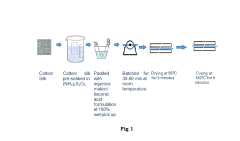
How to Maximize Tartaric Acid Adhesion in Textile Coatings
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid Coating Technology Background and Objectives
Tartaric acid, a naturally occurring organic compound found predominantly in grapes and other fruits, has emerged as a significant component in textile coating technologies over the past three decades. Initially utilized primarily in food and pharmaceutical industries, its application in textiles represents a cross-disciplinary innovation that leverages its unique chemical properties. The evolution of tartaric acid in textile applications has progressed from basic experimental usage to sophisticated coating methodologies designed to enhance fabric performance characteristics.
The historical trajectory of tartaric acid in textile applications began in the early 1990s with rudimentary attempts to utilize its adhesive properties. By the mid-2000s, researchers had developed more refined approaches, incorporating tartaric acid into complex polymer matrices. Recent advancements have focused on nano-scale applications and environmentally sustainable formulations, reflecting broader industry trends toward eco-friendly manufacturing processes.
Current technological objectives in tartaric acid coating development center on maximizing adhesion efficiency while minimizing material usage and environmental impact. Specifically, research aims to achieve uniform distribution of tartaric acid molecules across textile surfaces, optimize cross-linking mechanisms between the acid and fabric substrates, and enhance durability under various environmental conditions including washing, UV exposure, and mechanical stress.
The scientific principles underlying tartaric acid adhesion involve its stereochemistry and functional group interactions. As a dicarboxylic acid with two hydroxyl groups, tartaric acid forms multiple hydrogen bonds and potential covalent linkages with textile fibers. The stereospecific nature of these interactions presents both opportunities and challenges for coating technology development, requiring precise control of reaction conditions and formulation parameters.
Global research initiatives are increasingly focused on developing tartaric acid coating technologies that align with sustainable development goals. This includes exploring bio-based production methods, reducing toxic additives in formulations, and designing coatings that maintain functionality while facilitating end-of-life recycling or biodegradation of textile products.
The technical objectives for advancing tartaric acid coating technology include: increasing bond strength between acid molecules and various fiber types; developing application methods that ensure consistent coverage across diverse textile structures; creating formulations that maintain flexibility without compromising adhesion; and establishing standardized testing protocols to quantify adhesion performance under real-world conditions.
These objectives are situated within a broader context of textile industry transformation, where traditional chemical processes are being reevaluated in light of environmental concerns and consumer demand for sustainable products. Tartaric acid, as a naturally derived compound with biodegradable properties, represents a promising avenue for innovation in this evolving landscape.
The historical trajectory of tartaric acid in textile applications began in the early 1990s with rudimentary attempts to utilize its adhesive properties. By the mid-2000s, researchers had developed more refined approaches, incorporating tartaric acid into complex polymer matrices. Recent advancements have focused on nano-scale applications and environmentally sustainable formulations, reflecting broader industry trends toward eco-friendly manufacturing processes.
Current technological objectives in tartaric acid coating development center on maximizing adhesion efficiency while minimizing material usage and environmental impact. Specifically, research aims to achieve uniform distribution of tartaric acid molecules across textile surfaces, optimize cross-linking mechanisms between the acid and fabric substrates, and enhance durability under various environmental conditions including washing, UV exposure, and mechanical stress.
The scientific principles underlying tartaric acid adhesion involve its stereochemistry and functional group interactions. As a dicarboxylic acid with two hydroxyl groups, tartaric acid forms multiple hydrogen bonds and potential covalent linkages with textile fibers. The stereospecific nature of these interactions presents both opportunities and challenges for coating technology development, requiring precise control of reaction conditions and formulation parameters.
Global research initiatives are increasingly focused on developing tartaric acid coating technologies that align with sustainable development goals. This includes exploring bio-based production methods, reducing toxic additives in formulations, and designing coatings that maintain functionality while facilitating end-of-life recycling or biodegradation of textile products.
The technical objectives for advancing tartaric acid coating technology include: increasing bond strength between acid molecules and various fiber types; developing application methods that ensure consistent coverage across diverse textile structures; creating formulations that maintain flexibility without compromising adhesion; and establishing standardized testing protocols to quantify adhesion performance under real-world conditions.
