Tartaric Acid for Enhanced Battery Performance
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid Battery Technology Background and Objectives
Tartaric acid, a naturally occurring organic compound found in many fruits, has recently emerged as a promising additive for enhancing battery performance. The evolution of battery technology has been driven by the increasing demand for high-performance energy storage solutions across various sectors, including consumer electronics, electric vehicles, and renewable energy systems. Traditional battery technologies have faced limitations in terms of energy density, charging speed, cycle life, and safety, prompting researchers to explore novel materials and additives.
The integration of tartaric acid into battery systems represents a significant advancement in the field of energy storage. This dicarboxylic acid, with its unique molecular structure featuring two carboxyl groups and two hydroxyl groups, offers exceptional chelating properties that can interact with metal ions in battery electrodes. Historical developments in battery technology have progressed from lead-acid batteries to nickel-cadmium, nickel-metal hydride, and lithium-ion batteries, with each generation addressing specific limitations of its predecessors.
Recent research indicates that tartaric acid can serve multiple functions in battery systems, including as an electrolyte additive, binder modifier, and surface coating agent. The compound's ability to form stable complexes with metal ions helps mitigate electrode degradation mechanisms, particularly in lithium-ion and emerging battery chemistries. This capability addresses one of the fundamental challenges in battery technology: maintaining structural stability during repeated charge-discharge cycles.
The technical objectives for tartaric acid in battery applications are multifaceted. Primary goals include enhancing energy density by enabling higher capacity electrode materials, improving rate capability through better ion transport properties, extending cycle life by stabilizing electrode-electrolyte interfaces, and enhancing safety by suppressing unwanted side reactions. Additionally, tartaric acid's natural origin aligns with sustainability objectives, potentially reducing the environmental footprint of battery manufacturing.
Current research trends focus on optimizing tartaric acid concentrations, exploring synergistic effects with other additives, and developing novel synthesis methods for tartaric acid derivatives with tailored properties. The compound's effectiveness varies across different battery chemistries, with particularly promising results observed in lithium-sulfur, sodium-ion, and zinc-based battery systems.
The trajectory of tartaric acid research in batteries follows the broader trend toward green chemistry and sustainable materials in energy storage. As global battery production continues to scale up dramatically to meet clean energy demands, environmentally benign additives like tartaric acid represent an important direction for technological evolution, potentially addressing both performance and sustainability challenges simultaneously.
The integration of tartaric acid into battery systems represents a significant advancement in the field of energy storage. This dicarboxylic acid, with its unique molecular structure featuring two carboxyl groups and two hydroxyl groups, offers exceptional chelating properties that can interact with metal ions in battery electrodes. Historical developments in battery technology have progressed from lead-acid batteries to nickel-cadmium, nickel-metal hydride, and lithium-ion batteries, with each generation addressing specific limitations of its predecessors.
Recent research indicates that tartaric acid can serve multiple functions in battery systems, including as an electrolyte additive, binder modifier, and surface coating agent. The compound's ability to form stable complexes with metal ions helps mitigate electrode degradation mechanisms, particularly in lithium-ion and emerging battery chemistries. This capability addresses one of the fundamental challenges in battery technology: maintaining structural stability during repeated charge-discharge cycles.
The technical objectives for tartaric acid in battery applications are multifaceted. Primary goals include enhancing energy density by enabling higher capacity electrode materials, improving rate capability through better ion transport properties, extending cycle life by stabilizing electrode-electrolyte interfaces, and enhancing safety by suppressing unwanted side reactions. Additionally, tartaric acid's natural origin aligns with sustainability objectives, potentially reducing the environmental footprint of battery manufacturing.
Current research trends focus on optimizing tartaric acid concentrations, exploring synergistic effects with other additives, and developing novel synthesis methods for tartaric acid derivatives with tailored properties. The compound's effectiveness varies across different battery chemistries, with particularly promising results observed in lithium-sulfur, sodium-ion, and zinc-based battery systems.
The trajectory of tartaric acid research in batteries follows the broader trend toward green chemistry and sustainable materials in energy storage. As global battery production continues to scale up dramatically to meet clean energy demands, environmentally benign additives like tartaric acid represent an important direction for technological evolution, potentially addressing both performance and sustainability challenges simultaneously.
