strains capable of producing both acids simultaneously or sequentially. The interaction between different metabolic pathways can lead to improved substrate utilization, reduced inhibition effects, and enhanced productivity compared to single-acid fermentation processes.
- Novel microbial strains for improved acid fermentation: Development and selection of specialized microbial strains can significantly enhance the efficiency of tartaric and acetic acid fermentation. These include naturally occurring microorganisms with superior acid production capabilities as well as genetically modified strains engineered for improved substrate utilization, increased acid tolerance, and higher product yields. The use of these advanced strains can overcome traditional limitations in acid fermentation processes.
- Substrate and nutrient optimization for acid fermentation: The selection and preparation of appropriate substrates and nutrient formulations significantly impact the efficiency of tartaric and acetic acid fermentation. Various agricultural byproducts, industrial waste streams, and specifically formulated media can be used as cost-effective substrates. Supplementation with specific nutrients, vitamins, and minerals can enhance microbial growth and acid production. Pretreatment methods such as hydrolysis or sterilization can improve substrate accessibility and fermentation efficiency.
02 Acetic acid fermentation process improvements
Innovations in acetic acid fermentation focus on enhancing production efficiency through improved bacterial strains, optimized culture conditions, and novel reactor designs. Techniques include continuous fermentation systems, immobilized cell technology, and the use of specific substrates to increase yield. Temperature control, oxygen transfer rates, and pH management are critical factors that can be manipulated to improve the efficiency of acetic acid production.Expand Specific Solutions03 Combined fermentation systems for tartaric and acetic acids
Integrated fermentation systems can be designed to produce both tartaric and acetic acids either simultaneously or sequentially. These systems often utilize waste products from one fermentation process as substrates for the other, improving overall efficiency and reducing waste. Co-culture techniques with compatible microorganisms can enhance the production of both acids while minimizing inhibitory effects.Expand Specific Solutions04 Equipment and apparatus design for improved fermentation efficiency
Specialized equipment and apparatus designs can significantly improve the efficiency of tartaric and acetic acid fermentation processes. These include bioreactors with enhanced mixing capabilities, membrane separation systems for continuous product removal, and automated control systems for maintaining optimal fermentation conditions. Novel designs may incorporate features for improved oxygen transfer, temperature control, and reduced contamination risk.Expand Specific Solutions05 Recovery and purification methods for fermentation products
Efficient recovery and purification methods are essential for maximizing the yield of tartaric and acetic acids from fermentation processes. Techniques include crystallization, ion exchange, membrane filtration, and chromatography. Advanced separation technologies can reduce energy consumption and increase product purity. The integration of recovery systems directly with fermentation processes can improve overall efficiency by reducing processing steps and minimizing product loss.Expand Specific Solutions
Key Industry Players in Fermentation Acid Production
The fermentation industry utilizing tartaric and acetic acids is in a mature growth phase with an estimated global market size of $11.5 billion, expanding at 5-7% annually. The competitive landscape features established chemical manufacturers like BP Chemicals and Mizkan Co. alongside emerging biotechnology innovators such as Nourish Ingredients and Hangzhou Bioking. Traditional vinegar producers including Haitian Vinegar and Shanxi Liangfen Arawana maintain strong market positions, while research institutions like Tianjin University of Science & Technology and CSIC drive technological advancement. The industry demonstrates regional specialization with Asian companies dominating vinegar production and Western organizations focusing on novel fermentation applications in food technology, pharmaceuticals, and sustainable materials, creating a dynamic ecosystem balancing traditional practices with cutting-edge innovation.
Mizkan Co., Ltd.
Technical Solution: Mizkan has developed proprietary fermentation processes that utilize both acetic acid and tartaric acid in controlled environments. Their technology involves a two-stage fermentation process where tartaric acid is introduced during initial stages to optimize yeast activity and maintain pH stability, while acetic acid production is carefully managed during secondary fermentation. This approach allows for enhanced flavor development in vinegar products while minimizing unwanted byproducts. Mizkan's research has demonstrated that tartaric acid's presence during fermentation can reduce oxidation and improve the sensory qualities of the final product, particularly in rice vinegar production where delicate flavor profiles are desired. Their patented process includes precise temperature control systems that respond differently based on whether tartaric or acetic acid predominates at various fermentation stages.
