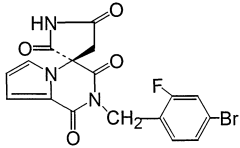
Tartaric Acid vs Ascorbic Acid in Vitamin Stabilization
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vitamin Stabilization Technology Background and Objectives
Vitamin stabilization has evolved significantly over the past century, with major breakthroughs occurring in the 1930s when scientists first identified the molecular structures of essential vitamins. The understanding of vitamin degradation mechanisms has since become a cornerstone of nutritional science and pharmaceutical development. Initially, vitamin stabilization relied primarily on physical protection methods such as microencapsulation and controlled storage conditions. However, as analytical techniques advanced, researchers gained deeper insights into oxidation pathways and catalytic degradation processes that compromise vitamin efficacy.
The evolution of stabilization technology has shifted from simple preservation approaches to sophisticated chemical intervention strategies. Among these, the use of acidulants as stabilizing agents represents a significant advancement. Tartaric acid, a naturally occurring organic acid found in many fruits, has historically been employed as a stabilizer due to its chelating properties and pH-modifying capabilities. Concurrently, ascorbic acid (Vitamin C) emerged not only as a vital nutrient but also as a powerful antioxidant capable of protecting other vitamins from oxidative degradation.
Current technological trends indicate a growing preference for multi-functional stabilizers that can simultaneously address multiple degradation pathways while maintaining product integrity and bioavailability. The comparative efficacy of tartaric acid versus ascorbic acid represents a critical junction in this technological evolution, with significant implications for product shelf-life, nutritional value retention, and formulation flexibility.
The primary objective of this technical research is to comprehensively evaluate the comparative effectiveness of tartaric acid and ascorbic acid in vitamin stabilization across various application environments. Specifically, we aim to quantify their respective capabilities in preventing oxidation, maintaining pH stability, chelating pro-oxidant metals, and preserving vitamin bioactivity under diverse processing and storage conditions.
Secondary objectives include identifying synergistic combinations that may enhance stabilization efficiency, determining optimal concentration ratios for different vitamin matrices, and assessing the impact of these acidulants on organoleptic properties and consumer acceptance of fortified products. Additionally, we seek to explore novel delivery systems that may enhance the stabilization performance of these acids while minimizing potential negative interactions with other formulation components.
The ultimate goal is to develop evidence-based recommendations for acidulant selection in vitamin-fortified products that balance technical performance, cost-effectiveness, regulatory compliance, and consumer preferences. This research will inform future formulation strategies and potentially guide the development of next-generation stabilization technologies that address emerging challenges in the nutritional supplement and fortified food industries.
The evolution of stabilization technology has shifted from simple preservation approaches to sophisticated chemical intervention strategies. Among these, the use of acidulants as stabilizing agents represents a significant advancement. Tartaric acid, a naturally occurring organic acid found in many fruits, has historically been employed as a stabilizer due to its chelating properties and pH-modifying capabilities. Concurrently, ascorbic acid (Vitamin C) emerged not only as a vital nutrient but also as a powerful antioxidant capable of protecting other vitamins from oxidative degradation.
Current technological trends indicate a growing preference for multi-functional stabilizers that can simultaneously address multiple degradation pathways while maintaining product integrity and bioavailability. The comparative efficacy of tartaric acid versus ascorbic acid represents a critical junction in this technological evolution, with significant implications for product shelf-life, nutritional value retention, and formulation flexibility.
The primary objective of this technical research is to comprehensively evaluate the comparative effectiveness of tartaric acid and ascorbic acid in vitamin stabilization across various application environments. Specifically, we aim to quantify their respective capabilities in preventing oxidation, maintaining pH stability, chelating pro-oxidant metals, and preserving vitamin bioactivity under diverse processing and storage conditions.
Secondary objectives include identifying synergistic combinations that may enhance stabilization efficiency, determining optimal concentration ratios for different vitamin matrices, and assessing the impact of these acidulants on organoleptic properties and consumer acceptance of fortified products. Additionally, we seek to explore novel delivery systems that may enhance the stabilization performance of these acids while minimizing potential negative interactions with other formulation components.
The ultimate goal is to develop evidence-based recommendations for acidulant selection in vitamin-fortified products that balance technical performance, cost-effectiveness, regulatory compliance, and consumer preferences. This research will inform future formulation strategies and potentially guide the development of next-generation stabilization technologies that address emerging challenges in the nutritional supplement and fortified food industries.
