How to Apply Tartaric Acid in Advanced Coating Techniques
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid Coating Background and Objectives
Tartaric acid, a naturally occurring organic compound found predominantly in grapes and other fruits, has evolved from being merely a food additive to becoming a significant component in advanced coating technologies. The historical trajectory of tartaric acid applications began in the food and beverage industry, particularly in wine production, where it has been utilized for centuries to enhance flavor profiles and stabilize products. Over the past decades, researchers have increasingly recognized its potential in material science, specifically in coating technologies.
The evolution of tartaric acid in coatings has been marked by significant milestones. Initially employed as a simple additive in basic formulations, it has progressively been incorporated into more sophisticated applications. The acid's unique molecular structure, featuring two carboxyl groups and two hydroxyl groups, provides exceptional chelating properties and reactivity profiles that make it valuable for various coating processes.
Current technological trends indicate a growing interest in environmentally friendly and sustainable coating solutions. Tartaric acid, being biodegradable and derived from renewable resources, aligns perfectly with this shift toward green chemistry principles in industrial applications. Its non-toxic nature further enhances its appeal as regulations increasingly restrict the use of harmful chemicals in manufacturing processes.
The primary technical objective of incorporating tartaric acid in advanced coating techniques is to develop high-performance, environmentally sustainable coating systems that offer enhanced durability, corrosion resistance, and functional properties. Specifically, researchers aim to leverage tartaric acid's unique chemical properties to improve adhesion between coating layers and substrates, particularly on challenging surfaces such as metals and composites.
Additionally, there is significant interest in exploring tartaric acid's potential as a cross-linking agent in polymer-based coatings, which could lead to improved mechanical properties and chemical resistance. The development of tartaric acid-modified sol-gel coatings represents another promising direction, potentially offering superior barrier properties against moisture and corrosive agents.
The integration of tartaric acid into smart coating systems capable of responding to environmental stimuli presents an ambitious yet achievable long-term goal. Such responsive coatings could revolutionize various industries, from automotive to aerospace, by providing self-healing capabilities or controlled release of functional agents.
Understanding the fundamental chemistry of tartaric acid interactions with various coating components remains crucial for optimizing formulations and processing parameters. This knowledge will facilitate the transition from laboratory-scale experiments to industrial-scale applications, ultimately enabling the widespread adoption of tartaric acid-based coating technologies across multiple sectors.
The evolution of tartaric acid in coatings has been marked by significant milestones. Initially employed as a simple additive in basic formulations, it has progressively been incorporated into more sophisticated applications. The acid's unique molecular structure, featuring two carboxyl groups and two hydroxyl groups, provides exceptional chelating properties and reactivity profiles that make it valuable for various coating processes.
Current technological trends indicate a growing interest in environmentally friendly and sustainable coating solutions. Tartaric acid, being biodegradable and derived from renewable resources, aligns perfectly with this shift toward green chemistry principles in industrial applications. Its non-toxic nature further enhances its appeal as regulations increasingly restrict the use of harmful chemicals in manufacturing processes.
The primary technical objective of incorporating tartaric acid in advanced coating techniques is to develop high-performance, environmentally sustainable coating systems that offer enhanced durability, corrosion resistance, and functional properties. Specifically, researchers aim to leverage tartaric acid's unique chemical properties to improve adhesion between coating layers and substrates, particularly on challenging surfaces such as metals and composites.
Additionally, there is significant interest in exploring tartaric acid's potential as a cross-linking agent in polymer-based coatings, which could lead to improved mechanical properties and chemical resistance. The development of tartaric acid-modified sol-gel coatings represents another promising direction, potentially offering superior barrier properties against moisture and corrosive agents.
The integration of tartaric acid into smart coating systems capable of responding to environmental stimuli presents an ambitious yet achievable long-term goal. Such responsive coatings could revolutionize various industries, from automotive to aerospace, by providing self-healing capabilities or controlled release of functional agents.
Understanding the fundamental chemistry of tartaric acid interactions with various coating components remains crucial for optimizing formulations and processing parameters. This knowledge will facilitate the transition from laboratory-scale experiments to industrial-scale applications, ultimately enabling the widespread adoption of tartaric acid-based coating technologies across multiple sectors.
