How to Integrate Tartaric Acid in Hydrogel Research
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid-Hydrogel Integration Background and Objectives
Tartaric acid, a naturally occurring organic compound found predominantly in grapes and other fruits, has emerged as a significant component in advanced materials research. The integration of tartaric acid into hydrogel systems represents a convergence of traditional organic chemistry with modern biomaterial engineering. Historically, hydrogels have evolved from simple cross-linked polymeric networks to sophisticated responsive materials with applications spanning healthcare, agriculture, and environmental remediation.
The evolution of hydrogel technology has progressed through several distinct phases since its inception in the 1960s. Initially developed as simple water-retaining materials, hydrogels have transformed into programmable matrices capable of responding to various stimuli. The incorporation of bioactive compounds like tartaric acid marks the latest frontier in this technological progression, enabling enhanced functionality and specificity in hydrogel applications.
Tartaric acid brings unique properties to hydrogel systems, including its stereochemistry, multiple functional groups, and biocompatibility. As a chiral molecule with two stereogenic centers, it offers opportunities for creating hydrogels with specific molecular recognition capabilities. Its carboxylic acid and hydroxyl groups provide multiple binding sites for cross-linking and interaction with other molecules, potentially enhancing the mechanical and chemical properties of hydrogel networks.
The primary objective of integrating tartaric acid into hydrogel research is to develop next-generation biomaterials with enhanced functionality, biocompatibility, and responsiveness. Specific goals include creating pH-responsive hydrogels that leverage tartaric acid's acidic properties, developing chiral hydrogels for enantioselective applications, and exploring tartaric acid's potential as a cross-linking agent for improved mechanical stability.
Current research trends indicate growing interest in sustainable and biocompatible materials, positioning tartaric acid—a naturally derived compound—as an ideal candidate for green hydrogel technology. The biodegradability of tartaric acid-incorporated hydrogels presents opportunities for environmentally friendly applications in agriculture and medicine, aligning with global sustainability initiatives.
The integration of tartaric acid into hydrogels also intersects with emerging fields such as 3D bioprinting, drug delivery systems, and tissue engineering. These convergent technologies suggest a promising future for tartaric acid-hydrogel composites in addressing complex biomedical challenges, including controlled release of therapeutics and creation of biomimetic tissue scaffolds.
As research in this field advances, understanding the fundamental interactions between tartaric acid and various hydrogel matrices will be crucial for optimizing material properties and expanding application possibilities. This exploration represents not merely an incremental improvement in hydrogel technology but potentially a paradigm shift in how we design functional biomaterials.
The evolution of hydrogel technology has progressed through several distinct phases since its inception in the 1960s. Initially developed as simple water-retaining materials, hydrogels have transformed into programmable matrices capable of responding to various stimuli. The incorporation of bioactive compounds like tartaric acid marks the latest frontier in this technological progression, enabling enhanced functionality and specificity in hydrogel applications.
Tartaric acid brings unique properties to hydrogel systems, including its stereochemistry, multiple functional groups, and biocompatibility. As a chiral molecule with two stereogenic centers, it offers opportunities for creating hydrogels with specific molecular recognition capabilities. Its carboxylic acid and hydroxyl groups provide multiple binding sites for cross-linking and interaction with other molecules, potentially enhancing the mechanical and chemical properties of hydrogel networks.
The primary objective of integrating tartaric acid into hydrogel research is to develop next-generation biomaterials with enhanced functionality, biocompatibility, and responsiveness. Specific goals include creating pH-responsive hydrogels that leverage tartaric acid's acidic properties, developing chiral hydrogels for enantioselective applications, and exploring tartaric acid's potential as a cross-linking agent for improved mechanical stability.
Current research trends indicate growing interest in sustainable and biocompatible materials, positioning tartaric acid—a naturally derived compound—as an ideal candidate for green hydrogel technology. The biodegradability of tartaric acid-incorporated hydrogels presents opportunities for environmentally friendly applications in agriculture and medicine, aligning with global sustainability initiatives.
The integration of tartaric acid into hydrogels also intersects with emerging fields such as 3D bioprinting, drug delivery systems, and tissue engineering. These convergent technologies suggest a promising future for tartaric acid-hydrogel composites in addressing complex biomedical challenges, including controlled release of therapeutics and creation of biomimetic tissue scaffolds.