Strengths: Superior flavor profile development with reduced harshness; better control over fermentation kinetics; improved product consistency across batches. Weaknesses: Higher production costs due to more complex process control requirements; longer fermentation times compared to traditional acetic acid-only methods; requires more sophisticated monitoring equipment.
BP Chemicals Ltd.
Technical Solution: BP Chemicals has pioneered industrial-scale fermentation technology that strategically balances tartaric and acetic acid concentrations for optimized chemical production. Their approach involves engineered microbial strains that can tolerate higher concentrations of both acids, allowing for increased production efficiency. The company has developed a proprietary membrane separation technology that selectively removes acetic acid during fermentation while retaining tartaric acid at beneficial levels, preventing inhibition of microbial growth. This continuous extraction system maintains optimal pH levels throughout the fermentation process, resulting in higher yields of target compounds. BP's research indicates that tartaric acid's chelating properties can be leveraged to reduce metal ion toxicity in industrial fermentations, extending the productive phase of microbial cultures by up to 30% compared to conventional methods.
Strengths: Higher production yields through optimized acid balance; reduced inhibitory effects on microbial cultures; more efficient use of raw materials. Weaknesses: Complex implementation requiring significant capital investment; higher energy consumption for separation processes; technology is less adaptable to smaller-scale operations.
Technical Innovations in Fermentation Acid Applications
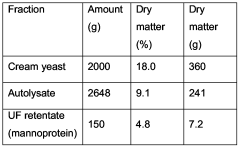
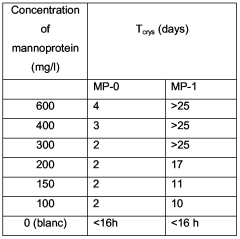
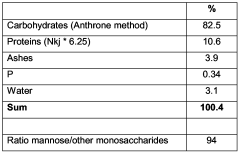
Process to improve activity of mannoprotein as wine stabiliser
PatentWO2006067147A1
Innovation
- Treating mannoprotein with a basic solution at a pH of at least 9 to enhance its stabilizing effect, which involves enzymatic hydrolysis of yeast cells, recovery of mannoprotein, and subsequent treatment with a basic solution to convert phosphomannan diesters to monoesters, improving its ability to prevent tartaric acid crystallization and protein haze formation.
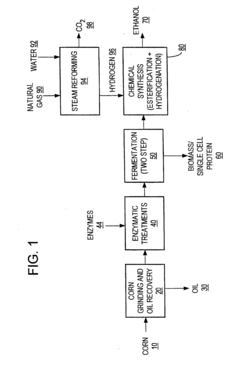
Efficient process for producing alcohol
PatentInactiveUS20130102043A1
Innovation
- A process combining homoacetic and homolactic fermentations to convert a wide range of carbohydrates into acetic acid, followed by esterification and hydrogenation to produce ethanol with high yield, eliminating carbon dioxide byproducts and generating high-value single cell protein as a coproduct.
Environmental Impact Assessment of Different Acid Usage
The environmental impact of acid selection in fermentation processes represents a critical consideration for sustainable industrial practices. When comparing tartaric acid and acetic acid usage, several environmental factors must be evaluated comprehensively to determine the most ecologically sound option.
Production methods for these acids differ significantly in their environmental footprints. Tartaric acid, primarily derived from wine industry byproducts, offers a circular economy advantage by utilizing waste streams. This recovery process typically requires less energy than synthetic production methods. Conversely, acetic acid is often produced through petrochemical processes or biological fermentation, with the former having higher carbon emissions and resource consumption.
