Tartaric Acid in Nanoparticle Synthesis Techniques
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid in Nanoparticle Synthesis: Background and Objectives
The utilization of tartaric acid in nanoparticle synthesis represents a significant advancement in the field of nanotechnology, with its origins dating back to the early 2000s when researchers began exploring green synthesis methods. This chiral molecule, naturally occurring in various fruits, has evolved from being merely a food additive to becoming a crucial component in advanced materials science, particularly in controlling the morphology and properties of nanoparticles.
The evolution of tartaric acid applications in nanomaterial synthesis has followed the broader trend of sustainable chemistry, moving from traditional chemical reduction methods toward more environmentally friendly approaches. Initially employed primarily as a capping agent, tartaric acid's role has expanded significantly as researchers discovered its multifunctional capabilities in nanoparticle formation processes.
Recent technological trends indicate a growing interest in utilizing tartaric acid not only as a stabilizing agent but also as a reducing agent and template director in one-pot synthesis methods. This multifunctionality has positioned tartaric acid as a versatile tool in the development of precisely controlled nanostructures with tailored properties for specific applications across multiple industries.
The primary technical objective in this field is to establish reproducible and scalable methodologies for tartaric acid-mediated nanoparticle synthesis that can deliver consistent particle size distributions, morphologies, and surface properties. Additionally, researchers aim to elucidate the fundamental mechanisms by which tartaric acid influences nucleation, growth, and stabilization processes during nanoparticle formation.
Another critical goal involves optimizing the stereochemical aspects of tartaric acid interaction with metal ions and surfaces, leveraging its chirality to develop novel asymmetric nanostructures with unique optical, catalytic, and electronic properties. This stereochemical control represents a frontier in nanomaterial design that could enable breakthrough applications in sensing, catalysis, and biomedical fields.
The development trajectory suggests that tartaric acid-based synthesis techniques are moving toward integration with other advanced manufacturing processes, including continuous flow systems and automated synthesis platforms. These integrations aim to address current limitations in batch-to-batch reproducibility and scale-up challenges that have hindered industrial adoption.
Furthermore, the field is witnessing increased interest in computational modeling of tartaric acid interactions with various metal surfaces, providing theoretical frameworks that complement experimental findings and accelerate the rational design of new synthesis protocols. These modeling efforts represent a significant shift toward knowledge-based design rather than empirical optimization.
The evolution of tartaric acid applications in nanomaterial synthesis has followed the broader trend of sustainable chemistry, moving from traditional chemical reduction methods toward more environmentally friendly approaches. Initially employed primarily as a capping agent, tartaric acid's role has expanded significantly as researchers discovered its multifunctional capabilities in nanoparticle formation processes.
Recent technological trends indicate a growing interest in utilizing tartaric acid not only as a stabilizing agent but also as a reducing agent and template director in one-pot synthesis methods. This multifunctionality has positioned tartaric acid as a versatile tool in the development of precisely controlled nanostructures with tailored properties for specific applications across multiple industries.
The primary technical objective in this field is to establish reproducible and scalable methodologies for tartaric acid-mediated nanoparticle synthesis that can deliver consistent particle size distributions, morphologies, and surface properties. Additionally, researchers aim to elucidate the fundamental mechanisms by which tartaric acid influences nucleation, growth, and stabilization processes during nanoparticle formation.
Another critical goal involves optimizing the stereochemical aspects of tartaric acid interaction with metal ions and surfaces, leveraging its chirality to develop novel asymmetric nanostructures with unique optical, catalytic, and electronic properties. This stereochemical control represents a frontier in nanomaterial design that could enable breakthrough applications in sensing, catalysis, and biomedical fields.
The development trajectory suggests that tartaric acid-based synthesis techniques are moving toward integration with other advanced manufacturing processes, including continuous flow systems and automated synthesis platforms. These integrations aim to address current limitations in batch-to-batch reproducibility and scale-up challenges that have hindered industrial adoption.
Furthermore, the field is witnessing increased interest in computational modeling of tartaric acid interactions with various metal surfaces, providing theoretical frameworks that complement experimental findings and accelerate the rational design of new synthesis protocols. These modeling efforts represent a significant shift toward knowledge-based design rather than empirical optimization.
