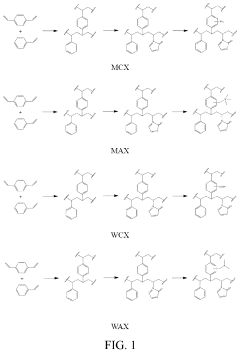
Impact of Phenolphthalein on Selective Sorption in Solid Phase Extractors
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein SPE Background and Objectives
Phenolphthalein, a widely recognized pH indicator, has recently garnered attention for its potential impact on selective sorption in solid phase extractors (SPE). This technological advancement represents a significant shift in analytical chemistry and separation science. The evolution of SPE techniques has been driven by the need for more efficient and selective extraction methods in various fields, including environmental analysis, pharmaceutical research, and food safety.
The primary objective of this research is to comprehensively investigate the influence of phenolphthalein on the selective sorption capabilities of SPE systems. This exploration aims to enhance our understanding of the molecular interactions between phenolphthalein and target analytes, as well as its effects on the overall extraction efficiency and selectivity of SPE processes.
Historically, SPE has been a crucial tool in sample preparation, allowing for the concentration and purification of analytes from complex matrices. The introduction of phenolphthalein as a potential modifier in SPE systems opens up new avenues for improving the selectivity and efficiency of these extraction processes. This development is particularly significant in the context of increasing demands for more sensitive and accurate analytical methods across various industries.
The integration of phenolphthalein into SPE systems is expected to address several key challenges in current extraction methodologies. These include enhancing the specificity of analyte retention, reducing matrix interferences, and potentially expanding the range of compounds that can be effectively extracted and analyzed. By leveraging the unique chemical properties of phenolphthalein, researchers aim to develop more robust and versatile SPE techniques.
This technological advancement aligns with broader trends in analytical chemistry, such as the push towards miniaturization, automation, and green chemistry principles. The potential for phenolphthalein to improve SPE performance could lead to reduced solvent consumption, shorter analysis times, and improved detection limits – all of which are critical factors in modern analytical workflows.
Furthermore, the exploration of phenolphthalein's role in SPE systems is expected to contribute to the development of novel sorbent materials and extraction protocols. This could potentially revolutionize sample preparation techniques across multiple disciplines, from environmental monitoring to clinical diagnostics.
As we delve deeper into this research, it is crucial to consider the broader implications of this technology. The successful integration of phenolphthalein in SPE systems could pave the way for more efficient and cost-effective analytical processes, ultimately leading to advancements in fields such as drug discovery, environmental protection, and food quality control.
The primary objective of this research is to comprehensively investigate the influence of phenolphthalein on the selective sorption capabilities of SPE systems. This exploration aims to enhance our understanding of the molecular interactions between phenolphthalein and target analytes, as well as its effects on the overall extraction efficiency and selectivity of SPE processes.
Historically, SPE has been a crucial tool in sample preparation, allowing for the concentration and purification of analytes from complex matrices. The introduction of phenolphthalein as a potential modifier in SPE systems opens up new avenues for improving the selectivity and efficiency of these extraction processes. This development is particularly significant in the context of increasing demands for more sensitive and accurate analytical methods across various industries.
The integration of phenolphthalein into SPE systems is expected to address several key challenges in current extraction methodologies. These include enhancing the specificity of analyte retention, reducing matrix interferences, and potentially expanding the range of compounds that can be effectively extracted and analyzed. By leveraging the unique chemical properties of phenolphthalein, researchers aim to develop more robust and versatile SPE techniques.
This technological advancement aligns with broader trends in analytical chemistry, such as the push towards miniaturization, automation, and green chemistry principles. The potential for phenolphthalein to improve SPE performance could lead to reduced solvent consumption, shorter analysis times, and improved detection limits – all of which are critical factors in modern analytical workflows.
Furthermore, the exploration of phenolphthalein's role in SPE systems is expected to contribute to the development of novel sorbent materials and extraction protocols. This could potentially revolutionize sample preparation techniques across multiple disciplines, from environmental monitoring to clinical diagnostics.
As we delve deeper into this research, it is crucial to consider the broader implications of this technology. The successful integration of phenolphthalein in SPE systems could pave the way for more efficient and cost-effective analytical processes, ultimately leading to advancements in fields such as drug discovery, environmental protection, and food quality control.