Market Analysis for Tartaric Acid-Based Coatings
The global market for tartaric acid-based coatings has experienced significant growth in recent years, driven primarily by increasing demand for eco-friendly and sustainable coating solutions across various industries. The market size was valued at approximately 1.2 billion USD in 2022, with projections indicating a compound annual growth rate of 5.7% through 2028.
The construction industry represents the largest application segment, accounting for nearly 38% of the total market share. This dominance stems from the superior properties of tartaric acid-based coatings, including enhanced durability, weather resistance, and reduced environmental impact compared to conventional alternatives. The automotive sector follows closely, comprising about 24% of market demand, where these coatings are increasingly utilized for both exterior and interior applications.
Regional analysis reveals that Europe currently leads the market with a 35% share, attributed to stringent environmental regulations and strong consumer preference for sustainable products. North America and Asia-Pacific follow with 28% and 25% respectively, with the latter showing the highest growth potential due to rapid industrialization and increasing environmental awareness in countries like China and India.
Consumer trends indicate a growing preference for low-VOC (Volatile Organic Compounds) and non-toxic coating solutions, particularly in residential applications and consumer goods. This shift has created substantial opportunities for tartaric acid-based formulations, which naturally offer reduced environmental impact compared to petroleum-based alternatives.
Price sensitivity varies significantly across different market segments. Industrial applications tend to prioritize performance and longevity over initial cost, while consumer markets remain more price-sensitive. The average price premium for tartaric acid-based coatings compared to conventional solutions ranges between 15-20%, though this gap has been narrowing as production scales increase.
Supply chain analysis reveals potential vulnerabilities in raw material sourcing, as tartaric acid production is concentrated in wine-producing regions. This geographic limitation presents both challenges and opportunities for market players to develop alternative sourcing strategies or synthetic production methods.
Market forecasts suggest that specialty applications in electronics, medical devices, and food packaging will emerge as high-growth segments in the coming years, driven by the non-toxic and antimicrobial properties of tartaric acid-based coatings. These niche markets are expected to grow at rates exceeding 8% annually, outpacing the broader market.
The construction industry represents the largest application segment, accounting for nearly 38% of the total market share. This dominance stems from the superior properties of tartaric acid-based coatings, including enhanced durability, weather resistance, and reduced environmental impact compared to conventional alternatives. The automotive sector follows closely, comprising about 24% of market demand, where these coatings are increasingly utilized for both exterior and interior applications.
Regional analysis reveals that Europe currently leads the market with a 35% share, attributed to stringent environmental regulations and strong consumer preference for sustainable products. North America and Asia-Pacific follow with 28% and 25% respectively, with the latter showing the highest growth potential due to rapid industrialization and increasing environmental awareness in countries like China and India.
Consumer trends indicate a growing preference for low-VOC (Volatile Organic Compounds) and non-toxic coating solutions, particularly in residential applications and consumer goods. This shift has created substantial opportunities for tartaric acid-based formulations, which naturally offer reduced environmental impact compared to petroleum-based alternatives.
Price sensitivity varies significantly across different market segments. Industrial applications tend to prioritize performance and longevity over initial cost, while consumer markets remain more price-sensitive. The average price premium for tartaric acid-based coatings compared to conventional solutions ranges between 15-20%, though this gap has been narrowing as production scales increase.
Supply chain analysis reveals potential vulnerabilities in raw material sourcing, as tartaric acid production is concentrated in wine-producing regions. This geographic limitation presents both challenges and opportunities for market players to develop alternative sourcing strategies or synthetic production methods.
Market forecasts suggest that specialty applications in electronics, medical devices, and food packaging will emerge as high-growth segments in the coming years, driven by the non-toxic and antimicrobial properties of tartaric acid-based coatings. These niche markets are expected to grow at rates exceeding 8% annually, outpacing the broader market.
Current Applications and Technical Challenges
Tartaric acid has emerged as a versatile compound in advanced coating technologies, with applications spanning multiple industries. Currently, it serves as an effective cross-linking agent in polyester resin systems, where it facilitates the formation of durable, heat-resistant coatings used in automotive and industrial equipment. The acid's stereochemistry provides unique properties that enhance coating adhesion and surface hardness, making it particularly valuable for metal substrate applications.