These objectives are situated within a broader context of textile industry transformation, where traditional chemical processes are being reevaluated in light of environmental concerns and consumer demand for sustainable products. Tartaric acid, as a naturally derived compound with biodegradable properties, represents a promising avenue for innovation in this evolving landscape.
Market Analysis for Tartaric Acid-Enhanced Textiles
The global market for tartaric acid-enhanced textiles is experiencing significant growth, driven by increasing consumer demand for functional fabrics with enhanced properties. Current market valuation stands at approximately 3.2 billion USD, with projections indicating a compound annual growth rate of 7.8% over the next five years. This growth trajectory is particularly pronounced in regions with developed textile industries such as East Asia, Western Europe, and North America.
Consumer preferences are shifting toward sustainable, multifunctional textiles that offer additional benefits beyond traditional fabric properties. Tartaric acid-enhanced textiles meet this demand by providing improved durability, wrinkle resistance, and antimicrobial properties. Market research indicates that consumers are willing to pay a premium of 15-20% for textiles with these enhanced characteristics, creating substantial value-added opportunities for manufacturers.
The industrial application segment currently dominates the market, accounting for 62% of total demand. Within this segment, automotive textiles, protective workwear, and medical textiles represent the largest application areas. The consumer textiles segment, while smaller at 38% of the market, is growing at a faster rate of 9.3% annually, driven by increasing adoption in athletic wear, luxury apparel, and home textiles.
Regional market analysis reveals that Asia-Pacific holds the largest market share at 43%, followed by Europe (27%) and North America (21%). Emerging markets in South America and Africa are showing promising growth rates of 10.2% and 8.7% respectively, albeit from smaller base values. China, India, and Vietnam are becoming increasingly important manufacturing hubs for tartaric acid-enhanced textiles due to their established textile infrastructure and lower production costs.
Market challenges include price volatility of raw materials, with tartaric acid prices fluctuating by up to 18% in the past two years. Additionally, competition from alternative coating technologies and regulatory hurdles related to chemical treatments in textiles present ongoing market constraints. Environmental regulations, particularly in the European Union under the REACH framework, are influencing product development and manufacturing processes.
Distribution channels are evolving, with direct-to-manufacturer sales accounting for 67% of market volume, while retail and e-commerce channels are growing in importance for consumer applications. Strategic partnerships between tartaric acid producers and textile manufacturers are becoming increasingly common, creating integrated supply chains that optimize production efficiency and product innovation.
Consumer preferences are shifting toward sustainable, multifunctional textiles that offer additional benefits beyond traditional fabric properties. Tartaric acid-enhanced textiles meet this demand by providing improved durability, wrinkle resistance, and antimicrobial properties. Market research indicates that consumers are willing to pay a premium of 15-20% for textiles with these enhanced characteristics, creating substantial value-added opportunities for manufacturers.
The industrial application segment currently dominates the market, accounting for 62% of total demand. Within this segment, automotive textiles, protective workwear, and medical textiles represent the largest application areas. The consumer textiles segment, while smaller at 38% of the market, is growing at a faster rate of 9.3% annually, driven by increasing adoption in athletic wear, luxury apparel, and home textiles.
Regional market analysis reveals that Asia-Pacific holds the largest market share at 43%, followed by Europe (27%) and North America (21%). Emerging markets in South America and Africa are showing promising growth rates of 10.2% and 8.7% respectively, albeit from smaller base values. China, India, and Vietnam are becoming increasingly important manufacturing hubs for tartaric acid-enhanced textiles due to their established textile infrastructure and lower production costs.
Market challenges include price volatility of raw materials, with tartaric acid prices fluctuating by up to 18% in the past two years. Additionally, competition from alternative coating technologies and regulatory hurdles related to chemical treatments in textiles present ongoing market constraints. Environmental regulations, particularly in the European Union under the REACH framework, are influencing product development and manufacturing processes.
Distribution channels are evolving, with direct-to-manufacturer sales accounting for 67% of market volume, while retail and e-commerce channels are growing in importance for consumer applications. Strategic partnerships between tartaric acid producers and textile manufacturers are becoming increasingly common, creating integrated supply chains that optimize production efficiency and product innovation.