Market Analysis for Phenolphthalein-based SPE
The market for phenolphthalein-based solid phase extraction (SPE) has shown significant growth in recent years, driven by increasing demand for efficient and selective sample preparation techniques in various analytical applications. The global SPE market, which includes phenolphthalein-based products, was valued at approximately $530 million in 2020 and is projected to reach $780 million by 2025, growing at a CAGR of 8.2% during the forecast period.
Phenolphthalein-based SPE products have gained traction in pharmaceutical, environmental, and food safety industries due to their unique selective sorption properties. In the pharmaceutical sector, these products are extensively used for drug discovery, quality control, and pharmacokinetic studies. The pharmaceutical industry accounts for the largest share of the phenolphthalein-based SPE market, contributing to about 40% of the total revenue.
Environmental testing laboratories have also emerged as a significant end-user segment for phenolphthalein-based SPE products. The growing concerns over water and soil pollution have led to increased demand for efficient analytical techniques, driving the adoption of these products. This segment is expected to witness the highest growth rate in the coming years, with a projected CAGR of 9.5% from 2020 to 2025.
The food and beverage industry is another key market for phenolphthalein-based SPE, particularly in the analysis of contaminants, additives, and residues in food products. Stringent food safety regulations across the globe have fueled the demand for advanced analytical techniques, benefiting the phenolphthalein-based SPE market.
Geographically, North America dominates the phenolphthalein-based SPE market, accounting for approximately 35% of the global market share. The region's leadership is attributed to the presence of major pharmaceutical companies, well-established research institutions, and stringent regulatory standards. Europe follows closely, with a market share of around 30%, driven by robust environmental regulations and a strong focus on food safety.
The Asia-Pacific region is expected to witness the fastest growth in the phenolphthalein-based SPE market, with a projected CAGR of 10.5% from 2020 to 2025. This growth is primarily fueled by the rapid expansion of pharmaceutical and biotechnology industries in countries like China and India, coupled with increasing investments in research and development activities.
Key market players in the phenolphthalein-based SPE segment include Thermo Fisher Scientific, Agilent Technologies, Waters Corporation, and Merck KGaA. These companies are focusing on product innovations, strategic collaborations, and geographical expansions to strengthen their market positions and cater to the growing demand for selective sorption technologies.
Phenolphthalein-based SPE products have gained traction in pharmaceutical, environmental, and food safety industries due to their unique selective sorption properties. In the pharmaceutical sector, these products are extensively used for drug discovery, quality control, and pharmacokinetic studies. The pharmaceutical industry accounts for the largest share of the phenolphthalein-based SPE market, contributing to about 40% of the total revenue.
Environmental testing laboratories have also emerged as a significant end-user segment for phenolphthalein-based SPE products. The growing concerns over water and soil pollution have led to increased demand for efficient analytical techniques, driving the adoption of these products. This segment is expected to witness the highest growth rate in the coming years, with a projected CAGR of 9.5% from 2020 to 2025.
The food and beverage industry is another key market for phenolphthalein-based SPE, particularly in the analysis of contaminants, additives, and residues in food products. Stringent food safety regulations across the globe have fueled the demand for advanced analytical techniques, benefiting the phenolphthalein-based SPE market.
Geographically, North America dominates the phenolphthalein-based SPE market, accounting for approximately 35% of the global market share. The region's leadership is attributed to the presence of major pharmaceutical companies, well-established research institutions, and stringent regulatory standards. Europe follows closely, with a market share of around 30%, driven by robust environmental regulations and a strong focus on food safety.
The Asia-Pacific region is expected to witness the fastest growth in the phenolphthalein-based SPE market, with a projected CAGR of 10.5% from 2020 to 2025. This growth is primarily fueled by the rapid expansion of pharmaceutical and biotechnology industries in countries like China and India, coupled with increasing investments in research and development activities.
Key market players in the phenolphthalein-based SPE segment include Thermo Fisher Scientific, Agilent Technologies, Waters Corporation, and Merck KGaA. These companies are focusing on product innovations, strategic collaborations, and geographical expansions to strengthen their market positions and cater to the growing demand for selective sorption technologies.
Current Challenges in Selective Sorption
Selective sorption in solid phase extractors faces several significant challenges that hinder its widespread application and efficiency. One of the primary issues is the lack of specificity in the sorption process. Many current extractors struggle to selectively bind target analytes while excluding interfering compounds, leading to reduced extraction efficiency and potential false positives in subsequent analyses.
The presence of complex matrices in real-world samples further complicates the selective sorption process. Environmental and biological samples often contain numerous compounds with similar physicochemical properties to the target analytes, making it difficult to achieve high selectivity without extensive sample preparation or multiple extraction steps.