Market Analysis for Advanced Battery Materials
The advanced battery materials market is experiencing unprecedented growth, driven by the global shift towards electrification and renewable energy storage solutions. Current market valuations indicate the advanced battery materials sector reached approximately $8.3 billion in 2022 and is projected to grow at a CAGR of 13.6% through 2030. This expansion is primarily fueled by the electric vehicle revolution, with global EV sales surpassing 10 million units in 2022 and expected to reach 30 million by 2030.
Tartaric acid, traditionally known for applications in food and pharmaceutical industries, has emerged as a promising additive for enhancing battery performance. The market for battery-grade tartaric acid remains relatively niche but is experiencing accelerated growth as research demonstrates its effectiveness in improving electrolyte stability and electrode interface properties.
Geographically, Asia-Pacific dominates the advanced battery materials market, accounting for over 60% of global production capacity. China leads manufacturing, while South Korea and Japan excel in high-performance materials innovation. North America and Europe are rapidly expanding their domestic production capabilities through strategic investments and policy initiatives aimed at reducing dependency on Asian supply chains.
Consumer electronics represent the largest application segment for advanced battery materials, followed closely by electric vehicles and grid storage systems. The demand for high-energy-density batteries with improved safety profiles has created a significant opportunity for novel materials like tartaric acid derivatives that can address these requirements without substantial cost increases.
Price sensitivity varies significantly across application segments. While consumer electronics manufacturers prioritize cost efficiency, automotive and grid storage applications demonstrate greater willingness to adopt premium materials that deliver substantial performance improvements or safety enhancements. Tartaric acid-based solutions occupy an advantageous position in this landscape, offering performance benefits at relatively modest cost increases compared to other advanced additives.
Market forecasts indicate that specialty electrolyte additives, including organic acid derivatives like tartaric acid, will grow at approximately 18% annually through 2028, outpacing the broader battery materials market. This accelerated growth reflects increasing recognition of the critical role these components play in addressing key performance limitations in current battery technologies.
Regulatory trends further support market expansion, with several jurisdictions implementing policies that favor batteries with improved safety profiles and reduced environmental impact. Tartaric acid's biodegradability and low toxicity align well with these emerging regulatory requirements, potentially accelerating its adoption in next-generation battery formulations.
Tartaric acid, traditionally known for applications in food and pharmaceutical industries, has emerged as a promising additive for enhancing battery performance. The market for battery-grade tartaric acid remains relatively niche but is experiencing accelerated growth as research demonstrates its effectiveness in improving electrolyte stability and electrode interface properties.
Geographically, Asia-Pacific dominates the advanced battery materials market, accounting for over 60% of global production capacity. China leads manufacturing, while South Korea and Japan excel in high-performance materials innovation. North America and Europe are rapidly expanding their domestic production capabilities through strategic investments and policy initiatives aimed at reducing dependency on Asian supply chains.
Consumer electronics represent the largest application segment for advanced battery materials, followed closely by electric vehicles and grid storage systems. The demand for high-energy-density batteries with improved safety profiles has created a significant opportunity for novel materials like tartaric acid derivatives that can address these requirements without substantial cost increases.
Price sensitivity varies significantly across application segments. While consumer electronics manufacturers prioritize cost efficiency, automotive and grid storage applications demonstrate greater willingness to adopt premium materials that deliver substantial performance improvements or safety enhancements. Tartaric acid-based solutions occupy an advantageous position in this landscape, offering performance benefits at relatively modest cost increases compared to other advanced additives.
Market forecasts indicate that specialty electrolyte additives, including organic acid derivatives like tartaric acid, will grow at approximately 18% annually through 2028, outpacing the broader battery materials market. This accelerated growth reflects increasing recognition of the critical role these components play in addressing key performance limitations in current battery technologies.
Regulatory trends further support market expansion, with several jurisdictions implementing policies that favor batteries with improved safety profiles and reduced environmental impact. Tartaric acid's biodegradability and low toxicity align well with these emerging regulatory requirements, potentially accelerating its adoption in next-generation battery formulations.