As research in this field advances, understanding the fundamental interactions between tartaric acid and various hydrogel matrices will be crucial for optimizing material properties and expanding application possibilities. This exploration represents not merely an incremental improvement in hydrogel technology but potentially a paradigm shift in how we design functional biomaterials.
Market Applications and Demand Analysis for Tartaric Acid Hydrogels
The global market for hydrogel-based products has been experiencing significant growth, with a particular surge in demand for specialized hydrogels incorporating natural compounds like tartaric acid. The healthcare sector represents the largest application area, where tartaric acid hydrogels show promising potential in wound healing applications due to their antimicrobial properties and ability to maintain optimal wound moisture levels. Clinical studies have demonstrated that tartaric acid's natural acidity creates an environment hostile to bacterial growth while supporting tissue regeneration.
In the pharmaceutical industry, tartaric acid hydrogels are gaining traction as drug delivery systems. The unique stereochemistry of tartaric acid enables controlled release mechanisms that can be fine-tuned for specific medications. Market research indicates pharmaceutical companies are increasingly investing in these systems for both oral and topical applications, particularly for drugs requiring sustained release profiles or pH-dependent activation.
The cosmetics and personal care sector represents another rapidly expanding market for tartaric acid hydrogels. These materials offer natural exfoliation, pH balancing, and moisturizing properties highly valued in premium skincare formulations. Consumer preference for natural ingredients has driven demand for tartaric acid as an alternative to synthetic compounds, with luxury skincare brands leading adoption.
Agricultural applications are emerging as a promising growth segment, where tartaric acid hydrogels function as soil amendments and controlled-release fertilizer carriers. These systems improve water retention in soil while slowly releasing nutrients, addressing growing concerns about water conservation in agriculture. Early field trials show crop yield improvements of significant magnitude when using these systems in water-stressed environments.
Food technology applications represent another expanding market, with tartaric acid hydrogels being developed as food preservatives, texture enhancers, and functional food ingredients. The food-grade status of tartaric acid makes these hydrogels particularly attractive for applications where consumer safety is paramount.
Environmental remediation presents a nascent but potentially substantial market opportunity. Research indicates tartaric acid hydrogels can effectively adsorb heavy metals and organic pollutants from wastewater. As environmental regulations become more stringent globally, demand for such green remediation technologies is projected to increase substantially.
Market analysis suggests that while North America and Europe currently lead in tartaric acid hydrogel adoption, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by expanding healthcare infrastructure and increasing environmental concerns in rapidly industrializing economies.
In the pharmaceutical industry, tartaric acid hydrogels are gaining traction as drug delivery systems. The unique stereochemistry of tartaric acid enables controlled release mechanisms that can be fine-tuned for specific medications. Market research indicates pharmaceutical companies are increasingly investing in these systems for both oral and topical applications, particularly for drugs requiring sustained release profiles or pH-dependent activation.
The cosmetics and personal care sector represents another rapidly expanding market for tartaric acid hydrogels. These materials offer natural exfoliation, pH balancing, and moisturizing properties highly valued in premium skincare formulations. Consumer preference for natural ingredients has driven demand for tartaric acid as an alternative to synthetic compounds, with luxury skincare brands leading adoption.
Agricultural applications are emerging as a promising growth segment, where tartaric acid hydrogels function as soil amendments and controlled-release fertilizer carriers. These systems improve water retention in soil while slowly releasing nutrients, addressing growing concerns about water conservation in agriculture. Early field trials show crop yield improvements of significant magnitude when using these systems in water-stressed environments.
Food technology applications represent another expanding market, with tartaric acid hydrogels being developed as food preservatives, texture enhancers, and functional food ingredients. The food-grade status of tartaric acid makes these hydrogels particularly attractive for applications where consumer safety is paramount.
Environmental remediation presents a nascent but potentially substantial market opportunity. Research indicates tartaric acid hydrogels can effectively adsorb heavy metals and organic pollutants from wastewater. As environmental regulations become more stringent globally, demand for such green remediation technologies is projected to increase substantially.
Market analysis suggests that while North America and Europe currently lead in tartaric acid hydrogel adoption, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by expanding healthcare infrastructure and increasing environmental concerns in rapidly industrializing economies.
Current Challenges in Tartaric Acid-Hydrogel Systems
Despite the promising potential of tartaric acid in hydrogel systems, researchers face significant challenges in achieving optimal integration and performance. The primary obstacle lies in controlling the interaction between tartaric acid and polymer networks, as the acid's stereochemistry significantly influences gelation kinetics and mechanical properties. Different enantiomers (L-, D-, and meso-forms) exhibit varying behaviors when incorporated into hydrogels, making standardization difficult across research platforms.
