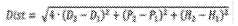How to Apply Tartaric Acid in Textile Dyeing
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid in Textile Dyeing: Background and Objectives
Tartaric acid, a naturally occurring organic acid found predominantly in grapes and other fruits, has a rich history dating back to ancient times. Its application in various industries has evolved significantly over centuries, with its use in textile dyeing representing one of the more specialized yet promising applications. The evolution of tartaric acid in textile processing can be traced from traditional craft dyeing methods to modern industrial applications, reflecting broader trends in sustainable and eco-friendly manufacturing processes.
The textile dyeing industry has historically relied on various mordants and auxiliaries to enhance color fastness and brightness. Tartaric acid emerged as a notable component in this technical ecosystem due to its unique chemical properties, including its ability to form complexes with metal ions and its acidic nature that can modify fiber surfaces to improve dye uptake. The progression from basic applications to more sophisticated uses parallels advancements in textile chemistry and environmental regulations.
Current technological trends in textile dyeing are increasingly focused on sustainability, reduced water consumption, and elimination of harmful chemicals. Tartaric acid aligns well with these trends as a biodegradable, non-toxic alternative to conventional synthetic auxiliaries. Its potential to replace environmentally problematic substances while maintaining or improving dyeing quality positions it as a technology of growing interest in the industry's evolution toward greener practices.
The primary technical objectives for tartaric acid application in textile dyeing include optimizing its role as a mordant for natural dyes, exploring its potential as a pH regulator in various dyeing processes, investigating its effectiveness in improving color fastness properties, and developing standardized methodologies for its incorporation into industrial dyeing protocols. These objectives address both performance enhancement and environmental sustainability goals.
Research into tartaric acid applications has accelerated in recent years, with particular emphasis on its synergistic effects with various dye classes and fiber types. The acid's stereochemistry and multiple functional groups offer unique binding opportunities that can be exploited for improved dyeing outcomes. Understanding these molecular interactions represents a key technical goal for advancing its practical applications.
The expected outcomes from tartaric acid research include the development of novel dyeing formulations with reduced environmental impact, improved color consistency across different fiber types, enhanced wash and light fastness properties, and potentially reduced energy consumption in dyeing processes. These outcomes align with broader industry goals of creating more sustainable and efficient textile processing methods while maintaining or improving product quality.
The textile dyeing industry has historically relied on various mordants and auxiliaries to enhance color fastness and brightness. Tartaric acid emerged as a notable component in this technical ecosystem due to its unique chemical properties, including its ability to form complexes with metal ions and its acidic nature that can modify fiber surfaces to improve dye uptake. The progression from basic applications to more sophisticated uses parallels advancements in textile chemistry and environmental regulations.
Current technological trends in textile dyeing are increasingly focused on sustainability, reduced water consumption, and elimination of harmful chemicals. Tartaric acid aligns well with these trends as a biodegradable, non-toxic alternative to conventional synthetic auxiliaries. Its potential to replace environmentally problematic substances while maintaining or improving dyeing quality positions it as a technology of growing interest in the industry's evolution toward greener practices.
The primary technical objectives for tartaric acid application in textile dyeing include optimizing its role as a mordant for natural dyes, exploring its potential as a pH regulator in various dyeing processes, investigating its effectiveness in improving color fastness properties, and developing standardized methodologies for its incorporation into industrial dyeing protocols. These objectives address both performance enhancement and environmental sustainability goals.
Research into tartaric acid applications has accelerated in recent years, with particular emphasis on its synergistic effects with various dye classes and fiber types. The acid's stereochemistry and multiple functional groups offer unique binding opportunities that can be exploited for improved dyeing outcomes. Understanding these molecular interactions represents a key technical goal for advancing its practical applications.
The expected outcomes from tartaric acid research include the development of novel dyeing formulations with reduced environmental impact, improved color consistency across different fiber types, enhanced wash and light fastness properties, and potentially reduced energy consumption in dyeing processes. These outcomes align with broader industry goals of creating more sustainable and efficient textile processing methods while maintaining or improving product quality.