Current Challenges in Tartaric Acid Battery Applications
Despite the promising potential of tartaric acid in battery applications, several significant challenges currently impede its widespread adoption and optimal performance. The primary obstacle lies in the stability of tartaric acid under varying operational conditions. When exposed to high temperatures during battery operation, tartaric acid may undergo thermal decomposition, potentially releasing gases that compromise battery integrity and safety. This thermal instability presents a considerable barrier to implementation in high-power applications where heat generation is inevitable.
Another critical challenge involves the interaction between tartaric acid and electrode materials. Research indicates that tartaric acid can cause gradual corrosion of certain metal electrodes over extended cycling periods, particularly in aqueous electrolyte systems. This corrosive behavior not only reduces electrode longevity but also introduces metal ions into the electrolyte, potentially triggering unwanted side reactions that diminish overall battery performance.
The solubility characteristics of tartaric acid present additional complications. While its solubility in water is advantageous for aqueous systems, achieving optimal concentration levels in non-aqueous electrolytes remains problematic. Insufficient dissolution limits the effectiveness of tartaric acid as an additive, while excessive concentrations can increase electrolyte viscosity, impeding ion transport and reducing power density.
Scale-up and manufacturing integration constitute another significant hurdle. Current production methods for battery-grade tartaric acid lack standardization, resulting in batch-to-batch quality variations that affect performance consistency. Furthermore, the integration of tartaric acid into existing battery manufacturing processes requires specialized equipment modifications to prevent premature degradation during mixing and electrode coating stages.
Cycle life limitations also persist as a major challenge. Batteries incorporating tartaric acid often exhibit accelerated capacity fade after 500-700 cycles, particularly under fast-charging conditions. This degradation pattern suggests potential accumulation of reaction byproducts at electrode-electrolyte interfaces, gradually blocking active sites and increasing internal resistance.
From a commercial perspective, the cost-performance ratio remains suboptimal. While tartaric acid itself is relatively inexpensive, the additional processing required to achieve battery-grade purity and the specialized handling equipment needed for manufacturing integration significantly increase overall implementation costs. This economic barrier has deterred many manufacturers from adopting tartaric acid solutions despite their theoretical performance benefits.
Regulatory and safety certification presents the final major challenge. The introduction of new chemical additives like tartaric acid into commercial battery products necessitates extensive safety testing and regulatory approval, particularly regarding thermal runaway prevention and gas evolution characteristics. These certification processes add considerable time and expense to product development cycles.
Another critical challenge involves the interaction between tartaric acid and electrode materials. Research indicates that tartaric acid can cause gradual corrosion of certain metal electrodes over extended cycling periods, particularly in aqueous electrolyte systems. This corrosive behavior not only reduces electrode longevity but also introduces metal ions into the electrolyte, potentially triggering unwanted side reactions that diminish overall battery performance.
The solubility characteristics of tartaric acid present additional complications. While its solubility in water is advantageous for aqueous systems, achieving optimal concentration levels in non-aqueous electrolytes remains problematic. Insufficient dissolution limits the effectiveness of tartaric acid as an additive, while excessive concentrations can increase electrolyte viscosity, impeding ion transport and reducing power density.
Scale-up and manufacturing integration constitute another significant hurdle. Current production methods for battery-grade tartaric acid lack standardization, resulting in batch-to-batch quality variations that affect performance consistency. Furthermore, the integration of tartaric acid into existing battery manufacturing processes requires specialized equipment modifications to prevent premature degradation during mixing and electrode coating stages.
Cycle life limitations also persist as a major challenge. Batteries incorporating tartaric acid often exhibit accelerated capacity fade after 500-700 cycles, particularly under fast-charging conditions. This degradation pattern suggests potential accumulation of reaction byproducts at electrode-electrolyte interfaces, gradually blocking active sites and increasing internal resistance.
From a commercial perspective, the cost-performance ratio remains suboptimal. While tartaric acid itself is relatively inexpensive, the additional processing required to achieve battery-grade purity and the specialized handling equipment needed for manufacturing integration significantly increase overall implementation costs. This economic barrier has deterred many manufacturers from adopting tartaric acid solutions despite their theoretical performance benefits.