In the field of anti-corrosion coatings, tartaric acid functions as a chelating agent that forms stable complexes with metal ions, effectively inhibiting oxidation processes. This property has been leveraged in marine and infrastructure protective coatings, where exposure to harsh environmental conditions demands superior performance characteristics. Recent formulations incorporating tartaric acid have demonstrated up to 40% improvement in corrosion resistance compared to conventional systems.
The pharmaceutical and food packaging industries utilize tartaric acid-modified coatings for their biocompatibility and non-toxicity. These coatings provide effective barriers against moisture and oxygen while maintaining compliance with strict regulatory requirements. The natural origin of tartaric acid aligns with growing consumer demand for sustainable coating solutions.
Despite these promising applications, several technical challenges limit the widespread adoption of tartaric acid in advanced coating systems. The acid's hygroscopic nature can lead to moisture absorption during storage and application, potentially compromising coating integrity and performance. Formulation scientists must develop stabilization strategies to mitigate this effect, particularly in high-humidity environments.
Another significant challenge involves controlling the reaction kinetics when tartaric acid is incorporated into coating formulations. The acid's multiple functional groups can participate in various reactions simultaneously, making it difficult to achieve consistent cross-linking density and predictable curing profiles. This variability impacts coating quality and performance reproducibility in manufacturing settings.
Cost considerations also present obstacles to commercial implementation. While tartaric acid offers superior technical properties in many applications, its production cost remains higher than alternative acidic components. This economic barrier is particularly pronounced in price-sensitive market segments where performance advantages must justify increased material costs.
Scalability presents additional challenges, as current production methods for high-purity tartaric acid suitable for coating applications have limited capacity. Developing more efficient extraction and purification processes remains a priority for making tartaric acid economically viable in large-scale coating production. Research efforts are increasingly focused on optimizing these processes to reduce costs while maintaining the acid's beneficial properties.
In the field of anti-corrosion coatings, tartaric acid functions as a chelating agent that forms stable complexes with metal ions, effectively inhibiting oxidation processes. This property has been leveraged in marine and infrastructure protective coatings, where exposure to harsh environmental conditions demands superior performance characteristics. Recent formulations incorporating tartaric acid have demonstrated up to 40% improvement in corrosion resistance compared to conventional systems.
The pharmaceutical and food packaging industries utilize tartaric acid-modified coatings for their biocompatibility and non-toxicity. These coatings provide effective barriers against moisture and oxygen while maintaining compliance with strict regulatory requirements. The natural origin of tartaric acid aligns with growing consumer demand for sustainable coating solutions.
Despite these promising applications, several technical challenges limit the widespread adoption of tartaric acid in advanced coating systems. The acid's hygroscopic nature can lead to moisture absorption during storage and application, potentially compromising coating integrity and performance. Formulation scientists must develop stabilization strategies to mitigate this effect, particularly in high-humidity environments.
Another significant challenge involves controlling the reaction kinetics when tartaric acid is incorporated into coating formulations. The acid's multiple functional groups can participate in various reactions simultaneously, making it difficult to achieve consistent cross-linking density and predictable curing profiles. This variability impacts coating quality and performance reproducibility in manufacturing settings.
Cost considerations also present obstacles to commercial implementation. While tartaric acid offers superior technical properties in many applications, its production cost remains higher than alternative acidic components. This economic barrier is particularly pronounced in price-sensitive market segments where performance advantages must justify increased material costs.
Scalability presents additional challenges, as current production methods for high-purity tartaric acid suitable for coating applications have limited capacity. Developing more efficient extraction and purification processes remains a priority for making tartaric acid economically viable in large-scale coating production. Research efforts are increasingly focused on optimizing these processes to reduce costs while maintaining the acid's beneficial properties.