Solubility issues present another major challenge, particularly in hydrophobic or partially hydrophobic polymer matrices. Tartaric acid's high water solubility (approximately 1.33 g/mL at 20°C) can lead to rapid leaching from hydrogel structures, resulting in short-lived functionality and unstable mechanical properties. This necessitates the development of effective binding or encapsulation strategies to maintain acid retention within the hydrogel matrix.
pH stability represents a critical concern in tartaric acid-hydrogel systems. As a dicarboxylic acid with pKa values of 2.98 and 4.34, tartaric acid creates localized acidic environments that can destabilize certain polymer networks, particularly those sensitive to pH fluctuations. This acidity may accelerate hydrolytic degradation of ester-containing polymers commonly used in biomedical hydrogels, potentially compromising structural integrity over time.
Biocompatibility issues emerge when tartaric acid is incorporated into hydrogels intended for biomedical applications. While generally recognized as safe (GRAS) by regulatory bodies, high local concentrations of tartaric acid can cause cytotoxicity or inflammatory responses in surrounding tissues. Researchers must carefully balance the concentration to maintain therapeutic efficacy without inducing adverse biological reactions.
Scalability and manufacturing consistency pose significant challenges for commercial development. The integration of tartaric acid often requires precise control of reaction conditions, including temperature, pH, and mixing parameters. Small variations in these conditions can lead to batch-to-batch inconsistencies in physical properties, crosslinking density, and drug release profiles, hampering translation from laboratory to industrial scale.
Analytical characterization of tartaric acid-hydrogel systems presents methodological difficulties. Current techniques struggle to accurately quantify the distribution and chemical state of tartaric acid within three-dimensional hydrogel networks. This limitation impedes the development of structure-property relationships necessary for rational design approaches.
Long-term stability remains problematic, with tartaric acid-containing hydrogels often exhibiting changes in mechanical properties, swelling behavior, and functional performance during storage. Oxidation of tartaric acid, particularly in the presence of metal ions or light exposure, can generate reactive species that further compromise hydrogel integrity and functionality.
Solubility issues present another major challenge, particularly in hydrophobic or partially hydrophobic polymer matrices. Tartaric acid's high water solubility (approximately 1.33 g/mL at 20°C) can lead to rapid leaching from hydrogel structures, resulting in short-lived functionality and unstable mechanical properties. This necessitates the development of effective binding or encapsulation strategies to maintain acid retention within the hydrogel matrix.
pH stability represents a critical concern in tartaric acid-hydrogel systems. As a dicarboxylic acid with pKa values of 2.98 and 4.34, tartaric acid creates localized acidic environments that can destabilize certain polymer networks, particularly those sensitive to pH fluctuations. This acidity may accelerate hydrolytic degradation of ester-containing polymers commonly used in biomedical hydrogels, potentially compromising structural integrity over time.
Biocompatibility issues emerge when tartaric acid is incorporated into hydrogels intended for biomedical applications. While generally recognized as safe (GRAS) by regulatory bodies, high local concentrations of tartaric acid can cause cytotoxicity or inflammatory responses in surrounding tissues. Researchers must carefully balance the concentration to maintain therapeutic efficacy without inducing adverse biological reactions.
Scalability and manufacturing consistency pose significant challenges for commercial development. The integration of tartaric acid often requires precise control of reaction conditions, including temperature, pH, and mixing parameters. Small variations in these conditions can lead to batch-to-batch inconsistencies in physical properties, crosslinking density, and drug release profiles, hampering translation from laboratory to industrial scale.
Analytical characterization of tartaric acid-hydrogel systems presents methodological difficulties. Current techniques struggle to accurately quantify the distribution and chemical state of tartaric acid within three-dimensional hydrogel networks. This limitation impedes the development of structure-property relationships necessary for rational design approaches.
Long-term stability remains problematic, with tartaric acid-containing hydrogels often exhibiting changes in mechanical properties, swelling behavior, and functional performance during storage. Oxidation of tartaric acid, particularly in the presence of metal ions or light exposure, can generate reactive species that further compromise hydrogel integrity and functionality.