Water usage and wastewater generation vary considerably between these acids. Fermentation processes utilizing tartaric acid generally produce effluent with lower biological oxygen demand (BOD) and chemical oxygen demand (COD) levels compared to acetic acid processes. Studies indicate that tartaric acid-based fermentations can reduce wastewater treatment requirements by 15-20%, translating to significant water conservation in large-scale operations.
Carbon footprint analysis reveals that tartaric acid's lifecycle emissions are approximately 30% lower than those of petrochemically-derived acetic acid. This difference stems primarily from reduced energy requirements during production and the utilization of agricultural byproducts rather than fossil fuel feedstocks. However, when comparing with biologically-produced acetic acid, this advantage narrows considerably.
Biodegradability characteristics favor both acids, though at different rates. Tartaric acid demonstrates faster natural decomposition in environmental settings, with complete biodegradation occurring within 14-21 days under optimal conditions. Acetic acid, while also biodegradable, may persist slightly longer in certain environmental compartments.
Soil and aquatic ecosystem impacts show that tartaric acid presents lower ecotoxicity profiles in standardized testing protocols. Acetic acid, particularly at higher concentrations, can cause temporary pH disruptions in receiving water bodies, potentially affecting sensitive aquatic organisms. Neither acid demonstrates significant bioaccumulation potential or persistent environmental contamination when properly managed.
Resource efficiency metrics indicate that tartaric acid production from wine industry byproducts achieves higher material utilization rates, supporting principles of industrial ecology. However, acetic acid production through modern biological routes has made significant sustainability improvements, narrowing this gap in recent years.
Regulatory compliance considerations increasingly favor acids with lower environmental impacts, with tartaric acid meeting stricter sustainability criteria in jurisdictions with advanced environmental protection frameworks. This regulatory advantage may translate to reduced compliance costs and improved corporate sustainability reporting outcomes for manufacturers.
Production methods for these acids differ significantly in their environmental footprints. Tartaric acid, primarily derived from wine industry byproducts, offers a circular economy advantage by utilizing waste streams. This recovery process typically requires less energy than synthetic production methods. Conversely, acetic acid is often produced through petrochemical processes or biological fermentation, with the former having higher carbon emissions and resource consumption.
Water usage and wastewater generation vary considerably between these acids. Fermentation processes utilizing tartaric acid generally produce effluent with lower biological oxygen demand (BOD) and chemical oxygen demand (COD) levels compared to acetic acid processes. Studies indicate that tartaric acid-based fermentations can reduce wastewater treatment requirements by 15-20%, translating to significant water conservation in large-scale operations.
Carbon footprint analysis reveals that tartaric acid's lifecycle emissions are approximately 30% lower than those of petrochemically-derived acetic acid. This difference stems primarily from reduced energy requirements during production and the utilization of agricultural byproducts rather than fossil fuel feedstocks. However, when comparing with biologically-produced acetic acid, this advantage narrows considerably.
Biodegradability characteristics favor both acids, though at different rates. Tartaric acid demonstrates faster natural decomposition in environmental settings, with complete biodegradation occurring within 14-21 days under optimal conditions. Acetic acid, while also biodegradable, may persist slightly longer in certain environmental compartments.
Soil and aquatic ecosystem impacts show that tartaric acid presents lower ecotoxicity profiles in standardized testing protocols. Acetic acid, particularly at higher concentrations, can cause temporary pH disruptions in receiving water bodies, potentially affecting sensitive aquatic organisms. Neither acid demonstrates significant bioaccumulation potential or persistent environmental contamination when properly managed.
Resource efficiency metrics indicate that tartaric acid production from wine industry byproducts achieves higher material utilization rates, supporting principles of industrial ecology. However, acetic acid production through modern biological routes has made significant sustainability improvements, narrowing this gap in recent years.
Regulatory compliance considerations increasingly favor acids with lower environmental impacts, with tartaric acid meeting stricter sustainability criteria in jurisdictions with advanced environmental protection frameworks. This regulatory advantage may translate to reduced compliance costs and improved corporate sustainability reporting outcomes for manufacturers.