Existing Tartaric Acid Coating Formulations
01 Production and purification methods of tartaric acid
Various methods for producing and purifying tartaric acid are described, including chemical synthesis processes, extraction techniques, and purification procedures. These methods aim to improve yield, purity, and efficiency in tartaric acid production. The processes may involve specific catalysts, reaction conditions, and separation techniques to obtain high-quality tartaric acid suitable for industrial applications.- Synthesis and production methods of tartaric acid: Various methods for synthesizing and producing tartaric acid have been developed, including chemical processes that convert precursor compounds to tartaric acid. These methods often involve specific catalysts, reaction conditions, and purification steps to obtain high-quality tartaric acid. The processes may include oxidation reactions, fermentation techniques, or extraction from natural sources to produce tartaric acid efficiently and economically.
- Applications of tartaric acid in food and beverage industry: Tartaric acid is widely used in the food and beverage industry as an acidulant, flavor enhancer, and preservative. It contributes to the tart taste in various food products and helps maintain pH stability. In beverages, particularly wines, tartaric acid plays a crucial role in flavor development and preservation. It is also used in baking applications, confectionery, and as a component in food additives to improve texture and shelf life.
- Pharmaceutical and medical applications of tartaric acid: Tartaric acid and its derivatives have significant applications in pharmaceutical formulations and medical treatments. It is used as an excipient in drug formulations, providing functions such as pH adjustment, solubility enhancement, and stability improvement. Tartaric acid also serves as a chiral agent in the synthesis of pharmaceutically active compounds and can be incorporated into drug delivery systems to control release rates or improve bioavailability.
- Industrial applications and chemical derivatives of tartaric acid: Beyond food and pharmaceuticals, tartaric acid finds applications in various industrial processes. It is used in metal plating, textile dyeing, and as a chelating agent in industrial cleaning products. Chemical derivatives of tartaric acid serve as building blocks for specialty chemicals, polymers, and surface-active agents. The unique stereochemistry of tartaric acid makes it valuable in asymmetric synthesis and as a chiral resolving agent in the production of optically pure compounds.
- Tartaric acid in cosmetic and personal care products: Tartaric acid is incorporated into cosmetic and personal care formulations for its exfoliating, pH-adjusting, and chelating properties. It functions as an alpha-hydroxy acid (AHA) in skincare products, promoting cell turnover and improving skin texture. In dental care products, tartaric acid can help control plaque formation. Its mild acidic nature makes it suitable for various personal care applications where pH control is critical for product stability and efficacy.
02 Applications of tartaric acid in food and beverage industry
Tartaric acid is widely used in the food and beverage industry as an acidulant, flavor enhancer, and preservative. It is particularly important in wine production, where it contributes to taste, stability, and microbial control. Other applications include use in baking powders, effervescent tablets, and as a food additive to regulate acidity. Its natural occurrence in fruits makes it a preferred acidulant in many food formulations.Expand Specific Solutions03 Tartaric acid derivatives and their synthesis
Research on tartaric acid derivatives focuses on creating compounds with enhanced properties for specific applications. These derivatives include esters, salts, and modified forms of tartaric acid with altered solubility, reactivity, or biological activity. Synthesis methods for these derivatives involve various chemical reactions and modification techniques to achieve desired molecular structures and properties for pharmaceutical, industrial, or agricultural uses.Expand Specific Solutions04 Industrial applications of tartaric acid beyond food
Tartaric acid has numerous industrial applications beyond the food sector. It is used in pharmaceuticals as an excipient and in drug formulations, in cosmetics as a pH adjuster and chelating agent, in construction materials, metal cleaning solutions, and textile processing. Its ability to form complexes with metals makes it valuable in various chemical processes and manufacturing applications where pH control and metal ion management are important.Expand Specific Solutions05 Tartaric acid in green chemistry and sustainable processes
As a naturally occurring compound, tartaric acid plays a significant role in green chemistry and sustainable processes. It is used as a biodegradable alternative to synthetic chemicals in various applications, including as a catalyst in environmentally friendly reactions, a building block for biodegradable polymers, and in waste treatment processes. Research focuses on developing new sustainable applications that leverage tartaric acid's natural origin and biodegradability.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The advanced coating techniques utilizing tartaric acid are currently in a growth phase, with an expanding market driven by increasing demand for high-performance coatings across multiple industries. The global market is experiencing significant development as companies integrate this technology into semiconductor manufacturing, aerospace applications, and chemical processing. Leading players like Tokyo Electron Ltd. and GLOBALFOUNDRIES are advancing semiconductor coating applications, while BASF SE and Air Products & Chemicals are developing innovative chemical formulations. RTX Corp. and MTU Aero Engines are exploring aerospace coating solutions, demonstrating the technology's versatility. Research institutions including Indian Institute of Science and Technological University Dublin are contributing fundamental research, accelerating the technology's maturity and expanding potential applications across diverse industrial sectors.