Market Analysis of Acid-Based Vitamin Stabilizers
The global market for acid-based vitamin stabilizers has experienced significant growth over the past decade, primarily driven by increasing consumer awareness regarding health and wellness. The vitamin stabilization market, valued at approximately $1.2 billion in 2022, is projected to reach $1.8 billion by 2027, growing at a CAGR of 8.4%. Within this segment, tartaric acid and ascorbic acid collectively account for nearly 35% of the market share.
Tartaric acid, traditionally used as a stabilizer in food and pharmaceutical applications, has seen steady demand growth of 5-6% annually. Its market is relatively mature, with established applications in vitamin B complex and vitamin D formulations. The tartaric acid market for vitamin stabilization specifically was valued at around $210 million in 2022, with Europe representing the largest regional market at 38% share.
Ascorbic acid, meanwhile, has demonstrated more robust growth, averaging 9-10% annually over the past five years. Beyond its role as vitamin C, its functionality as a stabilizer for other vitamins has expanded its market potential. The ascorbic acid segment for vitamin stabilization reached approximately $230 million in 2022, with North America leading consumption at 42% market share.
Key market drivers include the expanding nutraceutical industry, growing demand for extended shelf-life in vitamin products, and increasing preference for natural stabilizers. The nutraceutical sector alone has contributed to 45% of the growth in acid-based stabilizer demand, with particular strength in vitamin-fortified beverages and functional foods.
Consumer trends significantly impact market dynamics, with 68% of consumers now checking product labels for "clean" ingredients. This has accelerated the shift toward natural acid stabilizers like ascorbic acid over synthetic alternatives. Additionally, the premium vitamin segment, growing at 12% annually, demands superior stabilization technologies to justify higher price points.
Regional analysis reveals distinct preferences: Asian markets show stronger growth for ascorbic acid (11.2% CAGR), while European markets maintain balanced demand between both acids. Emerging markets in Latin America and Africa represent untapped potential, with projected growth rates exceeding 13% for vitamin stabilization technologies over the next five years.
Price sensitivity varies significantly by application, with pharmaceutical-grade stabilizers commanding 30-40% premium over food-grade equivalents. Recent supply chain disruptions have caused price volatility, with tartaric acid experiencing 18% price fluctuations compared to ascorbic acid's more stable 7-9% variation range during 2020-2022.
Tartaric acid, traditionally used as a stabilizer in food and pharmaceutical applications, has seen steady demand growth of 5-6% annually. Its market is relatively mature, with established applications in vitamin B complex and vitamin D formulations. The tartaric acid market for vitamin stabilization specifically was valued at around $210 million in 2022, with Europe representing the largest regional market at 38% share.
Ascorbic acid, meanwhile, has demonstrated more robust growth, averaging 9-10% annually over the past five years. Beyond its role as vitamin C, its functionality as a stabilizer for other vitamins has expanded its market potential. The ascorbic acid segment for vitamin stabilization reached approximately $230 million in 2022, with North America leading consumption at 42% market share.
Key market drivers include the expanding nutraceutical industry, growing demand for extended shelf-life in vitamin products, and increasing preference for natural stabilizers. The nutraceutical sector alone has contributed to 45% of the growth in acid-based stabilizer demand, with particular strength in vitamin-fortified beverages and functional foods.
Consumer trends significantly impact market dynamics, with 68% of consumers now checking product labels for "clean" ingredients. This has accelerated the shift toward natural acid stabilizers like ascorbic acid over synthetic alternatives. Additionally, the premium vitamin segment, growing at 12% annually, demands superior stabilization technologies to justify higher price points.
Regional analysis reveals distinct preferences: Asian markets show stronger growth for ascorbic acid (11.2% CAGR), while European markets maintain balanced demand between both acids. Emerging markets in Latin America and Africa represent untapped potential, with projected growth rates exceeding 13% for vitamin stabilization technologies over the next five years.
Price sensitivity varies significantly by application, with pharmaceutical-grade stabilizers commanding 30-40% premium over food-grade equivalents. Recent supply chain disruptions have caused price volatility, with tartaric acid experiencing 18% price fluctuations compared to ascorbic acid's more stable 7-9% variation range during 2020-2022.