Market Analysis of Eco-Friendly Textile Auxiliaries
The global market for eco-friendly textile auxiliaries has experienced significant growth in recent years, driven by increasing environmental awareness and stringent regulations. The textile industry, traditionally known for its high water consumption and chemical usage, is now shifting towards sustainable practices. Within this context, tartaric acid, a natural organic acid, has emerged as a promising eco-friendly alternative in textile dyeing processes.
Consumer demand for environmentally responsible textile products has created a robust market for green auxiliaries, with annual growth rates exceeding traditional chemical auxiliaries. Major textile manufacturing regions including Asia-Pacific, Europe, and North America are witnessing increased adoption of bio-based auxiliaries like tartaric acid. The market value of eco-friendly textile chemicals is projected to continue its upward trajectory as sustainability becomes a core business strategy rather than a niche consideration.
Regulatory frameworks worldwide are increasingly favoring environmentally benign chemicals in textile processing. The European Union's REACH regulations, along with similar initiatives in other regions, have accelerated the transition away from harmful chemicals. This regulatory landscape has created favorable market conditions for tartaric acid applications in textile dyeing, as manufacturers seek compliant alternatives to conventional mordants and pH regulators.
The competitive landscape reveals that companies investing in green chemistry innovations are gaining market share. Tartaric acid, being biodegradable and derived from renewable resources, aligns perfectly with this market direction. Its application in textile dyeing represents a growing segment within the broader eco-friendly auxiliaries market, particularly for natural fiber dyeing where chemical reduction is highly valued by consumers.
Price sensitivity remains a challenge in this market segment, as eco-friendly alternatives typically command premium pricing. However, analysis indicates that consumers and brands are increasingly willing to absorb moderate price increases for demonstrably sustainable products. The cost-benefit equation for tartaric acid in textile dyeing is becoming more favorable as production scales increase and more manufacturers enter this space.
Market segmentation shows particular strength in premium and luxury textile segments, where environmental credentials carry significant marketing value. The fast fashion segment, traditionally less concerned with environmental impacts, is now also pivoting towards greener processes due to consumer pressure and brand reputation considerations. This broadening market base suggests expanding opportunities for tartaric acid applications across diverse textile product categories.
Consumer demand for environmentally responsible textile products has created a robust market for green auxiliaries, with annual growth rates exceeding traditional chemical auxiliaries. Major textile manufacturing regions including Asia-Pacific, Europe, and North America are witnessing increased adoption of bio-based auxiliaries like tartaric acid. The market value of eco-friendly textile chemicals is projected to continue its upward trajectory as sustainability becomes a core business strategy rather than a niche consideration.
Regulatory frameworks worldwide are increasingly favoring environmentally benign chemicals in textile processing. The European Union's REACH regulations, along with similar initiatives in other regions, have accelerated the transition away from harmful chemicals. This regulatory landscape has created favorable market conditions for tartaric acid applications in textile dyeing, as manufacturers seek compliant alternatives to conventional mordants and pH regulators.
The competitive landscape reveals that companies investing in green chemistry innovations are gaining market share. Tartaric acid, being biodegradable and derived from renewable resources, aligns perfectly with this market direction. Its application in textile dyeing represents a growing segment within the broader eco-friendly auxiliaries market, particularly for natural fiber dyeing where chemical reduction is highly valued by consumers.
Price sensitivity remains a challenge in this market segment, as eco-friendly alternatives typically command premium pricing. However, analysis indicates that consumers and brands are increasingly willing to absorb moderate price increases for demonstrably sustainable products. The cost-benefit equation for tartaric acid in textile dyeing is becoming more favorable as production scales increase and more manufacturers enter this space.
Market segmentation shows particular strength in premium and luxury textile segments, where environmental credentials carry significant marketing value. The fast fashion segment, traditionally less concerned with environmental impacts, is now also pivoting towards greener processes due to consumer pressure and brand reputation considerations. This broadening market base suggests expanding opportunities for tartaric acid applications across diverse textile product categories.