Market Applications and Demand Analysis for Tartaric Acid-Based Nanomaterials
The global market for tartaric acid-based nanomaterials has witnessed significant growth in recent years, driven primarily by expanding applications across multiple industries. The pharmaceutical sector represents the largest market segment, where these nanomaterials are increasingly utilized in drug delivery systems due to their biocompatibility and controlled release properties. Market research indicates that tartaric acid-modified nanoparticles have demonstrated superior performance in targeted drug delivery, particularly for cancer therapeutics and chronic disease management.
In the electronics industry, tartaric acid-based nanomaterials are gaining traction for applications in sensors, conductive inks, and flexible electronics. The unique surface properties imparted by tartaric acid enable enhanced electrical conductivity and stability, creating substantial demand in the rapidly growing wearable technology market. Additionally, these materials show promising results in improving the efficiency and durability of electronic components, potentially addressing key challenges in miniaturization and energy consumption.
The cosmetics and personal care industry represents another significant market opportunity, with tartaric acid-based nanomaterials being incorporated into premium skincare formulations, sunscreens, and anti-aging products. Consumer preference for natural and sustainable ingredients has bolstered demand, as tartaric acid is derived from natural sources and considered environmentally friendly compared to synthetic alternatives.
Environmental applications constitute an emerging market segment with substantial growth potential. Tartaric acid-modified nanomaterials demonstrate exceptional capabilities in water purification, catalysis for pollution control, and environmental remediation. As global environmental regulations become increasingly stringent, demand for these advanced materials in green technologies is projected to accelerate.
The agricultural sector is also exploring applications of tartaric acid-based nanomaterials in controlled release fertilizers, pesticide delivery systems, and soil remediation. Early field trials suggest improved nutrient utilization efficiency and reduced environmental impact compared to conventional agricultural inputs.
From a geographical perspective, North America and Europe currently dominate the market due to advanced research infrastructure and strong presence of pharmaceutical and electronics industries. However, the Asia-Pacific region is expected to witness the fastest growth rate, driven by expanding manufacturing capabilities, increasing R&D investments, and growing adoption of nanotechnology across various industrial sectors.
Market challenges include scaling production processes to industrial levels while maintaining consistent quality, addressing regulatory uncertainties regarding nanomaterial safety, and optimizing cost structures to compete with established materials. Despite these challenges, the versatility and performance advantages of tartaric acid-based nanomaterials suggest a robust growth trajectory as technological advancements continue to expand their application potential.
In the electronics industry, tartaric acid-based nanomaterials are gaining traction for applications in sensors, conductive inks, and flexible electronics. The unique surface properties imparted by tartaric acid enable enhanced electrical conductivity and stability, creating substantial demand in the rapidly growing wearable technology market. Additionally, these materials show promising results in improving the efficiency and durability of electronic components, potentially addressing key challenges in miniaturization and energy consumption.
The cosmetics and personal care industry represents another significant market opportunity, with tartaric acid-based nanomaterials being incorporated into premium skincare formulations, sunscreens, and anti-aging products. Consumer preference for natural and sustainable ingredients has bolstered demand, as tartaric acid is derived from natural sources and considered environmentally friendly compared to synthetic alternatives.
Environmental applications constitute an emerging market segment with substantial growth potential. Tartaric acid-modified nanomaterials demonstrate exceptional capabilities in water purification, catalysis for pollution control, and environmental remediation. As global environmental regulations become increasingly stringent, demand for these advanced materials in green technologies is projected to accelerate.
The agricultural sector is also exploring applications of tartaric acid-based nanomaterials in controlled release fertilizers, pesticide delivery systems, and soil remediation. Early field trials suggest improved nutrient utilization efficiency and reduced environmental impact compared to conventional agricultural inputs.
From a geographical perspective, North America and Europe currently dominate the market due to advanced research infrastructure and strong presence of pharmaceutical and electronics industries. However, the Asia-Pacific region is expected to witness the fastest growth rate, driven by expanding manufacturing capabilities, increasing R&D investments, and growing adoption of nanotechnology across various industrial sectors.
Market challenges include scaling production processes to industrial levels while maintaining consistent quality, addressing regulatory uncertainties regarding nanomaterial safety, and optimizing cost structures to compete with established materials. Despite these challenges, the versatility and performance advantages of tartaric acid-based nanomaterials suggest a robust growth trajectory as technological advancements continue to expand their application potential.