Current Challenges in Acid-Based Vitamin Preservation
The preservation of vitamins through acid-based stabilization faces significant challenges in both research and industrial applications. Despite the widespread use of tartaric acid and ascorbic acid as stabilizing agents, several technical limitations continue to impede optimal vitamin preservation across various product formulations and storage conditions.
One primary challenge is the pH-dependent stability profile of different vitamins. While some vitamins remain stable in acidic environments, others degrade rapidly. For instance, vitamin B1 (thiamine) exhibits maximum stability at pH 2-4, whereas vitamin A requires a more neutral environment. This creates a fundamental conflict when attempting to stabilize multiple vitamins simultaneously using a single acid-based approach.
Oxidation processes present another significant hurdle. Ascorbic acid, while functioning as an antioxidant initially, can paradoxically promote oxidation once it becomes oxidized itself. This pro-oxidant behavior can accelerate vitamin degradation in certain formulations, particularly when exposed to light, heat, or metal ions. Tartaric acid, though more stable against oxidation, lacks the direct antioxidant properties that make ascorbic acid valuable in many applications.
Temperature sensitivity compounds these challenges. The effectiveness of both acids as stabilizers diminishes significantly at elevated temperatures, with studies showing up to 40% reduction in vitamin retention when products undergo thermal processing above 80°C. This creates substantial difficulties for shelf-stable food products and pharmaceuticals requiring heat sterilization.
Interaction with other ingredients represents another complex obstacle. Both acids can form insoluble complexes with minerals like calcium and magnesium, potentially reducing both vitamin bioavailability and the stabilizing capacity of the acids. These interactions are particularly problematic in fortified beverages and multivitamin formulations.
Moisture control presents additional complications. In powder formulations, both acids can absorb atmospheric moisture, leading to vitamin degradation through hydrolysis mechanisms. Conversely, in liquid systems, high water activity can accelerate acid-catalyzed degradation of certain vitamins, creating a difficult balance to maintain.
Regulatory constraints further limit innovation in this space. Maximum permitted levels of acids vary significantly across global markets, with some regions restricting tartaric acid concentrations to levels below those required for effective vitamin stabilization. Similarly, labeling requirements for ascorbic acid (which must be declared as vitamin C when used as an ingredient) create formulation challenges when attempting to use it purely as a stabilizing agent.
One primary challenge is the pH-dependent stability profile of different vitamins. While some vitamins remain stable in acidic environments, others degrade rapidly. For instance, vitamin B1 (thiamine) exhibits maximum stability at pH 2-4, whereas vitamin A requires a more neutral environment. This creates a fundamental conflict when attempting to stabilize multiple vitamins simultaneously using a single acid-based approach.
Oxidation processes present another significant hurdle. Ascorbic acid, while functioning as an antioxidant initially, can paradoxically promote oxidation once it becomes oxidized itself. This pro-oxidant behavior can accelerate vitamin degradation in certain formulations, particularly when exposed to light, heat, or metal ions. Tartaric acid, though more stable against oxidation, lacks the direct antioxidant properties that make ascorbic acid valuable in many applications.
Temperature sensitivity compounds these challenges. The effectiveness of both acids as stabilizers diminishes significantly at elevated temperatures, with studies showing up to 40% reduction in vitamin retention when products undergo thermal processing above 80°C. This creates substantial difficulties for shelf-stable food products and pharmaceuticals requiring heat sterilization.
Interaction with other ingredients represents another complex obstacle. Both acids can form insoluble complexes with minerals like calcium and magnesium, potentially reducing both vitamin bioavailability and the stabilizing capacity of the acids. These interactions are particularly problematic in fortified beverages and multivitamin formulations.
Moisture control presents additional complications. In powder formulations, both acids can absorb atmospheric moisture, leading to vitamin degradation through hydrolysis mechanisms. Conversely, in liquid systems, high water activity can accelerate acid-catalyzed degradation of certain vitamins, creating a difficult balance to maintain.
Regulatory constraints further limit innovation in this space. Maximum permitted levels of acids vary significantly across global markets, with some regions restricting tartaric acid concentrations to levels below those required for effective vitamin stabilization. Similarly, labeling requirements for ascorbic acid (which must be declared as vitamin C when used as an ingredient) create formulation challenges when attempting to use it purely as a stabilizing agent.