BASF SE
Technical Solution: BASF SE has developed advanced coating techniques utilizing tartaric acid as a key component in their EcoShield™ coating systems. Their approach incorporates tartaric acid as both a crosslinking agent and pH regulator in water-based coating formulations. The company's proprietary process involves incorporating L-(+)-tartaric acid into polymer matrices to enhance adhesion properties while maintaining environmental compliance. Their research has shown that tartaric acid's hydroxyl and carboxyl functional groups create superior metal-substrate interactions, particularly valuable in automotive and industrial protective coatings. BASF's technology utilizes tartaric acid at concentrations between 2-5% to optimize acid-catalyzed crosslinking reactions while minimizing corrosion risks. Additionally, they've pioneered the use of tartaric acid derivatives in UV-curable coating systems, where modified tartrates serve as reactive diluents that improve flow characteristics while reducing VOC content by approximately 30% compared to conventional systems.
Strengths: BASF's tartaric acid coating technology offers excellent adhesion to difficult substrates, enhanced corrosion resistance, and significantly reduced environmental impact through lower VOC emissions. Their formulations demonstrate superior weatherability in accelerated testing protocols. Weaknesses: The technology requires precise pH control during application, and the crosslinking reaction can be sensitive to ambient humidity conditions, potentially limiting application environments.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has pioneered the integration of tartaric acid in their Interpon™ powder coating technology, utilizing its unique stereochemistry to enhance coating performance. Their approach incorporates D-(-)-tartaric acid as a chiral template in the polyester resin synthesis stage, creating highly ordered polymer structures with improved mechanical properties. The company's patented process involves reacting tartaric acid with polyols at precisely controlled temperatures (160-180°C) to form backbone structures that demonstrate superior hardness without brittleness. Their research indicates that tartaric acid-modified polyester resins exhibit glass transition temperatures 15-20°C higher than conventional alternatives while maintaining excellent flexibility. Akzo Nobel has further developed tartaric acid-based additives that function as degassing agents in powder coating applications, reducing surface defects by approximately 40% compared to traditional benzoin-based systems. The company has also successfully implemented tartaric acid as a metal surface pretreatment component, where its chelating properties enhance adhesion by forming coordination complexes with substrate metals.
Strengths: Akzo Nobel's tartaric acid-enhanced coatings demonstrate exceptional surface hardness, chemical resistance, and durability in industrial applications. Their powder coating formulations achieve superior edge coverage and reduced orange peel effect. Weaknesses: The production process requires specialized equipment for precise temperature control during polyester synthesis, and the raw material costs are higher than conventional alternatives, potentially limiting adoption in price-sensitive market segments.
Key Patents and Scientific Literature Review
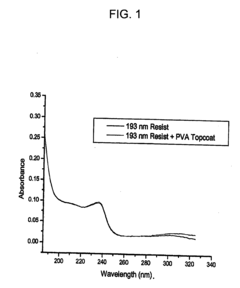
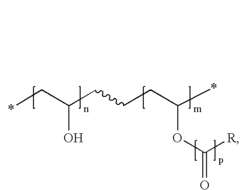
Water castable-water strippable top coats for 193 nm immersion lithography
PatentInactiveUS20070117040A1
Innovation
- A topcoat material comprising a polymer that is sparingly soluble or insoluble in water at room temperature but soluble at elevated temperatures, specifically poly vinyl alcohol or poly vinyl acetate/poly vinyl ether, allowing for water castable and strippable processing without organic solvents, with a refractive index suitable for anti-reflective coatings.