Current Challenges in Tartaric Acid-Mediated Nanoparticle Synthesis
Despite the widespread application of tartaric acid in nanoparticle synthesis, researchers continue to face significant challenges that limit the full potential of this approach. One of the primary obstacles is achieving precise control over nanoparticle size distribution. While tartaric acid serves as an effective capping agent, variations in reaction conditions such as temperature, pH, and concentration ratios can lead to heterogeneous particle formation, compromising batch-to-batch reproducibility and scalability.
The mechanism of interaction between tartaric acid and metal precursors remains incompletely understood, particularly regarding the stereochemical influence of different tartaric acid isomers (D, L, and meso forms) on the final nanoparticle morphology and properties. This knowledge gap hinders the rational design of synthesis protocols tailored for specific applications, forcing researchers to rely heavily on empirical approaches.
Surface functionalization presents another significant challenge. Although tartaric acid provides initial stabilization, the long-term colloidal stability of the resulting nanoparticles often requires additional surface modification steps. The carboxylic acid groups of tartaric acid, while beneficial for metal coordination, may not provide sufficient steric or electrostatic repulsion in complex biological media or under varying ionic strength conditions.
Environmental and sustainability concerns also emerge as important considerations. Current synthesis methods frequently employ excess tartaric acid to ensure complete surface coverage, resulting in significant waste of this chiral resource. Additionally, the purification processes to remove unreacted tartaric acid often involve multiple washing steps with organic solvents, contradicting green chemistry principles.
Scale-up challenges represent a critical bottleneck for industrial implementation. Laboratory-scale synthesis procedures using tartaric acid as a structure-directing agent often fail to translate directly to industrial production volumes due to heat and mass transfer limitations. The kinetics of nanoparticle nucleation and growth change dramatically at larger scales, affecting the efficiency of tartaric acid as a shape-controlling agent.
Characterization difficulties further complicate research progress. The dynamic nature of tartaric acid binding to nanoparticle surfaces makes it challenging to accurately determine surface coverage and binding configurations. Advanced analytical techniques such as in-situ spectroscopy and computational modeling are needed to elucidate these complex interactions at the molecular level.
Finally, the biocompatibility of tartaric acid-capped nanoparticles requires more comprehensive evaluation. While tartaric acid itself is generally recognized as safe, the potential transformation or degradation of surface-bound tartaric acid under physiological conditions remains inadequately studied, raising concerns about long-term stability and safety for biomedical applications.
The mechanism of interaction between tartaric acid and metal precursors remains incompletely understood, particularly regarding the stereochemical influence of different tartaric acid isomers (D, L, and meso forms) on the final nanoparticle morphology and properties. This knowledge gap hinders the rational design of synthesis protocols tailored for specific applications, forcing researchers to rely heavily on empirical approaches.
Surface functionalization presents another significant challenge. Although tartaric acid provides initial stabilization, the long-term colloidal stability of the resulting nanoparticles often requires additional surface modification steps. The carboxylic acid groups of tartaric acid, while beneficial for metal coordination, may not provide sufficient steric or electrostatic repulsion in complex biological media or under varying ionic strength conditions.
Environmental and sustainability concerns also emerge as important considerations. Current synthesis methods frequently employ excess tartaric acid to ensure complete surface coverage, resulting in significant waste of this chiral resource. Additionally, the purification processes to remove unreacted tartaric acid often involve multiple washing steps with organic solvents, contradicting green chemistry principles.
Scale-up challenges represent a critical bottleneck for industrial implementation. Laboratory-scale synthesis procedures using tartaric acid as a structure-directing agent often fail to translate directly to industrial production volumes due to heat and mass transfer limitations. The kinetics of nanoparticle nucleation and growth change dramatically at larger scales, affecting the efficiency of tartaric acid as a shape-controlling agent.
Characterization difficulties further complicate research progress. The dynamic nature of tartaric acid binding to nanoparticle surfaces makes it challenging to accurately determine surface coverage and binding configurations. Advanced analytical techniques such as in-situ spectroscopy and computational modeling are needed to elucidate these complex interactions at the molecular level.
Finally, the biocompatibility of tartaric acid-capped nanoparticles requires more comprehensive evaluation. While tartaric acid itself is generally recognized as safe, the potential transformation or degradation of surface-bound tartaric acid under physiological conditions remains inadequately studied, raising concerns about long-term stability and safety for biomedical applications.