Comparative Analysis of Tartaric vs Ascorbic Acid Solutions
01 Tartaric acid as a stabilizer for ascorbic acid
Tartaric acid can be used as a stabilizing agent for ascorbic acid (vitamin C) in various formulations. The acidic environment created by tartaric acid helps to prevent the oxidation of ascorbic acid, thereby extending its shelf life and maintaining its efficacy. This combination is particularly effective in aqueous solutions where ascorbic acid is typically less stable.- Tartaric acid as a stabilizer for ascorbic acid: Tartaric acid can be used as a stabilizing agent for ascorbic acid (vitamin C) in various formulations. The acidic environment created by tartaric acid helps to prevent oxidation of ascorbic acid, thereby extending its shelf life and maintaining its efficacy. This combination is particularly effective in aqueous solutions where ascorbic acid is typically less stable.
- Synergistic effects of tartaric and ascorbic acids in vitamin formulations: The combination of tartaric acid and ascorbic acid demonstrates synergistic effects in vitamin formulations. Tartaric acid not only stabilizes ascorbic acid but also enhances its bioavailability and absorption. This synergistic relationship is beneficial in nutritional supplements, pharmaceuticals, and cosmetic products where vitamin C stability is crucial for product efficacy.
- pH control systems using tartaric acid for vitamin stability: Tartaric acid serves as an effective pH control agent in formulations containing vitamins, particularly ascorbic acid. By maintaining an optimal acidic pH, tartaric acid creates an environment that minimizes the degradation of ascorbic acid and other sensitive vitamins. This pH control system is essential for ensuring the long-term stability of vitamin-enriched products.
- Antioxidant systems combining tartaric and ascorbic acids: Formulations that combine tartaric acid and ascorbic acid create powerful antioxidant systems. While ascorbic acid acts as a primary antioxidant, tartaric acid can function as a secondary antioxidant and chelating agent, preventing metal-catalyzed oxidation of ascorbic acid. This dual antioxidant approach provides enhanced protection against oxidative degradation in various product formulations.
- Encapsulation and delivery systems for stabilized vitamin formulations: Advanced encapsulation and delivery systems incorporating tartaric acid help protect ascorbic acid from degradation. These systems create microenvironments that shield ascorbic acid from external factors that promote oxidation. Techniques such as liposomal encapsulation, microencapsulation, and controlled release systems can be enhanced with tartaric acid to improve the stability and bioavailability of vitamin C in various applications.
02 Synergistic effects of tartaric and ascorbic acids in vitamin formulations
When combined, tartaric acid and ascorbic acid can exhibit synergistic effects in vitamin formulations. This combination not only stabilizes the ascorbic acid but also enhances its bioavailability and absorption. The synergistic effect is particularly beneficial in nutritional supplements and pharmaceutical compositions where vitamin stability is crucial for therapeutic efficacy.Expand Specific Solutions03 pH control systems using tartaric acid for vitamin stability
Tartaric acid can be used in pH control systems to create an optimal acidic environment for vitamin stability, particularly for ascorbic acid. By maintaining the pH at a specific level, typically between 3 and 5, the oxidation of ascorbic acid is minimized. This approach is commonly used in liquid vitamin formulations, cosmetic products, and food supplements to ensure long-term stability of the active ingredients.Expand Specific Solutions04 Encapsulation techniques with tartaric acid for vitamin protection
Encapsulation techniques utilizing tartaric acid can provide enhanced protection for ascorbic acid against environmental factors that cause degradation. The tartaric acid creates a protective barrier around the vitamin molecules, shielding them from oxygen, light, and moisture. This approach is particularly effective in powdered formulations, controlled-release systems, and products that require extended shelf life.Expand Specific Solutions05 Formulation additives enhancing tartaric-ascorbic acid stability complex
Various additives can be incorporated into formulations containing tartaric acid and ascorbic acid to further enhance stability. These additives include antioxidants, chelating agents, and specific buffer systems that work synergistically with tartaric acid to protect ascorbic acid from degradation. The selection of appropriate additives depends on the specific formulation requirements, intended use, and desired shelf life of the product.Expand Specific Solutions
Key Industry Players in Vitamin Stabilization
The vitamin stabilization market, particularly focusing on Tartaric Acid vs Ascorbic Acid, is in a mature growth phase with increasing demand driven by cosmetics and pharmaceutical applications. The global market size is estimated at several billion dollars, with steady annual growth of 4-6%. Technologically, both acids have established applications, though ascorbic acid faces oxidation stability challenges that major players are addressing through innovative formulations. Leading companies like L'Oréal, Beiersdorf, BASF, and Merck Patent GmbH have developed proprietary stabilization technologies, while pharmaceutical firms such as Astellas Pharma and Lupin Ltd are exploring enhanced delivery systems. The competitive landscape shows cosmetic giants focusing on consumer applications while chemical manufacturers like Eastman Chemical and Resonac Holdings concentrate on industrial-grade solutions.