Current Applications and Technical Challenges
Tartaric acid has emerged as a versatile auxiliary in textile dyeing processes, with applications spanning multiple fabric types and dyeing methodologies. Currently, it serves primarily as a mordant in natural dyeing processes, where it forms coordination complexes with metal ions and dye molecules, enhancing color fastness and brightness. The acid's ability to modify pH levels makes it particularly valuable in reactive dyeing processes, where it helps maintain optimal acidic conditions for dye-fiber reactions, resulting in improved color yield and reduced processing time.
In protein-based textiles such as wool and silk, tartaric acid functions as an effective pre-treatment agent, modifying fiber surfaces to increase dye receptivity. Research indicates that tartaric acid pre-treatments can improve dye uptake by 15-20% compared to conventional methods, while simultaneously reducing water consumption during the rinsing phase.
The eco-friendly profile of tartaric acid has positioned it as a sustainable alternative to conventional chemical auxiliaries in textile processing. Being biodegradable and derived from natural sources, it aligns with growing industry demands for greener production methods. Several manufacturers have incorporated tartaric acid into their sustainable dyeing processes, reporting reduced environmental impact without compromising color quality or production efficiency.
Despite these promising applications, significant technical challenges persist in the widespread adoption of tartaric acid in textile dyeing. Foremost among these is the issue of scalability - laboratory successes have proven difficult to translate to industrial-scale operations due to process control complexities and economic considerations. The relatively higher cost of tartaric acid compared to conventional auxiliaries presents a commercial barrier, particularly for mass-market textile producers operating on thin margins.
Compatibility issues also arise when integrating tartaric acid into existing dyeing systems. The acid can interact unpredictably with certain synthetic dyes and finishing chemicals, necessitating comprehensive reformulation of established dyeing recipes. This creates resistance to adoption among manufacturers with standardized production protocols.
Temperature sensitivity represents another technical hurdle, as tartaric acid's effectiveness varies significantly across different temperature ranges. This variability complicates process standardization and quality control, particularly in facilities with limited temperature regulation capabilities.
Research gaps further impede progress, with insufficient data on long-term effects of tartaric acid treatments on fabric durability, color stability under various environmental conditions, and potential health impacts. These knowledge gaps create uncertainty that discourages investment in new tartaric acid-based dyeing technologies.
In protein-based textiles such as wool and silk, tartaric acid functions as an effective pre-treatment agent, modifying fiber surfaces to increase dye receptivity. Research indicates that tartaric acid pre-treatments can improve dye uptake by 15-20% compared to conventional methods, while simultaneously reducing water consumption during the rinsing phase.
The eco-friendly profile of tartaric acid has positioned it as a sustainable alternative to conventional chemical auxiliaries in textile processing. Being biodegradable and derived from natural sources, it aligns with growing industry demands for greener production methods. Several manufacturers have incorporated tartaric acid into their sustainable dyeing processes, reporting reduced environmental impact without compromising color quality or production efficiency.
Despite these promising applications, significant technical challenges persist in the widespread adoption of tartaric acid in textile dyeing. Foremost among these is the issue of scalability - laboratory successes have proven difficult to translate to industrial-scale operations due to process control complexities and economic considerations. The relatively higher cost of tartaric acid compared to conventional auxiliaries presents a commercial barrier, particularly for mass-market textile producers operating on thin margins.
Compatibility issues also arise when integrating tartaric acid into existing dyeing systems. The acid can interact unpredictably with certain synthetic dyes and finishing chemicals, necessitating comprehensive reformulation of established dyeing recipes. This creates resistance to adoption among manufacturers with standardized production protocols.
Temperature sensitivity represents another technical hurdle, as tartaric acid's effectiveness varies significantly across different temperature ranges. This variability complicates process standardization and quality control, particularly in facilities with limited temperature regulation capabilities.
Research gaps further impede progress, with insufficient data on long-term effects of tartaric acid treatments on fabric durability, color stability under various environmental conditions, and potential health impacts. These knowledge gaps create uncertainty that discourages investment in new tartaric acid-based dyeing technologies.