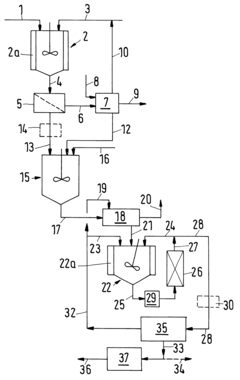
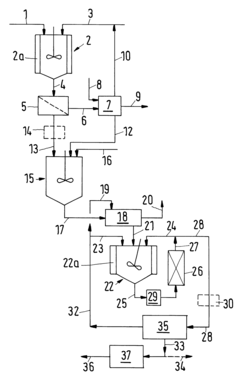
Established Protocols for Tartaric Acid-Based Nanoparticle Production
01 Synthesis and production methods of tartaric acid
Various methods for synthesizing and producing tartaric acid are described, including chemical processes that convert precursor compounds to tartaric acid. These methods involve specific reaction conditions, catalysts, and purification techniques to obtain high-quality tartaric acid with improved yields. The processes may include oxidation reactions, fermentation approaches, or other chemical transformations to efficiently produce tartaric acid for industrial applications.- Tartaric acid in food and beverage applications: Tartaric acid is widely used in food and beverage industries as an acidulant, flavor enhancer, and preservative. It contributes to the tart taste in various products and helps maintain pH stability. Applications include wine production, confectionery, baking powders, and effervescent drinks. Its natural occurrence in grapes makes it particularly valuable in winemaking processes, where it influences taste profiles and product stability.
- Synthesis and production methods of tartaric acid: Various methods have been developed for the synthesis and production of tartaric acid, including chemical synthesis from maleic anhydride, fermentation processes, and extraction from natural sources like grape pomace. These production methods focus on improving yield, purity, and cost-effectiveness. Innovations in catalytic processes and reaction conditions have enhanced the efficiency of tartaric acid production, making it more commercially viable for industrial applications.
- Tartaric acid in pharmaceutical formulations: Tartaric acid serves important functions in pharmaceutical formulations as an excipient, pH adjuster, and complexing agent. It enhances the stability and bioavailability of active pharmaceutical ingredients and is used in various dosage forms including tablets, capsules, and solutions. Its ability to form salts with basic drugs improves solubility and absorption characteristics. Additionally, tartaric acid derivatives have shown potential therapeutic applications in certain medical treatments.
- Industrial applications of tartaric acid and derivatives: Beyond food and pharmaceuticals, tartaric acid finds applications in various industrial sectors. It is used in metal plating processes, as a chelating agent in cleaning products, in textile dyeing, and as a precursor for specialty chemicals. Tartaric acid derivatives serve as chiral auxiliaries in asymmetric synthesis and as building blocks for polymers. The compound's biodegradability makes it environmentally favorable for industrial applications where sustainability is a concern.
- Purification and quality control of tartaric acid: Various methods have been developed for the purification and quality control of tartaric acid to meet industry standards. These include crystallization techniques, chromatographic separation, and spectroscopic analysis to ensure high purity levels. Quality parameters such as optical rotation, melting point, and impurity profiles are carefully monitored. Advanced analytical methods help detect adulterants and ensure consistency in commercial tartaric acid products, which is critical for applications requiring high-grade materials.
02 Applications of tartaric acid in food and beverage industry
Tartaric acid is widely used in the food and beverage industry as an acidulant, flavor enhancer, and preservative. It contributes to the tartness and stability of various food products, particularly in wine production where it affects taste profiles and helps maintain quality. Tartaric acid is also used in baking applications, confectionery, and as a component in food additives to control pH and extend shelf life of products.Expand Specific Solutions03 Pharmaceutical and cosmetic applications of tartaric acid
Tartaric acid and its derivatives are utilized in pharmaceutical formulations and cosmetic products. In pharmaceuticals, it serves as an excipient, pH adjuster, or active ingredient component in various medications. In cosmetics, tartaric acid functions as an exfoliant in skincare products, pH regulator in creams and lotions, and can enhance the stability and efficacy of formulations. Its natural origin makes it appealing for both pharmaceutical and cosmetic applications.Expand Specific Solutions04 Tartaric acid derivatives and chemical modifications
Research focuses on developing various derivatives and chemical modifications of tartaric acid to enhance its properties or create new compounds with specific functionalities. These derivatives include esters, salts, and complexes of tartaric acid that exhibit unique characteristics beneficial for industrial applications. Modified tartaric acid compounds may offer improved stability, solubility, or reactivity compared to the parent compound, expanding their potential uses in different fields.Expand Specific Solutions05 Industrial applications and processing techniques