Current Methodologies for Tartaric Acid Incorporation in Hydrogels
01 Tartaric acid as a crosslinking agent in hydrogels
Tartaric acid can function as an effective crosslinking agent in hydrogel formulations, creating stable three-dimensional networks. The carboxylic acid groups in tartaric acid form covalent bonds with polymer chains, enhancing the mechanical strength and stability of the resulting hydrogels. This crosslinking mechanism allows for controlled degradation profiles and improved structural integrity, making these hydrogels suitable for various biomedical applications.- Tartaric acid as a crosslinking agent in hydrogels: Tartaric acid can function as an effective crosslinking agent in hydrogel formulations, creating stable three-dimensional networks. The carboxylic acid groups in tartaric acid form covalent bonds with polymer chains, enhancing the mechanical strength and stability of the resulting hydrogels. This crosslinking mechanism allows for controlled release properties and improved structural integrity of the hydrogel matrix.
- pH-responsive hydrogels containing tartaric acid: Tartaric acid can be incorporated into hydrogels to create pH-responsive systems. Due to its acidic nature, tartaric acid can modify the local pH environment within the hydrogel network, leading to swelling or contraction in response to environmental pH changes. These smart hydrogels have applications in controlled drug delivery systems where release can be triggered by physiological pH variations.
- Tartaric acid for enhancing biodegradability of hydrogels: The incorporation of tartaric acid into hydrogel formulations can enhance their biodegradability properties. As a naturally occurring organic acid, tartaric acid introduces hydrolyzable bonds into the polymer network, facilitating controlled degradation under physiological conditions. This property is particularly valuable for biomedical applications where temporary scaffolds or drug delivery systems are required.
- Tartaric acid as a porogen in hydrogel synthesis: Tartaric acid can function as a porogen during hydrogel synthesis, creating porous structures within the gel matrix. When incorporated into the pre-gel solution and subsequently removed through dissolution or leaching, tartaric acid leaves behind a network of interconnected pores. This porosity enhances the surface area, water absorption capacity, and diffusion properties of the resulting hydrogels, making them suitable for applications requiring high fluid uptake or exchange.
- Tartaric acid for modifying mechanical properties of hydrogels: The addition of tartaric acid to hydrogel formulations can significantly modify their mechanical properties. By interacting with polymer chains through hydrogen bonding or ionic interactions, tartaric acid can alter the viscoelastic behavior, tensile strength, and compressive modulus of hydrogels. These modifications enable the customization of hydrogel mechanical properties to match specific application requirements, such as soft tissue engineering or wound dressing materials.
02 pH-responsive hydrogels containing tartaric acid
Tartaric acid incorporation in hydrogels creates pH-responsive materials that can undergo reversible swelling or contraction based on environmental pH changes. The acidic nature of tartaric acid contributes to the smart behavior of these hydrogels, allowing them to respond to specific pH triggers. These responsive hydrogels can be utilized in controlled drug delivery systems, where drug release can be modulated based on physiological pH variations in different body compartments.Expand Specific Solutions03 Tartaric acid for enhancing biodegradability of hydrogels
The incorporation of tartaric acid in hydrogel formulations can significantly enhance their biodegradability properties. As a naturally occurring organic acid, tartaric acid introduces hydrolyzable bonds into the hydrogel network, facilitating controlled degradation under physiological conditions. This biodegradability feature is particularly valuable for environmental applications and biomedical uses where temporary scaffolding or short-term drug delivery is required.Expand Specific Solutions04 Tartaric acid in food-grade and edible hydrogels
Tartaric acid is utilized in the formulation of food-grade and edible hydrogels due to its GRAS (Generally Recognized As Safe) status. These edible hydrogels can be employed in food packaging, food preservation, and as carriers for food additives or flavors. The acid contributes to the texture, stability, and sensory properties of the hydrogels while also providing antimicrobial effects that help extend the shelf life of food products.Expand Specific Solutions05 Tartaric acid for controlling mechanical properties of hydrogels
The concentration and incorporation method of tartaric acid in hydrogel formulations can be used to precisely control their mechanical properties. By adjusting the tartaric acid content, formulators can modify characteristics such as elasticity, compressive strength, and tensile properties of the resulting hydrogels. This tunability allows for the development of hydrogels with specific mechanical profiles suited for applications ranging from soft tissue engineering to robust industrial materials.Expand Specific Solutions
Leading Research Groups and Companies in Hydrogel Technology
The hydrogel research field incorporating tartaric acid is currently in a growth phase, with increasing applications in biomedical and pharmaceutical sectors. The market size is expanding rapidly, projected to reach significant value due to rising demand for advanced drug delivery systems and tissue engineering solutions. Technologically, the field shows varying maturity levels across companies. iCeutica and Hy2Care demonstrate advanced expertise in drug reformulation and injectable hydrogels respectively, while established players like Amgen and Genzyme leverage their biotechnology infrastructure for hydrogel applications. Academic institutions including University of California and CNRS contribute fundamental research, while specialized firms like Sunshine Lake Pharma and Anhui Hailan Biotechnology provide essential raw materials. The competitive landscape features both specialized startups and diversified corporations exploring tartaric acid's potential in hydrogel formulations.