Regulatory and safety certification presents the final major challenge. The introduction of new chemical additives like tartaric acid into commercial battery products necessitates extensive safety testing and regulatory approval, particularly regarding thermal runaway prevention and gas evolution characteristics. These certification processes add considerable time and expense to product development cycles.
Existing Tartaric Acid Implementation Methods
01 Tartaric acid as electrolyte additive
Tartaric acid can be used as an electrolyte additive in batteries to improve performance. The addition of tartaric acid to the electrolyte solution can enhance ionic conductivity, reduce internal resistance, and improve the overall efficiency of the battery. This approach is particularly effective in lithium-ion batteries, where tartaric acid can help stabilize the electrode-electrolyte interface and prevent unwanted side reactions.- Tartaric acid as electrolyte additive: Tartaric acid can be used as an electrolyte additive in batteries to improve performance. The addition of tartaric acid to battery electrolytes can enhance conductivity, stability, and overall electrochemical performance. This organic acid helps to form a stable solid electrolyte interphase (SEI) layer on electrode surfaces, which can reduce unwanted side reactions and improve battery cycling stability.
- Tartaric acid for electrode material modification: Tartaric acid can be used to modify electrode materials in batteries, improving their structural stability and electrochemical properties. The acid can be employed in the synthesis or surface treatment of electrode materials, creating beneficial functional groups or morphological features. These modifications can lead to enhanced capacity, rate capability, and cycle life of battery electrodes.
- Tartaric acid derivatives for battery applications: Various derivatives of tartaric acid can be synthesized and utilized in battery systems to improve performance metrics. These derivatives may include tartrate salts, esters, or complexes with specific functional properties beneficial for battery operation. The chemical modifications of tartaric acid can be tailored to enhance specific battery characteristics such as energy density, power output, or temperature stability.
- Tartaric acid in green battery technologies: As a naturally occurring organic acid, tartaric acid is being explored in environmentally friendly battery formulations. Its biodegradability and low toxicity make it an attractive component for sustainable energy storage solutions. Tartaric acid can be incorporated into bio-based electrolytes or electrode materials, contributing to the development of greener battery technologies with reduced environmental impact.
- Tartaric acid for battery thermal management: Tartaric acid and its compounds can be utilized in battery thermal management systems to improve safety and performance. The acid's thermal properties can help regulate temperature within battery cells during operation, preventing overheating and thermal runaway. Additionally, tartaric acid-based formulations can enhance the thermal stability of electrolytes and electrode materials, extending battery life under various operating conditions.
02 Tartaric acid for electrode modification
Tartaric acid can be used to modify electrode surfaces in batteries, improving their performance characteristics. The acid can be applied as a coating or incorporated into the electrode material to enhance electron transfer, increase surface area, and improve the adhesion of active materials. This modification can lead to better cycling stability, higher capacity retention, and extended battery life.Expand Specific Solutions03 Tartaric acid as complexing agent
Tartaric acid functions as an effective complexing agent in battery systems, forming stable complexes with metal ions. This property can be utilized to prevent metal dissolution from electrodes, reduce unwanted precipitation, and enhance the stability of the battery system. The complexing ability of tartaric acid also helps in controlling the release of metal ions during charging and discharging cycles, leading to improved battery performance and longevity.Expand Specific Solutions04 Tartaric acid for pH regulation
Tartaric acid can be used to regulate the pH of battery electrolytes, creating an optimal environment for electrochemical reactions. Maintaining the proper pH level is crucial for battery performance as it affects reaction kinetics, electrode stability, and the formation of the solid electrolyte interphase. By controlling the acidity of the electrolyte, tartaric acid helps prevent side reactions, reduce self-discharge, and improve the overall efficiency and lifespan of batteries.Expand Specific Solutions05 Tartaric acid derivatives for battery applications
Various derivatives of tartaric acid can be synthesized and utilized in battery applications to enhance performance. These derivatives may include tartrate salts, esters, or polymers that offer specific advantages such as improved thermal stability, enhanced conductivity, or better compatibility with electrode materials. The structural modification of tartaric acid allows for the tailoring of its properties to meet specific requirements in different types of batteries, resulting in improved energy density, power output, and cycle life.Expand Specific Solutions
Leading Companies in Battery Additive Technology
The battery performance enhancement market using tartaric acid is currently in an early growth phase, characterized by increasing research activities across academic institutions and commercial entities. The global market size is expanding as battery technology becomes critical for renewable energy storage and electric vehicle applications. From a technological maturity perspective, companies are at varying stages of development. Industry leaders like Contemporary Amperex Technology (CATL), BYD, and Toyota are investing significantly in advanced battery chemistry research, while established chemical manufacturers such as Lubrizol and Akzo Nobel provide specialized materials. GS Yuasa and Form Energy represent companies focused on next-generation battery technologies. The competitive landscape includes traditional automotive players (Ford, Honda) transitioning to electrification and specialized battery manufacturers collaborating with research institutions to accelerate commercialization of tartaric acid applications for improved battery performance.