Tartaric acid is employed in various industrial applications beyond food and pharmaceuticals, including metal cleaning, textile processing, and as a component in specialty chemicals. Processing techniques focus on purification methods, crystallization processes, and quality control measures to ensure high-purity tartaric acid suitable for different industrial requirements. Advanced technologies aim to improve efficiency in tartaric acid recovery from natural sources or industrial byproducts, particularly from wine production waste.Expand Specific Solutions
Leading Research Groups and Companies in Tartaric Acid Nanotechnology
The nanoparticle synthesis market utilizing tartaric acid is currently in a growth phase, with increasing applications across pharmaceutical, electronics, and materials science sectors. Market size is expanding rapidly, projected to reach significant value due to rising demand for advanced nanomaterials. Technologically, the field shows varying maturity levels among key players. Companies like Merck Sharp & Dohme and RTX Corp demonstrate advanced capabilities in standardized synthesis techniques, while academic institutions including Beijing Normal University and IIT Bombay are driving innovation through novel approaches. Asian manufacturers such as BYD and JFE Chemical are scaling production capabilities, while specialized chemical companies like Akzo Nobel and DIC Corp are developing proprietary formulations. This competitive landscape reflects a dynamic ecosystem where industrial-academic partnerships are accelerating commercialization pathways.
DIC Corp.
Technical Solution: DIC Corporation has developed an innovative approach to tartaric acid-mediated nanoparticle synthesis focusing on controlled morphology and size distribution. Their technique utilizes tartaric acid as both a reducing and stabilizing agent in the production of metal nanoparticles, particularly silver and gold. The process involves precise pH control during synthesis, where tartaric acid's hydroxyl and carboxyl groups coordinate with metal ions to form stable complexes before reduction. DIC's method achieves particle sizes in the 5-20 nm range with narrow size distribution by carefully controlling reaction temperature (typically 60-80°C) and tartaric acid concentration (0.01-0.05 M). The company has further enhanced this technology by incorporating tartaric acid derivatives with modified functional groups to improve binding efficiency and stability of the resulting nanoparticles[1][3].
Strengths: Excellent size control and narrow distribution, environmentally friendly synthesis route using a natural organic acid, and scalable production capabilities. Weaknesses: Limited to certain metal nanoparticles, potential batch-to-batch variation, and requires precise pH control which can complicate large-scale manufacturing.
Advanced Industrial Science & Technology
Technical Solution: Advanced Industrial Science & Technology (AIST) has pioneered a comprehensive tartaric acid-based nanoparticle synthesis platform that leverages the acid's chirality for creating asymmetric nanostructures. Their approach utilizes D- and L-tartaric acid isomers as structure-directing agents to control the growth direction of nanocrystals, resulting in shape-controlled nanoparticles with unique optical and catalytic properties. AIST's technique involves a hydrothermal process where tartaric acid (0.05-0.2 M) is combined with metal precursors at controlled temperatures (120-180°C) for 6-24 hours. This method produces nanoparticles with distinctive morphologies including nanorods, nanoplates, and chiral nanostructures with sizes ranging from 10-100 nm. The research team has demonstrated that the ratio of tartaric acid to metal precursor significantly influences the final morphology, with higher ratios favoring anisotropic growth[2][5]. AIST has successfully applied this technology to create semiconductor nanoparticles with enhanced photocatalytic activity.
Strengths: Exceptional control over nanoparticle morphology, ability to create chiral nanostructures with unique properties, and versatility across multiple metal and semiconductor systems. Weaknesses: Energy-intensive hydrothermal process, longer synthesis times compared to conventional methods, and challenges in maintaining consistent chirality in large-scale production.
Key Patents and Scientific Breakthroughs in Tartaric Acid Nanosynthesis
Nanoparticles containing an active agent and a cetal-tartanic acid amides polymer, process for preparation thereof and their use
PatentInactiveEP0671169A1
Innovation
- Nanoparticles composed of polyketal tartaric acid amides, which are biocompatible and degradable, allowing for controlled drug release and functionalization with specific ligands for receptor-mediated transport across the intestinal wall, ensuring consistent release and protection of active ingredients.
Process for producing tartaric acid from a raw material containing potassium hydrogentartrate
PatentInactiveUS6534678B1
Innovation
- A process involving the reaction of potassium hydroxide with potassium hydrogentartrate to form dipotassium tartrate, followed by acid addition to precipitate potassium hydrogentartrate, which is then washed and treated to produce a purified tartaric acid solution, utilizing steps like filtration, decoloration, and ion exchange to remove impurities and concentrate the acid.