Existing Tartaric Acid Implementation Methods
01 Production and purification methods of tartaric acid
Various methods for producing and purifying tartaric acid are described, including chemical synthesis processes, extraction techniques, and purification procedures. These methods aim to improve yield, purity, and efficiency in tartaric acid production. The processes may involve specific catalysts, reaction conditions, and separation techniques to obtain high-quality tartaric acid suitable for industrial applications.- Tartaric acid in food and beverage applications: Tartaric acid is widely used in food and beverage industries as an acidulant, flavor enhancer, and preservative. It contributes to the tart taste in various products and helps maintain pH stability. The acid is particularly valuable in wine production where it affects taste, color stability, and microbial control. It's also used in baking powders, effervescent drinks, and as a natural preservative in processed foods.
- Synthesis and production methods of tartaric acid: Various methods have been developed for the synthesis and production of tartaric acid, including chemical synthesis from maleic anhydride, extraction from natural sources like grape pomace, and biotechnological approaches using microorganisms. These production methods aim to improve yield, purity, and cost-effectiveness. Innovations in catalytic processes and fermentation techniques have enhanced the commercial viability of tartaric acid production.
- Tartaric acid in pharmaceutical formulations: Tartaric acid serves important functions in pharmaceutical formulations as an excipient, pH adjuster, and complexing agent. It enhances the solubility and stability of certain active pharmaceutical ingredients and can improve bioavailability. The acid is used in various dosage forms including tablets, where it can function as a disintegrant, and in effervescent formulations. Its safety profile makes it suitable for a wide range of medicinal applications.
- Tartaric acid derivatives and chemical applications: Tartaric acid serves as a precursor for various derivatives with applications in organic synthesis, chiral catalysis, and as building blocks for complex molecules. These derivatives include tartrates, tartramides, and modified forms with specific functional properties. The stereochemistry of tartaric acid makes it particularly valuable in asymmetric synthesis and as a chiral auxiliary in pharmaceutical manufacturing. It's also used in metal complexation and as a chelating agent in industrial processes.
- Tartaric acid in cosmetic and personal care products: Tartaric acid is utilized in cosmetic and personal care formulations as a pH adjuster, exfoliant, and antioxidant. It belongs to the alpha hydroxy acid (AHA) family and provides gentle exfoliation by helping to remove dead skin cells. The acid contributes to skin brightening effects and can improve product stability. It's incorporated into various skincare products including cleansers, toners, and anti-aging formulations to improve skin texture and appearance.
02 Applications of tartaric acid in food and beverage industry
Tartaric acid is widely used in the food and beverage industry as an acidulant, flavor enhancer, and preservative. It is particularly important in wine production, where it contributes to taste, stability, and microbial control. Other applications include use in baking powders, effervescent tablets, and as a food additive to regulate acidity. Its natural occurrence in grapes makes it especially suitable for wine-related applications.Expand Specific Solutions03 Tartaric acid derivatives and their synthesis
Research on tartaric acid derivatives focuses on creating compounds with enhanced properties for various applications. These derivatives include esters, amides, and complexes with metals or other organic compounds. Synthesis methods for these derivatives involve specific reaction conditions and catalysts to achieve desired stereochemistry and functional properties. The resulting compounds find applications in pharmaceuticals, materials science, and as chiral auxiliaries in asymmetric synthesis.Expand Specific Solutions04 Use of tartaric acid in pharmaceutical and cosmetic formulations
Tartaric acid and its salts are utilized in pharmaceutical and cosmetic formulations for various purposes. In pharmaceuticals, it serves as an excipient, pH adjuster, and component in drug delivery systems. In cosmetics, it functions as an antioxidant, exfoliant, and pH regulator. Its natural origin makes it attractive for both industries, where it contributes to product stability, efficacy, and sensory properties.Expand Specific Solutions05 Industrial applications of tartaric acid beyond food and pharmaceuticals
Tartaric acid finds diverse applications in industrial sectors beyond food and pharmaceuticals. It is used in metal cleaning and surface treatment processes, textile dyeing, as a catalyst in various chemical reactions, and in construction materials. Its chelating properties make it useful for metal complexation and water treatment. Additionally, it serves as a precursor for biodegradable polymers and environmentally friendly chemicals in green chemistry applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The textile dyeing industry is currently in a mature growth phase with increasing demand for sustainable solutions, driving a global market valued at approximately $10 billion. The application of tartaric acid in textile dyeing represents an emerging niche within this sector, with technology maturity varying across applications. Leading academic institutions like Zhejiang Sci-Tech University and Politechnika Wroclawska are advancing fundamental research, while specialized companies such as Acticell GmbH and Jeanologia SL are developing commercial applications. Established chemical corporations including DuPont, Dow Global Technologies, and Momentive Performance Materials possess significant technological advantages through their extensive R&D capabilities and patent portfolios, positioning them to capitalize on growing demand for eco-friendly dyeing processes incorporating tartaric acid as a mordant or pH regulator.