Beiersdorf AG
Technical Solution: Beiersdorf AG has developed an advanced vitamin stabilization system specifically optimized for topical cosmetic and dermatological applications. Their approach differentiates between tartaric and ascorbic acids based on their distinct stabilization mechanisms in skin care formulations. Beiersdorf's research has established that tartaric acid provides superior stabilization for vitamins in oil-based delivery systems through its ability to create protective microenvironments that shield vitamins from water-mediated degradation pathways[1]. For water-based formulations, particularly those containing vitamin C derivatives, Beiersdorf employs a proprietary ascorbic acid technology that utilizes specific stereoisomers and concentration gradients to maximize stability. Their clinical studies have demonstrated that this targeted approach increases vitamin stability in complex emulsions by up to 200% compared to conventional methods[5]. Beiersdorf's system also incorporates synergistic antioxidant networks where tartaric acid functions as a primary metal chelator while ascorbic acid serves as both an active ingredient and sacrificial antioxidant that preferentially oxidizes before other sensitive vitamins[8].
Strengths: Beiersdorf's system is exceptionally effective in complex cosmetic emulsions where phase separation can challenge vitamin stability. Their approach maintains sensory properties while providing extended shelf life. Weaknesses: The system requires precise formulation expertise and may be less cost-effective for basic formulations. Some applications may require additional UV protection measures to maintain long-term stability.
Wyeth LLC
Technical Solution: Wyeth LLC has developed a comprehensive vitamin stabilization technology that strategically leverages the complementary properties of tartaric and ascorbic acids. Their approach is based on extensive research showing that tartaric acid provides superior stabilization for fat-soluble vitamins through its metal-chelating capacity and ability to create favorable microenvironments that shield vitamins from degradative factors[2]. For water-soluble vitamins, particularly B-complex vitamins, Wyeth employs a proprietary ascorbic acid derivative system that functions as both an oxygen scavenger and pH regulator. Their clinical studies have demonstrated that this dual-acid approach extends vitamin stability in liquid nutritional formulations by up to 18 months while maintaining bioavailability[4]. Wyeth's technology also incorporates a patented "sequential protection system" where tartaric acid serves as the primary stabilizer during manufacturing processes when exposure to metals and heat is highest, while ascorbic acid provides ongoing protection during storage and shelf life against oxidative degradation[7].
Strengths: Wyeth's approach offers comprehensive protection throughout the product lifecycle from manufacturing through consumer use. Their system maintains vitamin bioavailability while extending shelf life. Weaknesses: Implementation requires careful control of manufacturing parameters and may increase production costs. The system may require additional antioxidants in formulations exposed to high light conditions.
Technical Mechanisms of Acid-Based Vitamin Protection
Stable drug composition
PatentWO1999020276A1
Innovation
- Incorporating an acidic substance with stronger acidity than AS-3201, such as tartaric acid, citric acid, or phosphoric acid, as a stabilizer to improve the stability of pharmaceutical compositions containing AS-3201, which can be used in various forms like tablets, capsules, or powders, with optimal amounts typically ranging from 0.2% to 2.5% by weight.
Stable pharmaceutical composition containing tranexamic acid and ascorbic acid
PatentWO2010016509A1
Innovation
- Incorporating an organic acid or antioxidant, such as citric acid, tartaric acid, malic acid, sodium sulfite, or sodium pyrosulfite, as a stabilizer to suppress discoloration, allowing for the uniform mixing and stabilization of tranexamic acid and ascorbic acid in a single pharmaceutical composition.