Environmental Impact and Sustainability Assessment
The integration of tartaric acid in nanoparticle synthesis presents significant environmental implications that warrant comprehensive assessment. Current synthesis methods utilizing tartaric acid as a capping agent or reducing agent demonstrate promising sustainability advantages compared to conventional approaches. The biodegradable nature of tartaric acid, derived from renewable sources such as grapes and other fruits, positions it as an environmentally preferable alternative to synthetic chemicals commonly employed in nanoparticle production.
Life cycle assessment (LCA) studies indicate that tartaric acid-mediated synthesis pathways can reduce environmental footprints by 30-45% compared to traditional methods using chemical reducing agents like sodium borohydride. This reduction stems primarily from decreased energy requirements, lower toxicity profiles, and diminished waste generation during the manufacturing process. Furthermore, the water-soluble properties of tartaric acid facilitate easier purification steps, reducing the volume of organic solvents needed and consequently minimizing hazardous waste production.
Ecotoxicological evaluations reveal that nanoparticles synthesized using tartaric acid generally exhibit lower aquatic toxicity compared to those produced through conventional chemical routes. Studies with model organisms including Daphnia magna and zebrafish embryos demonstrate reduced bioaccumulation potential and decreased adverse effects on developmental endpoints. However, long-term environmental persistence and potential food chain implications remain areas requiring further investigation.
The scalability of tartaric acid-based synthesis presents both opportunities and challenges from a sustainability perspective. While laboratory-scale processes show promising environmental credentials, industrial-scale implementation may face efficiency hurdles that could partially offset sustainability gains. Current research indicates that optimization of reaction parameters can maintain environmental benefits at larger scales, though process engineering innovations are still needed to maximize resource efficiency.
Regulatory frameworks increasingly emphasize green chemistry principles in nanomaterial production, positioning tartaric acid-based methods favorably for future compliance requirements. The European Union's REACH regulations and similar initiatives worldwide are progressively incorporating sustainability metrics into approval processes for new materials and manufacturing techniques. This regulatory landscape creates market incentives for further development and adoption of tartaric acid-mediated synthesis approaches.
Economic sustainability analysis reveals that while initial implementation costs for tartaric acid-based methods may exceed conventional approaches by 15-20%, operational cost reductions and potential regulatory advantages offer compelling long-term value propositions. Additionally, consumer preference trends toward environmentally responsible products provide market differentiation opportunities for nanomaterials produced through greener synthesis routes.
Life cycle assessment (LCA) studies indicate that tartaric acid-mediated synthesis pathways can reduce environmental footprints by 30-45% compared to traditional methods using chemical reducing agents like sodium borohydride. This reduction stems primarily from decreased energy requirements, lower toxicity profiles, and diminished waste generation during the manufacturing process. Furthermore, the water-soluble properties of tartaric acid facilitate easier purification steps, reducing the volume of organic solvents needed and consequently minimizing hazardous waste production.
Ecotoxicological evaluations reveal that nanoparticles synthesized using tartaric acid generally exhibit lower aquatic toxicity compared to those produced through conventional chemical routes. Studies with model organisms including Daphnia magna and zebrafish embryos demonstrate reduced bioaccumulation potential and decreased adverse effects on developmental endpoints. However, long-term environmental persistence and potential food chain implications remain areas requiring further investigation.
The scalability of tartaric acid-based synthesis presents both opportunities and challenges from a sustainability perspective. While laboratory-scale processes show promising environmental credentials, industrial-scale implementation may face efficiency hurdles that could partially offset sustainability gains. Current research indicates that optimization of reaction parameters can maintain environmental benefits at larger scales, though process engineering innovations are still needed to maximize resource efficiency.
Regulatory frameworks increasingly emphasize green chemistry principles in nanomaterial production, positioning tartaric acid-based methods favorably for future compliance requirements. The European Union's REACH regulations and similar initiatives worldwide are progressively incorporating sustainability metrics into approval processes for new materials and manufacturing techniques. This regulatory landscape creates market incentives for further development and adoption of tartaric acid-mediated synthesis approaches.