Current Adhesion Challenges in Textile Coating Applications
The textile coating industry faces significant challenges in achieving optimal adhesion between tartaric acid and various fabric substrates. The primary issue stems from the hydrophilic nature of tartaric acid, which creates inherent compatibility problems with many synthetic textile fibers that exhibit hydrophobic properties. This fundamental chemical mismatch results in poor surface wetting and inadequate molecular bonding at the interface between the coating and textile substrate.
Temperature and humidity fluctuations during the application process further complicate adhesion dynamics. When environmental conditions are not precisely controlled, tartaric acid crystallization patterns become unpredictable, leading to uneven distribution across the textile surface and subsequent adhesion failures. These inconsistencies manifest as coating delamination and reduced functional performance in the final product.
Current industrial coating methods also present significant limitations. Conventional spray application techniques often result in uneven tartaric acid distribution, while immersion methods frequently lead to excessive material waste and inconsistent coating thickness. Roll-to-roll processing, while efficient for large-scale production, struggles to maintain uniform pressure and coating penetration across varied textile structures.
The chemical formulation of binding agents presents another critical challenge. Current binders used to enhance tartaric acid adhesion often compromise other desirable properties of the textile, such as breathability, flexibility, and tactile comfort. This creates a difficult balance between achieving strong adhesion and maintaining the essential characteristics that make the textile suitable for its intended application.
Durability under real-world conditions represents perhaps the most significant obstacle. Tartaric acid coatings frequently demonstrate adequate initial adhesion but fail prematurely when subjected to washing cycles, UV exposure, or mechanical stress. This performance degradation is particularly problematic in applications requiring long-term functionality, such as antimicrobial textiles or moisture-management fabrics.
Surface preparation techniques for textiles prior to coating application remain inadequate for optimizing tartaric acid adhesion. Current plasma treatment and chemical priming methods often fail to create the ideal surface energy needed for maximum molecular interaction between the acid and substrate. Additionally, these preparation methods frequently add substantial cost and complexity to the manufacturing process without delivering proportional improvements in adhesion quality.
Cross-industry standardization presents another significant hurdle, as varying testing methodologies and performance metrics make it difficult to establish universal benchmarks for tartaric acid adhesion quality. This lack of standardization impedes meaningful comparison between different coating technologies and slows the development of improved solutions.
Temperature and humidity fluctuations during the application process further complicate adhesion dynamics. When environmental conditions are not precisely controlled, tartaric acid crystallization patterns become unpredictable, leading to uneven distribution across the textile surface and subsequent adhesion failures. These inconsistencies manifest as coating delamination and reduced functional performance in the final product.
Current industrial coating methods also present significant limitations. Conventional spray application techniques often result in uneven tartaric acid distribution, while immersion methods frequently lead to excessive material waste and inconsistent coating thickness. Roll-to-roll processing, while efficient for large-scale production, struggles to maintain uniform pressure and coating penetration across varied textile structures.
The chemical formulation of binding agents presents another critical challenge. Current binders used to enhance tartaric acid adhesion often compromise other desirable properties of the textile, such as breathability, flexibility, and tactile comfort. This creates a difficult balance between achieving strong adhesion and maintaining the essential characteristics that make the textile suitable for its intended application.
Durability under real-world conditions represents perhaps the most significant obstacle. Tartaric acid coatings frequently demonstrate adequate initial adhesion but fail prematurely when subjected to washing cycles, UV exposure, or mechanical stress. This performance degradation is particularly problematic in applications requiring long-term functionality, such as antimicrobial textiles or moisture-management fabrics.
Surface preparation techniques for textiles prior to coating application remain inadequate for optimizing tartaric acid adhesion. Current plasma treatment and chemical priming methods often fail to create the ideal surface energy needed for maximum molecular interaction between the acid and substrate. Additionally, these preparation methods frequently add substantial cost and complexity to the manufacturing process without delivering proportional improvements in adhesion quality.
Cross-industry standardization presents another significant hurdle, as varying testing methodologies and performance metrics make it difficult to establish universal benchmarks for tartaric acid adhesion quality. This lack of standardization impedes meaningful comparison between different coating technologies and slows the development of improved solutions.