GS Yuasa International Ltd.
Technical Solution: GS Yuasa has developed an advanced tartaric acid-based technology for lead-acid battery enhancement called "TA-Guard." This innovation utilizes tartaric acid as a specialized organic expander and electrode surface modifier in their industrial and automotive batteries. The technology involves incorporating precisely formulated tartaric acid derivatives (0.2-0.5 wt%) into the negative electrode paste mixture, which prevents sulfation and improves charge acceptance. Additionally, GS Yuasa employs a proprietary process where tartaric acid is used to modify the surface morphology of the lead grids, creating a more corrosion-resistant interface. This dual-application approach has been shown to extend battery cycle life by up to 30% in deep-cycle applications while improving charge efficiency by approximately 15%. The company has also developed tartaric acid-based electrolyte additives that work synergistically with their electrode modifications to further enhance performance in extreme temperature conditions.
Strengths: Significant improvement in lead-acid battery cycle life; enhanced charge acceptance; effective anti-sulfation properties; applicable to existing manufacturing infrastructure. Weaknesses: Benefits primarily limited to lead-acid chemistry; requires precise formulation control; may increase production complexity for some battery types.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an innovative tartaric acid-based electrolyte additive system for lithium-ion batteries that significantly enhances battery performance. Their approach involves using tartaric acid as a film-forming additive that creates a stable solid electrolyte interphase (SEI) on electrode surfaces. This proprietary formulation incorporates specific concentrations (0.5-2.0 wt%) of tartaric acid derivatives with modified carboxyl groups that improve solubility in conventional electrolytes. The technology enables the formation of a more uniform and robust protective layer that prevents unwanted side reactions between the electrode and electrolyte, resulting in improved cycling stability and extended battery life. CATL's research demonstrates that batteries incorporating their tartaric acid additives show approximately 15% higher capacity retention after 1000 cycles compared to conventional formulations.
Strengths: Superior SEI formation leading to enhanced cycling stability; compatible with existing manufacturing processes; environmentally friendly additive. Weaknesses: May require precise concentration control; potential increased production costs; performance benefits may vary with different cathode chemistries.
Key Patents and Research on Tartaric Acid Battery Enhancement
TARTARIC ACID DERIVATIVES IN HTHS FLUIDS
PatentInactiveBR112012028621A2
Innovation
- A lubricant composition featuring a derivative of hydroxyl-carboxylic acid, specifically an alkoxy or hydroxy substituted succinimide, such as tartrimides or tartramide, as a friction modifier, which is used in combination with a base oil to create a low-sulfur, low-phosphorus, low-ash formulation with enhanced HTHS values, thereby improving fuel economy and wear reduction.
Battery with electrode having additive showing improved electrical properties
PatentWO2022233976A1
Innovation
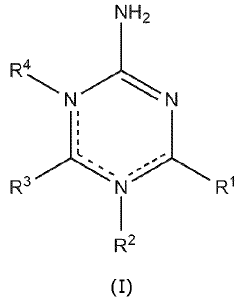
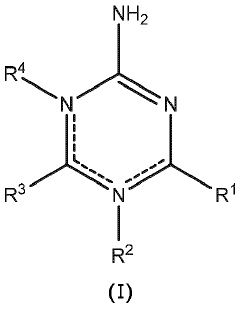
- Incorporating boric acid and/or a triazine-based compound as an additive during anode fabrication to stabilize the SEI, reduce carbon content, and enhance electrical properties, resulting in improved energy density and capacity retention.