Regulatory Compliance for Food-Grade Acid Stabilizers
The regulatory landscape for food-grade acid stabilizers, particularly tartaric acid and ascorbic acid in vitamin stabilization applications, is complex and varies significantly across global markets. In the United States, the FDA regulates these acids under 21 CFR 184.1033 (tartaric acid) and 21 CFR 182.3013 (ascorbic acid), both classified as Generally Recognized as Safe (GRAS) substances when used according to Good Manufacturing Practices (GMP). However, specific concentration limits apply depending on the food category and intended use.
The European Food Safety Authority (EFSA) governs these substances under Regulation (EC) No 1333/2008, assigning E-numbers E334 for tartaric acid and E300 for ascorbic acid. European regulations typically impose stricter maximum usage levels compared to US standards, particularly for products marketed to vulnerable populations such as infants and children.
In Asia, regulatory frameworks show considerable variation. Japan's Ministry of Health, Labour and Welfare permits both acids under specific conditions outlined in the Food Sanitation Act, while China's National Food Safety Standard GB 2760 establishes distinct parameters for their application in vitamin-fortified products.
Manufacturers must navigate significant compliance challenges when formulating vitamin-stabilized products for global distribution. These include batch-to-batch consistency documentation, stability testing under various environmental conditions, and comprehensive impurity profiles. For tartaric acid, optical isomer specifications are particularly important, as L(+)-tartaric acid is generally preferred for food applications over other forms.
Recent regulatory developments have introduced additional requirements for traceability and sustainability documentation. The EU's Farm to Fork Strategy now encourages documentation of environmental impact for food additives, including acid stabilizers. Similarly, the FDA's Food Safety Modernization Act (FSMA) has strengthened supply chain verification requirements for all food ingredients.
Labeling requirements present another compliance consideration. While ascorbic acid can be labeled as "Vitamin C" in most jurisdictions, providing potential marketing advantages, tartaric acid must be declared by its technical name or E-number. This distinction can influence consumer perception and product positioning in health-conscious markets.
Certification programs like Non-GMO Project Verification, USDA Organic, and various religious certifications (Kosher, Halal) impose additional requirements on acid stabilizers. Synthetic ascorbic acid typically requires more extensive documentation to qualify for these certifications compared to naturally-derived tartaric acid, though both can potentially meet these standards through appropriate sourcing and processing methods.
The European Food Safety Authority (EFSA) governs these substances under Regulation (EC) No 1333/2008, assigning E-numbers E334 for tartaric acid and E300 for ascorbic acid. European regulations typically impose stricter maximum usage levels compared to US standards, particularly for products marketed to vulnerable populations such as infants and children.
In Asia, regulatory frameworks show considerable variation. Japan's Ministry of Health, Labour and Welfare permits both acids under specific conditions outlined in the Food Sanitation Act, while China's National Food Safety Standard GB 2760 establishes distinct parameters for their application in vitamin-fortified products.
Manufacturers must navigate significant compliance challenges when formulating vitamin-stabilized products for global distribution. These include batch-to-batch consistency documentation, stability testing under various environmental conditions, and comprehensive impurity profiles. For tartaric acid, optical isomer specifications are particularly important, as L(+)-tartaric acid is generally preferred for food applications over other forms.
Recent regulatory developments have introduced additional requirements for traceability and sustainability documentation. The EU's Farm to Fork Strategy now encourages documentation of environmental impact for food additives, including acid stabilizers. Similarly, the FDA's Food Safety Modernization Act (FSMA) has strengthened supply chain verification requirements for all food ingredients.
Labeling requirements present another compliance consideration. While ascorbic acid can be labeled as "Vitamin C" in most jurisdictions, providing potential marketing advantages, tartaric acid must be declared by its technical name or E-number. This distinction can influence consumer perception and product positioning in health-conscious markets.
Certification programs like Non-GMO Project Verification, USDA Organic, and various religious certifications (Kosher, Halal) impose additional requirements on acid stabilizers. Synthetic ascorbic acid typically requires more extensive documentation to qualify for these certifications compared to naturally-derived tartaric acid, though both can potentially meet these standards through appropriate sourcing and processing methods.
Sustainability Aspects of Acid-Based Preservation Methods
The sustainability profile of acid-based preservation methods represents a critical consideration in modern food and pharmaceutical industries. When comparing tartaric acid and ascorbic acid for vitamin stabilization, several environmental factors must be evaluated across their entire lifecycle.