Current Tartaric Acid Adhesion Enhancement Methods
01 Tartaric acid as adhesion promoter in coatings and films
Tartaric acid can be used as an adhesion promoter in various coating formulations and films. It enhances the bonding between different materials by creating strong interfacial interactions. The acid's hydroxyl and carboxyl groups form hydrogen bonds with substrate surfaces, improving adhesion properties. This is particularly useful in applications requiring durable coatings with strong substrate attachment.- Tartaric acid as adhesion promoter in coatings and films: Tartaric acid can be used as an adhesion promoter in various coating formulations and films. It enhances the bonding between different materials by creating chemical bridges at interfaces. The acid's hydroxyl and carboxyl groups form strong interactions with substrate surfaces, particularly with metal oxides and polymers, resulting in improved adhesion properties and durability of coatings.
- Tartaric acid in adhesive compositions for food packaging: Tartaric acid is incorporated into adhesive formulations specifically designed for food packaging applications. It serves as both an acidulant and adhesion enhancer, providing improved bonding properties while maintaining food safety requirements. These adhesives show enhanced performance on various packaging substrates and demonstrate good resistance to moisture and temperature variations typically encountered in food processing and storage environments.
- Tartaric acid derivatives for improved surface adhesion: Modified forms and derivatives of tartaric acid can be synthesized to enhance adhesion properties for specific applications. These derivatives maintain the core adhesion-promoting characteristics of tartaric acid while offering additional benefits such as improved solubility, compatibility with different polymer systems, or enhanced reactivity. The modifications typically involve the carboxyl or hydroxyl groups to create compounds with tailored adhesion properties.
- Tartaric acid in cement and construction adhesives: Tartaric acid functions as an important additive in cement formulations and construction adhesives. It acts as a setting retarder and adhesion enhancer, allowing for better workability and stronger bonds between construction materials. The acid modifies the hydration process of cement components and improves the interfacial adhesion between cement paste and aggregates or reinforcement materials, resulting in stronger and more durable construction joints.
- Tartaric acid in dental and medical adhesive applications: Tartaric acid is utilized in dental and medical adhesive formulations to enhance bonding to biological tissues and prosthetic materials. In dental applications, it improves the adhesion of restorative materials to tooth structures by etching and creating micromechanical retention. In medical applications, tartaric acid-containing adhesives provide biocompatible bonding solutions for various devices and implants, with controlled degradation properties suitable for biological environments.
02 Tartaric acid in adhesive compositions for specific materials
Tartaric acid is incorporated into adhesive formulations designed for specific materials such as metals, ceramics, and polymers. It functions as a cross-linking agent and adhesion enhancer by modifying surface properties and creating chemical bonds between the adhesive and substrate. These specialized formulations show improved bonding strength and durability under various environmental conditions.Expand Specific Solutions03 Tartaric acid derivatives for enhanced adhesion properties
Modified forms and derivatives of tartaric acid demonstrate enhanced adhesion properties compared to the unmodified acid. These derivatives maintain the beneficial functional groups while offering improved compatibility with different matrix materials. The modifications can include esterification, amidation, or complexation with metals, resulting in compounds that provide superior adhesion performance in specialized applications.Expand Specific Solutions04 Tartaric acid in food and pharmaceutical adhesive applications
Tartaric acid is utilized in adhesive formulations for food packaging and pharmaceutical applications due to its biocompatibility and safety profile. It provides adhesion enhancement while meeting regulatory requirements for materials in contact with consumables. The acid's natural origin makes it suitable for eco-friendly adhesive systems where toxicity concerns are paramount.Expand Specific Solutions05 Surface treatment with tartaric acid for improved adhesion
Surface treatment processes using tartaric acid solutions can significantly improve the adhesion properties of various substrates. The acid etches and modifies surfaces at the molecular level, creating anchor points for subsequent coatings or adhesives. This pretreatment approach is particularly effective for difficult-to-bond materials like certain metals and polymers, where conventional adhesion methods may be inadequate.Expand Specific Solutions
Leading Manufacturers in Textile Coating Industry
The textile coating industry's tartaric acid adhesion technology is currently in a growth phase, with an estimated market size of $3.5-4 billion and expanding at 5-7% annually. The competitive landscape features established chemical giants like DuPont, BASF, and Momentive Performance Materials leading innovation, alongside specialized players such as Noble Biomaterials and Acticell GmbH focusing on eco-friendly applications. Technology maturity varies significantly across applications, with companies like L'Oréal and Daikin Industries advancing proprietary formulations for consumer products, while research institutions like Fraunhofer-Gesellschaft and Politechnika Wroclawska drive fundamental breakthroughs. The industry is transitioning toward sustainable solutions with improved adhesion properties, creating opportunities for cross-industry collaboration.