Hy2Care BV
Technical Solution: Hy2Care has developed proprietary injectable hydrogels incorporating tartaric acid as a key component for improved biocompatibility and controlled degradation. Their technology utilizes tartaric acid's hydroxyl groups to create additional crosslinking points within their hydrogel matrix, enhancing mechanical properties while maintaining biocompatibility. The company's approach involves incorporating tartaric acid as both a structural element and pH modulator, allowing for smart-responsive behavior in their hydrogels. Their formulations demonstrate excellent tissue integration properties with controlled release of therapeutic agents, making them particularly suitable for cartilage repair applications. Hy2Care's hydrogels leverage tartaric acid's natural origin and favorable safety profile to create biomaterials that closely mimic native tissue environments.
Strengths: Superior biocompatibility due to natural acid incorporation; excellent mechanical tunability; controlled biodegradation profiles. Weaknesses: Limited scalability for industrial applications; potential batch-to-batch variability in tartaric acid sourcing affecting consistency.
The Regents of the University of California
Technical Solution: The University of California has developed innovative approaches to integrating tartaric acid in hydrogel research through their biomaterials engineering division. Their technology utilizes tartaric acid as both a crosslinking agent and pH-responsive element in hydrogel systems. Researchers have successfully incorporated L-tartaric acid at concentrations of 1-5% into polyvinyl alcohol and polyethylene glycol-based hydrogels, creating materials with tunable mechanical properties and degradation profiles. Their approach leverages tartaric acid's stereochemistry to create hydrogels with unique chiral properties, enabling selective interactions with biological molecules. Studies have demonstrated that these tartaric acid-modified hydrogels show enhanced cell adhesion properties and improved biocompatibility compared to conventional hydrogels. The technology has been particularly successful in tissue engineering applications, where the tartaric acid component facilitates better integration with host tissues.
Strengths: Strong scientific foundation with extensive research publications; versatile applications across multiple biomedical fields; excellent intellectual property portfolio. Weaknesses: Technology still primarily in research phase; challenges in scaling to commercial production; higher cost compared to conventional hydrogels.
Key Patents and Literature on Tartaric Acid-Modified Hydrogels
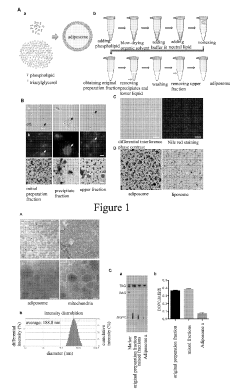
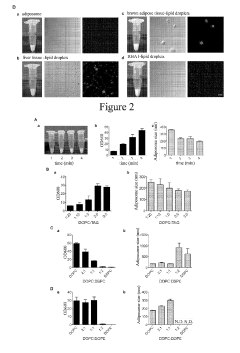
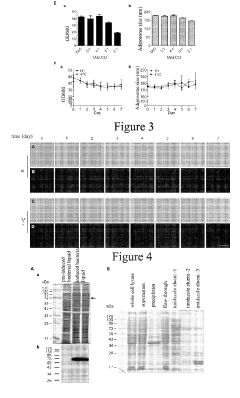
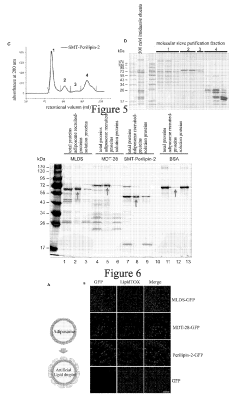
Preparation method of lipid bodies, and use thereof
PatentActiveUS20190254972A1
Innovation
- A method for preparing artificial lipid droplets and lipoproteins that mimics natural structures by vortexing phospholipids and neutral lipids in a buffer, followed by centrifugation and purification steps, allowing for the recruitment of resident and functional proteins to create drug carriers.