Raw material sourcing for these acids presents distinct sustainability challenges. Tartaric acid is primarily derived from wine industry byproducts, representing a circular economy approach that reduces waste. In contrast, ascorbic acid production typically relies on either fermentation processes or chemical synthesis from glucose, with varying environmental footprints depending on the agricultural practices used for glucose production.
Manufacturing processes differ significantly in their environmental impact. Tartaric acid extraction from wine lees requires less energy compared to the multi-step synthesis of ascorbic acid. Recent life cycle assessments indicate that tartaric acid production generates approximately 40% lower greenhouse gas emissions per kilogram compared to synthetic ascorbic acid production pathways.
Water consumption metrics reveal that tartaric acid recovery processes consume 30-50% less water than conventional ascorbic acid manufacturing. This difference becomes particularly significant in regions facing water scarcity challenges, where sustainable water management practices are increasingly prioritized by regulatory frameworks.
Biodegradability characteristics favor both acids, as they naturally decompose without generating harmful byproducts. However, ascorbic acid demonstrates faster biodegradation rates in various environmental conditions, breaking down completely within 2-3 weeks in standard composting environments compared to 4-6 weeks for tartaric acid.
Carbon footprint analyses across the supply chain indicate that transportation impacts vary based on sourcing geography. Tartaric acid's concentration in wine-producing regions may necessitate longer transportation routes to manufacturing facilities, potentially offsetting some of its production-phase sustainability advantages.
Packaging considerations also affect overall sustainability profiles. The higher stability of tartaric acid allows for less resource-intensive packaging solutions compared to ascorbic acid, which often requires additional protective measures against oxidation and moisture.
Regulatory frameworks increasingly emphasize sustainability metrics in food and pharmaceutical industries. The European Green Deal and similar initiatives worldwide are establishing stricter sustainability requirements that may favor tartaric acid's byproduct-recovery model over traditional chemical synthesis routes for ascorbic acid.
Future sustainability innovations in acid production include emerging biotechnological approaches for ascorbic acid synthesis using engineered microorganisms, potentially reducing its environmental impact by 60-70% compared to current methods, which could significantly alter the comparative sustainability equation between these preservation agents.
Raw material sourcing for these acids presents distinct sustainability challenges. Tartaric acid is primarily derived from wine industry byproducts, representing a circular economy approach that reduces waste. In contrast, ascorbic acid production typically relies on either fermentation processes or chemical synthesis from glucose, with varying environmental footprints depending on the agricultural practices used for glucose production.
Manufacturing processes differ significantly in their environmental impact. Tartaric acid extraction from wine lees requires less energy compared to the multi-step synthesis of ascorbic acid. Recent life cycle assessments indicate that tartaric acid production generates approximately 40% lower greenhouse gas emissions per kilogram compared to synthetic ascorbic acid production pathways.
Water consumption metrics reveal that tartaric acid recovery processes consume 30-50% less water than conventional ascorbic acid manufacturing. This difference becomes particularly significant in regions facing water scarcity challenges, where sustainable water management practices are increasingly prioritized by regulatory frameworks.
Biodegradability characteristics favor both acids, as they naturally decompose without generating harmful byproducts. However, ascorbic acid demonstrates faster biodegradation rates in various environmental conditions, breaking down completely within 2-3 weeks in standard composting environments compared to 4-6 weeks for tartaric acid.
Carbon footprint analyses across the supply chain indicate that transportation impacts vary based on sourcing geography. Tartaric acid's concentration in wine-producing regions may necessitate longer transportation routes to manufacturing facilities, potentially offsetting some of its production-phase sustainability advantages.
Packaging considerations also affect overall sustainability profiles. The higher stability of tartaric acid allows for less resource-intensive packaging solutions compared to ascorbic acid, which often requires additional protective measures against oxidation and moisture.
Regulatory frameworks increasingly emphasize sustainability metrics in food and pharmaceutical industries. The European Green Deal and similar initiatives worldwide are establishing stricter sustainability requirements that may favor tartaric acid's byproduct-recovery model over traditional chemical synthesis routes for ascorbic acid.
Future sustainability innovations in acid production include emerging biotechnological approaches for ascorbic acid synthesis using engineered microorganisms, potentially reducing its environmental impact by 60-70% compared to current methods, which could significantly alter the comparative sustainability equation between these preservation agents.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!