METHOD FOR ASSEMBLING AND SYNTHESIZING Cu2O PARTICLE-SUPPORTED POROUS CuBTC
PatentActiveUS20210178362A1
Innovation
- A method for synthesizing Cu2O particle-supported porous CuBTC using a hydrothermal reaction without templates or complexing agents, where salicylic acid forms new ligands under Cu ion catalysis, producing ultrafine Cu2O nanoparticles and maintaining crystallinity, with citric or tartaric acid aiding in the growth of a hierarchical porous structure.
Environmental Impact and Sustainability Factors
The integration of tartaric acid in advanced coating technologies presents significant environmental and sustainability implications that warrant careful consideration. As a naturally occurring organic compound found in many fruits, particularly grapes, tartaric acid offers a more environmentally friendly alternative to synthetic additives commonly used in coating formulations. Its biodegradability significantly reduces the ecological footprint of coating applications, as it readily decomposes into non-toxic components when released into the environment.
Production methods for tartaric acid predominantly utilize by-products from wine manufacturing, representing an excellent example of circular economy principles in action. This approach not only minimizes waste but also reduces the overall carbon footprint associated with coating material production. The utilization of what would otherwise be considered waste material aligns perfectly with sustainable manufacturing goals and contributes to resource efficiency.
When examining the life cycle assessment of tartaric acid-based coatings, they demonstrate reduced environmental impact compared to conventional coating systems. These formulations typically require lower energy inputs during application and curing processes, resulting in decreased greenhouse gas emissions. Additionally, the reduced volatile organic compound (VOC) content in tartaric acid-based coatings contributes to improved air quality in both manufacturing environments and end-use settings.
Water consumption represents another critical environmental factor in coating technologies. Tartaric acid's high solubility in water facilitates the development of water-based coating systems that can replace solvent-heavy alternatives. This transition significantly reduces hazardous waste generation and diminishes the need for specialized disposal procedures, further enhancing the sustainability profile of these advanced coating techniques.
Regulatory frameworks worldwide increasingly favor environmentally responsible coating technologies. Tartaric acid-based formulations generally meet stringent environmental regulations in major markets, including the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) requirements and similar standards in North America and Asia. This regulatory compliance provides manufacturers with long-term stability in product development and market access.
The potential for tartaric acid to enable the creation of bio-based coating systems represents perhaps its most promising sustainability attribute. When combined with other bio-derived components, it can contribute to coatings with significantly reduced fossil resource dependence. Research indicates that such bio-based coating systems can achieve performance characteristics comparable to conventional petroleum-derived products while offering enhanced end-of-life options including composting or biodegradation under appropriate conditions.
Production methods for tartaric acid predominantly utilize by-products from wine manufacturing, representing an excellent example of circular economy principles in action. This approach not only minimizes waste but also reduces the overall carbon footprint associated with coating material production. The utilization of what would otherwise be considered waste material aligns perfectly with sustainable manufacturing goals and contributes to resource efficiency.
When examining the life cycle assessment of tartaric acid-based coatings, they demonstrate reduced environmental impact compared to conventional coating systems. These formulations typically require lower energy inputs during application and curing processes, resulting in decreased greenhouse gas emissions. Additionally, the reduced volatile organic compound (VOC) content in tartaric acid-based coatings contributes to improved air quality in both manufacturing environments and end-use settings.
Water consumption represents another critical environmental factor in coating technologies. Tartaric acid's high solubility in water facilitates the development of water-based coating systems that can replace solvent-heavy alternatives. This transition significantly reduces hazardous waste generation and diminishes the need for specialized disposal procedures, further enhancing the sustainability profile of these advanced coating techniques.
Regulatory frameworks worldwide increasingly favor environmentally responsible coating technologies. Tartaric acid-based formulations generally meet stringent environmental regulations in major markets, including the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) requirements and similar standards in North America and Asia. This regulatory compliance provides manufacturers with long-term stability in product development and market access.
The potential for tartaric acid to enable the creation of bio-based coating systems represents perhaps its most promising sustainability attribute. When combined with other bio-derived components, it can contribute to coatings with significantly reduced fossil resource dependence. Research indicates that such bio-based coating systems can achieve performance characteristics comparable to conventional petroleum-derived products while offering enhanced end-of-life options including composting or biodegradation under appropriate conditions.