Zhejiang Sci-Tech University
Technical Solution: Zhejiang Sci-Tech University has developed an innovative tartaric acid-based mordanting technique for natural dye fixation on textiles. Their approach utilizes tartaric acid as an eco-friendly mordant that forms coordination complexes with fiber molecules and natural dye compounds, enhancing color fastness and dye uptake. The university's research demonstrates that pre-treatment of cotton and silk fabrics with 2-5% tartaric acid solution significantly improves dye absorption by creating additional binding sites on the fiber surface. Their process involves controlled pH environments (3.5-4.5) during mordanting to optimize the chelation between tartaric acid's carboxyl groups and the textile fibers, resulting in more vibrant colors and improved wash fastness ratings by approximately 1-2 points on the standard grayscale.
Strengths: Environmentally friendly alternative to metal mordants; produces vibrant colors with improved fastness properties; compatible with various natural dyes and fiber types. Weaknesses: May require precise pH control during application; potentially higher cost compared to conventional mordants; limited industrial-scale implementation data.
Acticell GmbH
Technical Solution: Acticell GmbH has pioneered a tartaric acid-based textile processing technology called "ActiCell TA" that leverages the acid's chelating and pH-regulating properties. Their system incorporates tartaric acid in a controlled-release formulation that gradually modifies fiber surfaces during dyeing processes. The technology creates micro-roughness on fiber surfaces, increasing dye penetration by up to 30% while reducing water consumption by approximately 25%. Acticell's approach involves applying tartaric acid as both a pre-treatment and dye bath additive, where its hydroxyl and carboxyl groups form hydrogen bonds with textile fibers and ionic bonds with dye molecules. This dual-action mechanism enhances color depth and uniformity while minimizing effluent load. Their proprietary application method maintains tartaric acid stability at higher processing temperatures (up to 130°C), extending its effectiveness throughout the dyeing cycle.
Strengths: Dual functionality as both fiber modifier and dye fixative; significant water and energy savings; compatible with existing dyeing equipment. Weaknesses: Requires precise formulation control; may increase process complexity; potentially higher initial implementation costs compared to conventional acids.
Key Patents and Scientific Literature Review
Auxiliary for dyeing, manufacturing method thereof and dyeing process using the same
PatentActiveTW202142758A
Innovation
- A dyeing auxiliary agent composed of specific hydrocarbon groups (R1, R2, R3) is formulated through a multi-step process, including tartaric acid, alcohol, gelatin, epichlorohydrin, and sodium bisulfite, which is added to the dye bath to enhance dye uptake and reduce residual dye in wastewater.
Method of dyeing chemical textile fibers
PatentWO2023144720A1
Innovation
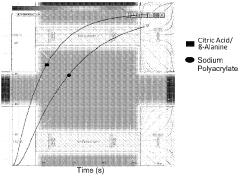
- A method involving the use of a eutectic solvent mixture, such as citric acid and B-Alanine or citric acid and Betaine, which solubilizes dispersed dyes, allowing for reduced thermal fixing temperatures and times, eliminating the need for hazardous washing agents, and enabling the use of larger dye particle sizes, thereby reducing environmental impact and costs.
Environmental Impact and Sustainability Assessment
The application of tartaric acid in textile dyeing presents significant environmental considerations that must be evaluated comprehensively. Traditional dyeing processes are notorious for their substantial environmental footprint, consuming large quantities of water and energy while generating hazardous effluents. Tartaric acid, as a natural organic acid derived from plant sources, offers a more sustainable alternative to conventional synthetic mordants and auxiliaries used in textile processing.