Quality Control Standards for Acids in Food Fermentation
Quality control standards for acids in food fermentation processes require rigorous monitoring and adherence to established parameters to ensure product safety, consistency, and quality. For tartaric and acetic acids specifically, different regulatory bodies worldwide have established varying acceptable limits and testing methodologies that manufacturers must follow.
The FDA and European Food Safety Authority (EFSA) have established specific concentration limits for both acids in fermented food products. Tartaric acid (E334) typically has maximum permitted levels ranging from 1,500-5,000 mg/kg depending on the food category, while acetic acid (E260) often has quantum satis status in many applications, though specific limits apply in certain products.
Testing protocols for acid quality control in fermentation processes typically involve titrimetric analysis, high-performance liquid chromatography (HPLC), and spectrophotometric methods. The AOAC International has established Method 986.13 specifically for tartaric acid determination in grape juice and wine products, while Method 930.35 addresses acetic acid in vinegars and fermented products.
Critical control points in acid monitoring include raw material verification, in-process testing during fermentation, and finished product analysis. Modern facilities increasingly implement real-time monitoring systems with automated sampling to ensure continuous compliance with quality parameters. Statistical Process Control (SPC) charts are commonly employed to track acid levels throughout production cycles.
Purity standards are particularly stringent, with food-grade tartaric acid requiring minimum 99.5% purity and heavy metal contamination below 10 ppm. Acetic acid for food applications must typically meet 99.8% purity with specific limits on aldehydes, formic acid, and non-volatile residues.
Documentation requirements include Certificate of Analysis (CoA) for each acid batch, detailed records of in-process testing, and validation studies demonstrating the effectiveness of the quality control system. Many manufacturers now implement electronic quality management systems (eQMS) that integrate with laboratory information management systems (LIMS) to ensure complete traceability.
Industry best practices increasingly focus on preventive controls rather than end-product testing alone. This includes supplier qualification programs, environmental monitoring, and comprehensive HACCP plans that specifically address acid control points. Third-party certification programs like FSSC 22000 and BRC Global Standards have developed specific modules addressing fermentation process controls, including acid management protocols.
The FDA and European Food Safety Authority (EFSA) have established specific concentration limits for both acids in fermented food products. Tartaric acid (E334) typically has maximum permitted levels ranging from 1,500-5,000 mg/kg depending on the food category, while acetic acid (E260) often has quantum satis status in many applications, though specific limits apply in certain products.
Testing protocols for acid quality control in fermentation processes typically involve titrimetric analysis, high-performance liquid chromatography (HPLC), and spectrophotometric methods. The AOAC International has established Method 986.13 specifically for tartaric acid determination in grape juice and wine products, while Method 930.35 addresses acetic acid in vinegars and fermented products.
Critical control points in acid monitoring include raw material verification, in-process testing during fermentation, and finished product analysis. Modern facilities increasingly implement real-time monitoring systems with automated sampling to ensure continuous compliance with quality parameters. Statistical Process Control (SPC) charts are commonly employed to track acid levels throughout production cycles.
Purity standards are particularly stringent, with food-grade tartaric acid requiring minimum 99.5% purity and heavy metal contamination below 10 ppm. Acetic acid for food applications must typically meet 99.8% purity with specific limits on aldehydes, formic acid, and non-volatile residues.
Documentation requirements include Certificate of Analysis (CoA) for each acid batch, detailed records of in-process testing, and validation studies demonstrating the effectiveness of the quality control system. Many manufacturers now implement electronic quality management systems (eQMS) that integrate with laboratory information management systems (LIMS) to ensure complete traceability.
Industry best practices increasingly focus on preventive controls rather than end-product testing alone. This includes supplier qualification programs, environmental monitoring, and comprehensive HACCP plans that specifically address acid control points. Third-party certification programs like FSSC 22000 and BRC Global Standards have developed specific modules addressing fermentation process controls, including acid management protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!