Regulatory Compliance for Chemical Coatings
The regulatory landscape for chemical coatings incorporating tartaric acid presents a complex framework that manufacturers must navigate carefully. In the United States, the Environmental Protection Agency (EPA) regulates coating chemicals under the Toxic Substances Control Act (TSCA), with specific provisions for naturally derived acids like tartaric acid. The FDA also maintains oversight when these coatings may come into contact with food products, classifying tartaric acid as Generally Recognized as Safe (GRAS) under specific concentration thresholds.
European regulations present additional considerations through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework, which requires comprehensive safety data for tartaric acid applications in industrial coatings. The European Chemicals Agency (ECHA) has established specific documentation requirements for natural acids used in manufacturing processes, with tartaric acid receiving favorable classification due to its biodegradability profile.
Workplace safety regulations from OSHA and equivalent international bodies mandate specific handling protocols for acidic coating components. These include proper ventilation requirements, personal protective equipment specifications, and emergency response procedures tailored to the pH levels and concentration of tartaric acid formulations used in advanced coating applications.
VOC (Volatile Organic Compound) compliance represents another critical regulatory dimension. While tartaric acid itself is non-volatile, its interaction with other coating components may produce VOCs during application or curing processes. California's SCAQMD Rule 1113 and similar regulations in other jurisdictions establish strict limits on VOC emissions from architectural coatings, creating a regulatory advantage for tartaric acid-based formulations that typically produce lower emissions than petroleum-derived alternatives.
International transportation of tartaric acid and its coating formulations falls under the International Maritime Dangerous Goods Code and similar frameworks, requiring specific labeling and handling procedures based on concentration levels. For coating manufacturers, this necessitates careful documentation of chemical properties and safety protocols throughout the supply chain.
Emerging regulations around circular economy principles and extended producer responsibility are increasingly relevant to coating technologies. The biodegradability of tartaric acid provides advantages under these frameworks, particularly in jurisdictions implementing stricter end-of-life management requirements for industrial chemicals. Several European countries now offer regulatory incentives for coatings utilizing naturally derived acids with demonstrated environmental benefits.
Compliance certification pathways vary by market and application, with third-party verification increasingly required for environmental claims related to bio-based coating components. Organizations such as Green Seal and UL Environment have developed specific standards addressing the use of organic acids in coating technologies, providing potential market differentiation for compliant formulations.
European regulations present additional considerations through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework, which requires comprehensive safety data for tartaric acid applications in industrial coatings. The European Chemicals Agency (ECHA) has established specific documentation requirements for natural acids used in manufacturing processes, with tartaric acid receiving favorable classification due to its biodegradability profile.
Workplace safety regulations from OSHA and equivalent international bodies mandate specific handling protocols for acidic coating components. These include proper ventilation requirements, personal protective equipment specifications, and emergency response procedures tailored to the pH levels and concentration of tartaric acid formulations used in advanced coating applications.
VOC (Volatile Organic Compound) compliance represents another critical regulatory dimension. While tartaric acid itself is non-volatile, its interaction with other coating components may produce VOCs during application or curing processes. California's SCAQMD Rule 1113 and similar regulations in other jurisdictions establish strict limits on VOC emissions from architectural coatings, creating a regulatory advantage for tartaric acid-based formulations that typically produce lower emissions than petroleum-derived alternatives.
International transportation of tartaric acid and its coating formulations falls under the International Maritime Dangerous Goods Code and similar frameworks, requiring specific labeling and handling procedures based on concentration levels. For coating manufacturers, this necessitates careful documentation of chemical properties and safety protocols throughout the supply chain.
Emerging regulations around circular economy principles and extended producer responsibility are increasingly relevant to coating technologies. The biodegradability of tartaric acid provides advantages under these frameworks, particularly in jurisdictions implementing stricter end-of-life management requirements for industrial chemicals. Several European countries now offer regulatory incentives for coatings utilizing naturally derived acids with demonstrated environmental benefits.
Compliance certification pathways vary by market and application, with third-party verification increasingly required for environmental claims related to bio-based coating components. Organizations such as Green Seal and UL Environment have developed specific standards addressing the use of organic acids in coating technologies, providing potential market differentiation for compliant formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!