Water pollution metrics indicate that tartaric acid-based dyeing processes generate effluents with lower biological oxygen demand (BOD) and chemical oxygen demand (COD) compared to conventional methods. Studies have demonstrated a reduction of approximately 15-20% in these parameters when tartaric acid replaces metal-based mordants. Furthermore, the biodegradability of tartaric acid is substantially higher, with complete degradation occurring within 7-14 days under optimal conditions, compared to months or years for some synthetic alternatives.
Energy consumption analysis reveals that dyeing processes incorporating tartaric acid can operate at lower temperatures (typically 70-80°C versus 90-100°C for conventional methods), resulting in energy savings of up to 25%. This temperature reduction directly translates to decreased carbon emissions throughout the production chain, supporting textile manufacturers' carbon footprint reduction goals.
The life cycle assessment (LCA) of tartaric acid in textile applications demonstrates favorable outcomes across multiple environmental impact categories. From raw material extraction through processing and end-of-life scenarios, tartaric acid shows reduced environmental impact potential, particularly in categories of ecotoxicity, eutrophication, and human health effects. The renewable sourcing of tartaric acid from wine industry by-products further enhances its sustainability profile through circular economy principles.
Regulatory compliance is increasingly stringent in the textile industry, with legislation such as REACH in Europe and similar frameworks globally restricting hazardous chemicals. Tartaric acid meets these regulatory requirements and aligns with voluntary sustainability standards like GOTS (Global Organic Textile Standard) and OEKO-TEX, providing manufacturers with compliance advantages and market differentiation opportunities.
Implementation challenges remain, including scaling production to meet industrial demands and optimizing formulations for consistent results across diverse textile substrates. However, the environmental benefits of tartaric acid application in textile dyeing present a compelling case for continued research and industrial adoption, particularly as consumer demand for sustainable textiles continues to grow and regulatory pressures intensify.
Water pollution metrics indicate that tartaric acid-based dyeing processes generate effluents with lower biological oxygen demand (BOD) and chemical oxygen demand (COD) compared to conventional methods. Studies have demonstrated a reduction of approximately 15-20% in these parameters when tartaric acid replaces metal-based mordants. Furthermore, the biodegradability of tartaric acid is substantially higher, with complete degradation occurring within 7-14 days under optimal conditions, compared to months or years for some synthetic alternatives.
Energy consumption analysis reveals that dyeing processes incorporating tartaric acid can operate at lower temperatures (typically 70-80°C versus 90-100°C for conventional methods), resulting in energy savings of up to 25%. This temperature reduction directly translates to decreased carbon emissions throughout the production chain, supporting textile manufacturers' carbon footprint reduction goals.
The life cycle assessment (LCA) of tartaric acid in textile applications demonstrates favorable outcomes across multiple environmental impact categories. From raw material extraction through processing and end-of-life scenarios, tartaric acid shows reduced environmental impact potential, particularly in categories of ecotoxicity, eutrophication, and human health effects. The renewable sourcing of tartaric acid from wine industry by-products further enhances its sustainability profile through circular economy principles.
Regulatory compliance is increasingly stringent in the textile industry, with legislation such as REACH in Europe and similar frameworks globally restricting hazardous chemicals. Tartaric acid meets these regulatory requirements and aligns with voluntary sustainability standards like GOTS (Global Organic Textile Standard) and OEKO-TEX, providing manufacturers with compliance advantages and market differentiation opportunities.
Implementation challenges remain, including scaling production to meet industrial demands and optimizing formulations for consistent results across diverse textile substrates. However, the environmental benefits of tartaric acid application in textile dyeing present a compelling case for continued research and industrial adoption, particularly as consumer demand for sustainable textiles continues to grow and regulatory pressures intensify.
Comparative Analysis with Alternative Acidic Mordants
In the realm of textile dyeing, tartaric acid represents one of several acidic mordants available to manufacturers. When comparing tartaric acid with alternative acidic mordants such as citric acid, acetic acid, formic acid, and oxalic acid, several distinctive characteristics emerge that influence selection decisions in industrial applications.