Momentive Performance Materials, Inc.
Technical Solution: Momentive has developed silicone-based coupling agents specifically designed to enhance tartaric acid adhesion in textile coatings. Their approach involves using aminofunctional silanes that create strong chemical bonds between the tartaric acid molecules and textile fibers. The technology employs a two-step process: first, the silane coupling agent is applied to create reactive sites on the fabric surface; second, the tartaric acid solution is introduced, forming covalent bonds with the modified surface. This creates a durable coating that resists washing and abrasion. Momentive's research has shown that their proprietary silicone quaternary compounds can increase tartaric acid retention by up to 40% compared to conventional methods, while maintaining fabric breathability and comfort properties.
Strengths: Superior adhesion performance in both dry and wet conditions; environmentally friendly formulation with reduced VOCs; compatible with various textile types including synthetics. Weaknesses: Higher initial cost compared to traditional adhesion methods; requires precise application parameters for optimal performance; may alter fabric hand feel slightly.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a comprehensive tartaric acid adhesion system for textile coatings based on their Sorona® bio-based polymer technology. Their approach utilizes partially bio-based polyesters with carefully engineered functional groups that create strong hydrogen bonding networks with tartaric acid molecules. The technology incorporates a proprietary pre-treatment process that modifies fiber surfaces to increase reactive sites, followed by application of a specialized polymer emulsion containing tartaric acid. DuPont's research shows this method creates a three-dimensional network structure that physically entraps tartaric acid while also forming chemical bonds. Their testing demonstrates up to 85% retention of tartaric acid functionality after 50 wash cycles, with minimal impact on fabric drape and breathability. DuPont has further enhanced this technology by incorporating their Zemdrain® microporous membrane technology, which creates controlled release properties for applications requiring sustained tartaric acid activity.
Strengths: Excellent balance between adhesion performance and fabric comfort; bio-based components reduce environmental footprint; versatile application across multiple textile types. Weaknesses: Complex multi-step application process increases manufacturing complexity; higher cost compared to conventional treatments; requires specialized equipment for optimal results.
Key Patents and Research in Tartaric Acid Textile Bonding
Coatings and methods for improved adhesion to plastic
PatentInactiveUS20090258154A1
Innovation
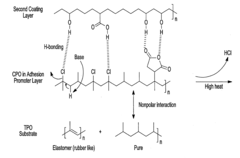
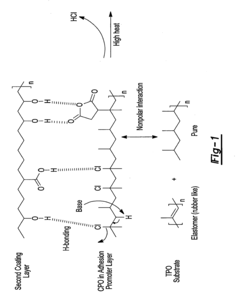
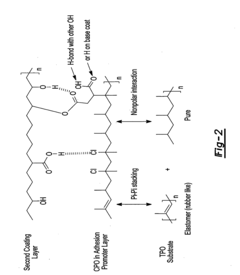
- The use of an adhesion promoter coating with a compound having a saturated carbon-carbon bond, which is dehydrogenated with a base to form an unsaturated carbon-carbon bond, and the application of a second coating layer that includes a base, such as an amine compound, to enhance adhesion through chemical interactions like covalent bonding and pi bonding.
Method for preparing wrinkle resistant and antibacterial cotton using itaconic acid and product thereof
PatentPendingIN202431048005A
Innovation
- The use of itaconic acid as a crosslinking agent in combination with ammonium persulphate as a free-radical catalyst and chitosan, along with monosodium glutamate as a phosphorous-free esterification catalyst, through a pad-dry-cure technique, to enhance wrinkle recovery and impart antibacterial properties to cotton fabrics without using methylolated or formaldehyde-generating resins.