Economic sustainability analysis reveals that while initial implementation costs for tartaric acid-based methods may exceed conventional approaches by 15-20%, operational cost reductions and potential regulatory advantages offer compelling long-term value propositions. Additionally, consumer preference trends toward environmentally responsible products provide market differentiation opportunities for nanomaterials produced through greener synthesis routes.
Scalability and Industrial Implementation Considerations
The scalability of tartaric acid-mediated nanoparticle synthesis represents a critical consideration for industrial implementation. Laboratory-scale synthesis methods often face significant challenges when transitioning to commercial production volumes. Current batch processes utilizing tartaric acid as a capping agent or structure-directing molecule typically yield gram-scale quantities, whereas industrial applications may require kilogram to ton-scale production capacities.
Process intensification strategies have emerged as promising approaches to address this scalability gap. Continuous flow reactors, for instance, offer advantages over traditional batch methods by enabling precise control over reaction parameters while facilitating larger throughput. Several research groups have demonstrated successful implementation of tartaric acid-based nanoparticle synthesis in microfluidic and millifluidic systems, achieving production rates up to 10-20 times higher than conventional methods while maintaining particle size distribution and morphological consistency.
Economic considerations present another crucial dimension for industrial implementation. The cost structure of tartaric acid-mediated synthesis must be carefully evaluated against alternative approaches. While tartaric acid itself is relatively inexpensive (approximately $5-15/kg for food-grade material), its stereochemical purity requirements for certain nanoparticle applications may necessitate higher-grade products, potentially increasing raw material costs by 3-5 times.
Regulatory compliance and environmental impact assessments constitute essential components of industrial implementation strategies. Tartaric acid benefits from its generally recognized as safe (GRAS) status in many jurisdictions, potentially streamlining regulatory approval processes compared to synthetic alternatives. However, waste management considerations remain significant, as large-scale production would generate substantial volumes of tartaric acid-containing effluents requiring appropriate treatment protocols.
Quality control mechanisms must be adapted for industrial-scale production. In-line monitoring technologies, including spectroscopic methods and automated sampling systems, have been developed to ensure consistent nanoparticle characteristics across production batches. These systems can detect deviations in tartaric acid concentration, pH levels, and reaction kinetics, enabling real-time process adjustments to maintain product specifications.
Storage stability and shelf-life considerations present additional challenges for commercialization. Nanoparticles synthesized using tartaric acid as a stabilizing agent may exhibit aggregation or surface chemistry alterations during prolonged storage. Research indicates that optimization of post-synthesis processing, including appropriate drying techniques and storage conditions, can extend product stability from weeks to several months, enhancing commercial viability.
Process intensification strategies have emerged as promising approaches to address this scalability gap. Continuous flow reactors, for instance, offer advantages over traditional batch methods by enabling precise control over reaction parameters while facilitating larger throughput. Several research groups have demonstrated successful implementation of tartaric acid-based nanoparticle synthesis in microfluidic and millifluidic systems, achieving production rates up to 10-20 times higher than conventional methods while maintaining particle size distribution and morphological consistency.
Economic considerations present another crucial dimension for industrial implementation. The cost structure of tartaric acid-mediated synthesis must be carefully evaluated against alternative approaches. While tartaric acid itself is relatively inexpensive (approximately $5-15/kg for food-grade material), its stereochemical purity requirements for certain nanoparticle applications may necessitate higher-grade products, potentially increasing raw material costs by 3-5 times.
Regulatory compliance and environmental impact assessments constitute essential components of industrial implementation strategies. Tartaric acid benefits from its generally recognized as safe (GRAS) status in many jurisdictions, potentially streamlining regulatory approval processes compared to synthetic alternatives. However, waste management considerations remain significant, as large-scale production would generate substantial volumes of tartaric acid-containing effluents requiring appropriate treatment protocols.
Quality control mechanisms must be adapted for industrial-scale production. In-line monitoring technologies, including spectroscopic methods and automated sampling systems, have been developed to ensure consistent nanoparticle characteristics across production batches. These systems can detect deviations in tartaric acid concentration, pH levels, and reaction kinetics, enabling real-time process adjustments to maintain product specifications.
Storage stability and shelf-life considerations present additional challenges for commercialization. Nanoparticles synthesized using tartaric acid as a stabilizing agent may exhibit aggregation or surface chemistry alterations during prolonged storage. Research indicates that optimization of post-synthesis processing, including appropriate drying techniques and storage conditions, can extend product stability from weeks to several months, enhancing commercial viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!