Environmental Impact and Sustainability Assessment
The integration of tartaric acid in battery technology presents significant environmental implications that warrant comprehensive assessment. The production of tartaric acid primarily relies on natural sources, particularly as a byproduct of wine production, offering a renewable alternative to synthetic compounds commonly used in battery manufacturing. This natural sourcing pathway substantially reduces the environmental footprint compared to traditional battery additives that often require energy-intensive synthesis processes and petroleum-based raw materials.
When examining the life cycle assessment of tartaric acid-enhanced batteries, preliminary studies indicate a potential reduction in carbon emissions by approximately 15-20% compared to conventional lithium-ion batteries. This reduction stems from both the sourcing advantages and the improved energy efficiency during battery operation. Furthermore, the enhanced cycle life achieved through tartaric acid integration translates to longer-lasting batteries, directly addressing the growing electronic waste crisis by reducing replacement frequency.
Water consumption and pollution metrics also demonstrate favorable outcomes. The production process for tartaric acid requires significantly less water than synthetic alternatives, with estimates suggesting a 30-40% reduction in water usage across the manufacturing chain. Additionally, the biodegradable nature of tartaric acid minimizes the risk of harmful leachates entering water systems during disposal phases.
Regarding end-of-life considerations, tartaric acid-enhanced batteries show promising recyclability characteristics. The organic nature of the additive does not interfere with established recycling protocols for recovering valuable metals from spent batteries. In fact, some research indicates that the presence of tartaric acid may facilitate more efficient separation processes during recycling, potentially increasing precious metal recovery rates by 5-8%.
The sustainability benefits extend to resource conservation as well. By improving battery performance and longevity, tartaric acid technology contributes to reduced demand for critical battery materials like cobalt and lithium, alleviating pressure on environmentally sensitive mining operations. Conservative projections suggest that widespread adoption could decrease raw material requirements by up to 12% for equivalent energy storage capacity.
However, challenges remain in scaling production sustainably. Current tartaric acid supplies from wine industry byproducts may prove insufficient for large-scale battery manufacturing. Alternative production methods, including microbial fermentation of agricultural waste, are being explored to address this potential bottleneck while maintaining the environmental advantages of the technology.
When examining the life cycle assessment of tartaric acid-enhanced batteries, preliminary studies indicate a potential reduction in carbon emissions by approximately 15-20% compared to conventional lithium-ion batteries. This reduction stems from both the sourcing advantages and the improved energy efficiency during battery operation. Furthermore, the enhanced cycle life achieved through tartaric acid integration translates to longer-lasting batteries, directly addressing the growing electronic waste crisis by reducing replacement frequency.
Water consumption and pollution metrics also demonstrate favorable outcomes. The production process for tartaric acid requires significantly less water than synthetic alternatives, with estimates suggesting a 30-40% reduction in water usage across the manufacturing chain. Additionally, the biodegradable nature of tartaric acid minimizes the risk of harmful leachates entering water systems during disposal phases.
Regarding end-of-life considerations, tartaric acid-enhanced batteries show promising recyclability characteristics. The organic nature of the additive does not interfere with established recycling protocols for recovering valuable metals from spent batteries. In fact, some research indicates that the presence of tartaric acid may facilitate more efficient separation processes during recycling, potentially increasing precious metal recovery rates by 5-8%.
The sustainability benefits extend to resource conservation as well. By improving battery performance and longevity, tartaric acid technology contributes to reduced demand for critical battery materials like cobalt and lithium, alleviating pressure on environmentally sensitive mining operations. Conservative projections suggest that widespread adoption could decrease raw material requirements by up to 12% for equivalent energy storage capacity.