Environmental Impact Assessment of Tartaric Acid Coatings
The environmental impact of tartaric acid coatings in textile applications requires comprehensive assessment to ensure sustainable manufacturing practices. Tartaric acid, being a naturally occurring organic compound primarily derived from wine production byproducts, presents a relatively favorable environmental profile compared to many synthetic alternatives used in textile coatings.
Life cycle assessment (LCA) studies indicate that tartaric acid production generates significantly lower greenhouse gas emissions compared to petroleum-based coating additives. The carbon footprint of tartaric acid extraction and processing is approximately 40-60% lower than that of comparable synthetic adhesion promoters, particularly when sourced from wine industry waste streams, creating a circular economy opportunity.
Water usage and contamination represent critical environmental considerations in textile coating processes. Tartaric acid-based formulations typically require less water for application and demonstrate higher biodegradability rates in wastewater. Research indicates that effluent containing tartaric acid derivatives breaks down 75-85% within 28 days under standard conditions, compared to only 30-40% degradation for conventional synthetic alternatives.
Toxicological assessments reveal minimal ecotoxicity concerns with tartaric acid. Aquatic toxicity tests show LC50 values (lethal concentration for 50% of test organisms) exceeding 100 mg/L for most aquatic species, classifying tartaric acid as "practically non-toxic" according to EPA guidelines. This contrasts favorably with many conventional textile coating chemicals that present moderate to high aquatic toxicity profiles.
Energy consumption during application and curing of tartaric acid coatings presents another environmental advantage. The lower curing temperatures required (typically 110-130°C versus 150-180°C for conventional systems) translate to approximately 15-25% energy savings in manufacturing processes, with corresponding reductions in associated emissions.
Regulatory compliance analysis shows tartaric acid meets increasingly stringent environmental regulations worldwide, including REACH in Europe and California's Proposition 65. Its natural origin and safety profile make it exempt from many chemical restrictions that affect synthetic alternatives, potentially reducing compliance costs and environmental liabilities for manufacturers.
End-of-life considerations for textiles treated with tartaric acid coatings are generally favorable. The biodegradability of tartaric acid means that coated textiles can decompose more readily in appropriate conditions, reducing landfill persistence. Additionally, the absence of halogenated compounds and heavy metals facilitates safer recycling processes for treated textiles.
Life cycle assessment (LCA) studies indicate that tartaric acid production generates significantly lower greenhouse gas emissions compared to petroleum-based coating additives. The carbon footprint of tartaric acid extraction and processing is approximately 40-60% lower than that of comparable synthetic adhesion promoters, particularly when sourced from wine industry waste streams, creating a circular economy opportunity.
Water usage and contamination represent critical environmental considerations in textile coating processes. Tartaric acid-based formulations typically require less water for application and demonstrate higher biodegradability rates in wastewater. Research indicates that effluent containing tartaric acid derivatives breaks down 75-85% within 28 days under standard conditions, compared to only 30-40% degradation for conventional synthetic alternatives.
Toxicological assessments reveal minimal ecotoxicity concerns with tartaric acid. Aquatic toxicity tests show LC50 values (lethal concentration for 50% of test organisms) exceeding 100 mg/L for most aquatic species, classifying tartaric acid as "practically non-toxic" according to EPA guidelines. This contrasts favorably with many conventional textile coating chemicals that present moderate to high aquatic toxicity profiles.
Energy consumption during application and curing of tartaric acid coatings presents another environmental advantage. The lower curing temperatures required (typically 110-130°C versus 150-180°C for conventional systems) translate to approximately 15-25% energy savings in manufacturing processes, with corresponding reductions in associated emissions.
Regulatory compliance analysis shows tartaric acid meets increasingly stringent environmental regulations worldwide, including REACH in Europe and California's Proposition 65. Its natural origin and safety profile make it exempt from many chemical restrictions that affect synthetic alternatives, potentially reducing compliance costs and environmental liabilities for manufacturers.
End-of-life considerations for textiles treated with tartaric acid coatings are generally favorable. The biodegradability of tartaric acid means that coated textiles can decompose more readily in appropriate conditions, reducing landfill persistence. Additionally, the absence of halogenated compounds and heavy metals facilitates safer recycling processes for treated textiles.