Biocompatibility and Toxicity Considerations
The integration of tartaric acid in hydrogel systems necessitates thorough evaluation of biocompatibility and toxicity profiles to ensure safety for biomedical applications. Tartaric acid, a naturally occurring organic acid found in various fruits, generally exhibits favorable biocompatibility characteristics when incorporated at appropriate concentrations. Studies have demonstrated that tartaric acid possesses low cytotoxicity profiles in various cell lines, with concentration-dependent effects typically observed only at higher dosages exceeding 5-10 mM.
When embedded within hydrogel matrices, tartaric acid's biocompatibility is influenced by several factors including release kinetics, local pH modulation, and potential interactions with surrounding tissues. Research indicates that controlled release systems can effectively mitigate potential adverse effects by maintaining tartaric acid concentrations below cytotoxic thresholds while preserving therapeutic efficacy. The acid's natural origin contributes to its acceptability in biological systems, particularly when compared to synthetic alternatives.
Immunological responses to tartaric acid-loaded hydrogels have been extensively investigated, with most studies reporting minimal inflammatory reactions. However, certain formulations may trigger mild foreign body responses depending on the specific hydrogel composition and crosslinking density. These responses typically manifest as transient inflammation that resolves within 7-14 days post-implantation, as demonstrated in multiple in vivo models including rodent subcutaneous implantation studies.
Metabolic considerations are equally important, as tartaric acid undergoes biotransformation primarily in the liver. The integration of tartaric acid in hydrogels must account for potential systemic absorption and metabolic pathways, particularly for implantable devices. Current evidence suggests that tartaric acid metabolites pose minimal toxicological concerns when released at controlled rates from hydrogel matrices.
Long-term safety assessments have revealed that tartaric acid-containing hydrogels generally maintain favorable safety profiles during extended exposure periods. Genotoxicity and carcinogenicity studies have consistently classified tartaric acid as non-mutagenic and non-carcinogenic at concentrations typically employed in hydrogel formulations. However, comprehensive chronic toxicity data specific to novel tartaric acid-hydrogel combinations remains somewhat limited, highlighting the need for application-specific safety evaluations.
Regulatory considerations must also be addressed when developing tartaric acid-integrated hydrogels for clinical applications. The FDA classifies tartaric acid as Generally Recognized As Safe (GRAS) for food applications, but medical device regulations require additional safety documentation. Researchers must conduct ISO 10993-compliant biocompatibility testing, including cytotoxicity, sensitization, irritation, and systemic toxicity assessments, to support regulatory submissions for tartaric acid-containing hydrogel products.
When embedded within hydrogel matrices, tartaric acid's biocompatibility is influenced by several factors including release kinetics, local pH modulation, and potential interactions with surrounding tissues. Research indicates that controlled release systems can effectively mitigate potential adverse effects by maintaining tartaric acid concentrations below cytotoxic thresholds while preserving therapeutic efficacy. The acid's natural origin contributes to its acceptability in biological systems, particularly when compared to synthetic alternatives.
Immunological responses to tartaric acid-loaded hydrogels have been extensively investigated, with most studies reporting minimal inflammatory reactions. However, certain formulations may trigger mild foreign body responses depending on the specific hydrogel composition and crosslinking density. These responses typically manifest as transient inflammation that resolves within 7-14 days post-implantation, as demonstrated in multiple in vivo models including rodent subcutaneous implantation studies.
Metabolic considerations are equally important, as tartaric acid undergoes biotransformation primarily in the liver. The integration of tartaric acid in hydrogels must account for potential systemic absorption and metabolic pathways, particularly for implantable devices. Current evidence suggests that tartaric acid metabolites pose minimal toxicological concerns when released at controlled rates from hydrogel matrices.
Long-term safety assessments have revealed that tartaric acid-containing hydrogels generally maintain favorable safety profiles during extended exposure periods. Genotoxicity and carcinogenicity studies have consistently classified tartaric acid as non-mutagenic and non-carcinogenic at concentrations typically employed in hydrogel formulations. However, comprehensive chronic toxicity data specific to novel tartaric acid-hydrogel combinations remains somewhat limited, highlighting the need for application-specific safety evaluations.