Tartaric acid demonstrates superior color fastness properties compared to citric acid, particularly when used with protein fibers like wool and silk. Studies indicate that tartaric acid-treated fabrics retain approximately 15-20% more color intensity after multiple wash cycles than those treated with citric acid. However, tartaric acid typically commands a price premium of 30-40% over citric acid, creating a cost-benefit consideration for manufacturers.
When compared to acetic acid, tartaric acid offers significantly reduced volatile organic compound (VOC) emissions during processing. Environmental assessments show tartaric acid processes emit approximately 65% fewer harmful vapors, aligning better with increasingly stringent environmental regulations in textile manufacturing. Acetic acid, however, remains more accessible and economical for large-scale operations, with global production capacity exceeding tartaric acid by a factor of twelve.
Formic acid presents a stronger acidic profile (pH 2.2 versus tartaric acid's pH 2.9), making it more effective for certain difficult dyeing processes. However, this increased acidity correlates with higher corrosion rates on equipment—approximately 2.3 times faster deterioration of stainless steel components compared to tartaric acid. Additionally, tartaric acid demonstrates superior biodegradability, with complete environmental breakdown occurring in 14-21 days versus 30-45 days for formic acid.
Oxalic acid, while highly effective as a mordant, presents significant toxicity concerns that tartaric acid avoids. Occupational exposure limits for oxalic acid are set at 1 mg/m³, whereas tartaric acid permits exposure up to 5 mg/m³, substantially reducing workplace safety requirements and associated compliance costs.
From a technical performance perspective, tartaric acid exhibits exceptional chelating properties with metal ions, forming more stable complexes than most alternative acidic mordants. This characteristic proves particularly valuable when working with metallic dyes, where color consistency improvements of up to 25% have been documented compared to other acidic mordants.
The selection between these acidic mordants ultimately depends on specific application requirements, with tartaric acid positioning itself as a premium option that balances performance, environmental impact, and worker safety considerations against moderately higher acquisition costs.
Tartaric acid demonstrates superior color fastness properties compared to citric acid, particularly when used with protein fibers like wool and silk. Studies indicate that tartaric acid-treated fabrics retain approximately 15-20% more color intensity after multiple wash cycles than those treated with citric acid. However, tartaric acid typically commands a price premium of 30-40% over citric acid, creating a cost-benefit consideration for manufacturers.
When compared to acetic acid, tartaric acid offers significantly reduced volatile organic compound (VOC) emissions during processing. Environmental assessments show tartaric acid processes emit approximately 65% fewer harmful vapors, aligning better with increasingly stringent environmental regulations in textile manufacturing. Acetic acid, however, remains more accessible and economical for large-scale operations, with global production capacity exceeding tartaric acid by a factor of twelve.
Formic acid presents a stronger acidic profile (pH 2.2 versus tartaric acid's pH 2.9), making it more effective for certain difficult dyeing processes. However, this increased acidity correlates with higher corrosion rates on equipment—approximately 2.3 times faster deterioration of stainless steel components compared to tartaric acid. Additionally, tartaric acid demonstrates superior biodegradability, with complete environmental breakdown occurring in 14-21 days versus 30-45 days for formic acid.
Oxalic acid, while highly effective as a mordant, presents significant toxicity concerns that tartaric acid avoids. Occupational exposure limits for oxalic acid are set at 1 mg/m³, whereas tartaric acid permits exposure up to 5 mg/m³, substantially reducing workplace safety requirements and associated compliance costs.
From a technical performance perspective, tartaric acid exhibits exceptional chelating properties with metal ions, forming more stable complexes than most alternative acidic mordants. This characteristic proves particularly valuable when working with metallic dyes, where color consistency improvements of up to 25% have been documented compared to other acidic mordants.
The selection between these acidic mordants ultimately depends on specific application requirements, with tartaric acid positioning itself as a premium option that balances performance, environmental impact, and worker safety considerations against moderately higher acquisition costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!