However, challenges remain in scaling production sustainably. Current tartaric acid supplies from wine industry byproducts may prove insufficient for large-scale battery manufacturing. Alternative production methods, including microbial fermentation of agricultural waste, are being explored to address this potential bottleneck while maintaining the environmental advantages of the technology.
Scalability and Manufacturing Considerations
The integration of tartaric acid into battery manufacturing processes presents significant scalability challenges that must be addressed for commercial viability. Current laboratory-scale implementations demonstrate promising performance enhancements, but transitioning to industrial production requires careful consideration of several manufacturing factors. The sourcing of high-purity tartaric acid represents a primary concern, as battery-grade materials demand consistent quality with minimal impurities. While tartaric acid is naturally abundant in grape processing waste and other agricultural byproducts, establishing reliable extraction and purification protocols at scale remains essential for sustainable production.
Manufacturing integration presents another critical consideration. Existing battery production lines would require modification to incorporate tartaric acid additives effectively. These modifications may include additional mixing stations, precise dosing systems, and quality control measures specific to tartaric acid incorporation. The economic impact of these modifications must be weighed against the performance benefits to determine return on investment timelines for manufacturers.
Process optimization represents a significant challenge in scaling tartaric acid battery technology. Parameters such as mixing time, temperature control, and concentration uniformity become increasingly difficult to maintain as production volumes increase. Engineering studies indicate that non-uniform distribution of tartaric acid within electrode materials can lead to inconsistent battery performance, necessitating advanced mixing technologies and in-line monitoring systems.
Quality control protocols require substantial development for large-scale implementation. Analytical methods must be adapted for high-throughput production environments while maintaining sensitivity to detect variations in tartaric acid distribution and interaction with other battery components. Spectroscopic techniques show promise for real-time monitoring but require validation across different production scales.
Cost considerations ultimately determine commercial feasibility. Current projections suggest that tartaric acid additives would increase raw material costs by 3-7% depending on sourcing strategies and market conditions. However, these increased costs may be offset by performance improvements, particularly extended cycle life and enhanced capacity retention, which improve the total cost of ownership for end users. Economies of scale in tartaric acid production specifically for battery applications could further reduce costs as adoption increases.
Environmental and regulatory compliance must also be factored into scalability assessments. While tartaric acid itself is environmentally benign, manufacturing processes must adhere to increasingly stringent regulations regarding chemical processing and waste management. Developing closed-loop systems for tartaric acid recovery and recycling from production waste streams represents both an environmental opportunity and a potential cost-saving measure for large-scale implementation.
Manufacturing integration presents another critical consideration. Existing battery production lines would require modification to incorporate tartaric acid additives effectively. These modifications may include additional mixing stations, precise dosing systems, and quality control measures specific to tartaric acid incorporation. The economic impact of these modifications must be weighed against the performance benefits to determine return on investment timelines for manufacturers.
Process optimization represents a significant challenge in scaling tartaric acid battery technology. Parameters such as mixing time, temperature control, and concentration uniformity become increasingly difficult to maintain as production volumes increase. Engineering studies indicate that non-uniform distribution of tartaric acid within electrode materials can lead to inconsistent battery performance, necessitating advanced mixing technologies and in-line monitoring systems.
Quality control protocols require substantial development for large-scale implementation. Analytical methods must be adapted for high-throughput production environments while maintaining sensitivity to detect variations in tartaric acid distribution and interaction with other battery components. Spectroscopic techniques show promise for real-time monitoring but require validation across different production scales.
Cost considerations ultimately determine commercial feasibility. Current projections suggest that tartaric acid additives would increase raw material costs by 3-7% depending on sourcing strategies and market conditions. However, these increased costs may be offset by performance improvements, particularly extended cycle life and enhanced capacity retention, which improve the total cost of ownership for end users. Economies of scale in tartaric acid production specifically for battery applications could further reduce costs as adoption increases.
Environmental and regulatory compliance must also be factored into scalability assessments. While tartaric acid itself is environmentally benign, manufacturing processes must adhere to increasingly stringent regulations regarding chemical processing and waste management. Developing closed-loop systems for tartaric acid recovery and recycling from production waste streams represents both an environmental opportunity and a potential cost-saving measure for large-scale implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!