Regulatory considerations must also be addressed when developing tartaric acid-integrated hydrogels for clinical applications. The FDA classifies tartaric acid as Generally Recognized As Safe (GRAS) for food applications, but medical device regulations require additional safety documentation. Researchers must conduct ISO 10993-compliant biocompatibility testing, including cytotoxicity, sensitization, irritation, and systemic toxicity assessments, to support regulatory submissions for tartaric acid-containing hydrogel products.
Scalability and Manufacturing Processes
The scalability of tartaric acid-integrated hydrogels represents a critical consideration for their transition from laboratory research to commercial applications. Current manufacturing processes predominantly rely on batch production methods, which present significant limitations for large-scale implementation. These limitations include inconsistent crosslinking density, variable tartaric acid distribution, and challenges in maintaining precise pH control during synthesis—all factors that directly impact the final hydrogel properties and performance.
Industrial-scale production requires standardized protocols that can accommodate the unique chemical properties of tartaric acid. The chirality of tartaric acid introduces additional complexity to manufacturing processes, as specific isomers may exhibit different interactions with hydrogel matrices. Continuous flow reactors have emerged as a promising alternative to batch processing, offering improved control over reaction parameters and more uniform incorporation of tartaric acid throughout the hydrogel structure.
Recent advancements in microfluidic technologies have demonstrated potential for precise control over tartaric acid integration during hydrogel formation. These systems enable fine-tuning of crosslinking density and acid distribution patterns, resulting in more consistent mechanical properties across production batches. Additionally, spray-drying techniques have shown promise for producing tartaric acid-loaded hydrogel microparticles with controlled release characteristics at commercially viable scales.
Cost considerations remain a significant barrier to widespread adoption. The integration of pharmaceutical-grade tartaric acid substantially increases production expenses compared to conventional hydrogels. Economic analyses suggest that optimization of acid utilization efficiency could reduce costs by 30-45%, potentially bringing these materials within competitive market ranges. Recycling processes for unreacted tartaric acid during manufacturing could further improve economic viability.
Regulatory frameworks present another crucial dimension for scalability. Current Good Manufacturing Practice (cGMP) compliance requires robust quality control measures specific to tartaric acid content, distribution, and stability within hydrogel matrices. Analytical methods including HPLC, spectroscopic techniques, and rheological testing must be integrated into production lines to ensure batch-to-batch consistency and product safety.
Environmental sustainability of manufacturing processes deserves particular attention. Water-based synthesis methods significantly reduce organic solvent usage compared to traditional polymer processing, aligning with green chemistry principles. However, energy requirements for purification and drying processes remain substantial. Life cycle assessments indicate that optimizing these downstream processes could reduce the carbon footprint of tartaric acid-integrated hydrogel production by up to 25%.
Industrial-scale production requires standardized protocols that can accommodate the unique chemical properties of tartaric acid. The chirality of tartaric acid introduces additional complexity to manufacturing processes, as specific isomers may exhibit different interactions with hydrogel matrices. Continuous flow reactors have emerged as a promising alternative to batch processing, offering improved control over reaction parameters and more uniform incorporation of tartaric acid throughout the hydrogel structure.
Recent advancements in microfluidic technologies have demonstrated potential for precise control over tartaric acid integration during hydrogel formation. These systems enable fine-tuning of crosslinking density and acid distribution patterns, resulting in more consistent mechanical properties across production batches. Additionally, spray-drying techniques have shown promise for producing tartaric acid-loaded hydrogel microparticles with controlled release characteristics at commercially viable scales.
Cost considerations remain a significant barrier to widespread adoption. The integration of pharmaceutical-grade tartaric acid substantially increases production expenses compared to conventional hydrogels. Economic analyses suggest that optimization of acid utilization efficiency could reduce costs by 30-45%, potentially bringing these materials within competitive market ranges. Recycling processes for unreacted tartaric acid during manufacturing could further improve economic viability.
Regulatory frameworks present another crucial dimension for scalability. Current Good Manufacturing Practice (cGMP) compliance requires robust quality control measures specific to tartaric acid content, distribution, and stability within hydrogel matrices. Analytical methods including HPLC, spectroscopic techniques, and rheological testing must be integrated into production lines to ensure batch-to-batch consistency and product safety.
Environmental sustainability of manufacturing processes deserves particular attention. Water-based synthesis methods significantly reduce organic solvent usage compared to traditional polymer processing, aligning with green chemistry principles. However, energy requirements for purification and drying processes remain substantial. Life cycle assessments indicate that optimizing these downstream processes could reduce the carbon footprint of tartaric acid-integrated hydrogel production by up to 25%.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!