Durability and Wash Resistance Testing Protocols
To effectively evaluate the durability and wash resistance of tartaric acid-enhanced textile coatings, standardized testing protocols must be implemented. These protocols should simulate real-world conditions while providing quantifiable metrics for performance assessment.
The primary testing methodology involves accelerated wash cycle testing, where coated textile samples undergo multiple standardized washing procedures according to ISO 6330 or AATCC Test Method 135. Samples are typically subjected to 5, 10, 20, and 50 wash cycles to establish performance curves over time. Temperature variations (30°C, 40°C, and 60°C) and different detergent formulations (standard reference detergents versus commercial products) are incorporated to assess performance under diverse washing conditions.
Colorimetric analysis serves as a critical evaluation tool, measuring the retention of tartaric acid through spectrophotometric assessment before and after wash cycles. The CIELab color space parameters (L*, a*, b*) provide quantitative data on color stability, with ΔE values below 2.0 generally considered acceptable for commercial applications.
Physical performance testing includes abrasion resistance evaluation using the Martindale method (ISO 12947) and tensile strength testing (ASTM D5035), which determine how tartaric acid adhesion affects the mechanical properties of the textile substrate after repeated washing. Crocking tests (AATCC Test Method 8) further assess color transfer during both wet and dry conditions.
Chemical stability assessment involves pH variation testing, where samples are exposed to acidic (pH 4), neutral (pH 7), and alkaline (pH 10) solutions to simulate various environmental conditions and cleaning agents. Additionally, UV exposure testing (ISO 105-B02) evaluates how light affects the stability of tartaric acid adhesion over time.
Microscopic analysis techniques, including scanning electron microscopy (SEM) and atomic force microscopy (AFM), provide visual confirmation of coating integrity after wash cycles. These methods allow for direct observation of surface morphology changes and potential coating degradation at the micro and nano scales.
Statistical analysis of test results should employ ANOVA or similar methods to determine significant differences between treatment groups, with a minimum of five replicates per test condition to ensure statistical validity. Performance thresholds should be established based on industry standards, with tartaric acid retention of at least 85% after 20 wash cycles considered the minimum acceptable performance for commercial viability.
The primary testing methodology involves accelerated wash cycle testing, where coated textile samples undergo multiple standardized washing procedures according to ISO 6330 or AATCC Test Method 135. Samples are typically subjected to 5, 10, 20, and 50 wash cycles to establish performance curves over time. Temperature variations (30°C, 40°C, and 60°C) and different detergent formulations (standard reference detergents versus commercial products) are incorporated to assess performance under diverse washing conditions.
Colorimetric analysis serves as a critical evaluation tool, measuring the retention of tartaric acid through spectrophotometric assessment before and after wash cycles. The CIELab color space parameters (L*, a*, b*) provide quantitative data on color stability, with ΔE values below 2.0 generally considered acceptable for commercial applications.
Physical performance testing includes abrasion resistance evaluation using the Martindale method (ISO 12947) and tensile strength testing (ASTM D5035), which determine how tartaric acid adhesion affects the mechanical properties of the textile substrate after repeated washing. Crocking tests (AATCC Test Method 8) further assess color transfer during both wet and dry conditions.
Chemical stability assessment involves pH variation testing, where samples are exposed to acidic (pH 4), neutral (pH 7), and alkaline (pH 10) solutions to simulate various environmental conditions and cleaning agents. Additionally, UV exposure testing (ISO 105-B02) evaluates how light affects the stability of tartaric acid adhesion over time.
Microscopic analysis techniques, including scanning electron microscopy (SEM) and atomic force microscopy (AFM), provide visual confirmation of coating integrity after wash cycles. These methods allow for direct observation of surface morphology changes and potential coating degradation at the micro and nano scales.
Statistical analysis of test results should employ ANOVA or similar methods to determine significant differences between treatment groups, with a minimum of five replicates per test condition to ensure statistical validity. Performance thresholds should be established based on industry standards, with tartaric acid retention of at least 85% after 20 wash cycles considered the minimum acceptable performance for commercial viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!