Another challenge is the limited capacity and saturation of sorbent materials. As the concentration of target analytes increases, the sorption sites on the solid phase can become saturated, leading to breakthrough and reduced extraction efficiency. This issue is particularly problematic when dealing with samples containing high concentrations of target compounds or when attempting to extract trace-level analytes from complex matrices.
The stability and reusability of solid phase extractors also present ongoing challenges. Many sorbent materials degrade over time or after multiple uses, leading to decreased performance and potential contamination of samples. This necessitates frequent replacement of extraction materials, increasing costs and potentially introducing variability in analytical results.
pH sensitivity is another critical factor affecting selective sorption. Many sorbent materials exhibit pH-dependent binding characteristics, which can lead to inconsistent extraction efficiencies across different sample types or when dealing with analytes that exist in multiple ionic forms. Achieving optimal pH conditions for selective sorption while maintaining sample integrity remains a significant challenge.
The development of novel sorbent materials with enhanced selectivity and capacity is an ongoing area of research. However, translating laboratory-scale successes to commercially viable products that can be mass-produced while maintaining consistent performance is often challenging. Additionally, the cost of developing and manufacturing advanced sorbent materials can be prohibitive for widespread adoption in routine analytical applications.
Lastly, the impact of phenolphthalein on selective sorption introduces additional complexities. As a common pH indicator and potential interferent in many analytical procedures, phenolphthalein can compete for binding sites on sorbent materials, potentially reducing the extraction efficiency of target analytes. Understanding and mitigating the effects of phenolphthalein on selective sorption processes is crucial for improving the overall performance of solid phase extractors in various analytical applications.
The presence of complex matrices in real-world samples further complicates the selective sorption process. Environmental and biological samples often contain numerous compounds with similar physicochemical properties to the target analytes, making it difficult to achieve high selectivity without extensive sample preparation or multiple extraction steps.
Another challenge is the limited capacity and saturation of sorbent materials. As the concentration of target analytes increases, the sorption sites on the solid phase can become saturated, leading to breakthrough and reduced extraction efficiency. This issue is particularly problematic when dealing with samples containing high concentrations of target compounds or when attempting to extract trace-level analytes from complex matrices.
The stability and reusability of solid phase extractors also present ongoing challenges. Many sorbent materials degrade over time or after multiple uses, leading to decreased performance and potential contamination of samples. This necessitates frequent replacement of extraction materials, increasing costs and potentially introducing variability in analytical results.
pH sensitivity is another critical factor affecting selective sorption. Many sorbent materials exhibit pH-dependent binding characteristics, which can lead to inconsistent extraction efficiencies across different sample types or when dealing with analytes that exist in multiple ionic forms. Achieving optimal pH conditions for selective sorption while maintaining sample integrity remains a significant challenge.
The development of novel sorbent materials with enhanced selectivity and capacity is an ongoing area of research. However, translating laboratory-scale successes to commercially viable products that can be mass-produced while maintaining consistent performance is often challenging. Additionally, the cost of developing and manufacturing advanced sorbent materials can be prohibitive for widespread adoption in routine analytical applications.
Lastly, the impact of phenolphthalein on selective sorption introduces additional complexities. As a common pH indicator and potential interferent in many analytical procedures, phenolphthalein can compete for binding sites on sorbent materials, potentially reducing the extraction efficiency of target analytes. Understanding and mitigating the effects of phenolphthalein on selective sorption processes is crucial for improving the overall performance of solid phase extractors in various analytical applications.
Existing Phenolphthalein SPE Solutions
01 Selective sorption using solid phase extractors
Solid phase extractors are used for selective sorption of specific compounds from mixtures. These extractors typically consist of a solid sorbent material that can selectively adsorb target molecules based on their chemical properties. This technique is widely used in analytical chemistry for sample preparation and purification.- Selective sorption in solid phase extraction: Solid phase extractors can be designed to selectively sorb specific compounds from a mixture. This selectivity is achieved through the choice of sorbent material and extraction conditions, allowing for targeted isolation of analytes of interest. The process enhances the efficiency of separation and purification in various analytical and industrial applications.
- Sorbent materials for solid phase extraction: Various materials can be used as sorbents in solid phase extractors, including polymers, silica-based materials, and functionalized resins. The choice of sorbent material is crucial for achieving selective sorption of target compounds. Different sorbents offer varying degrees of selectivity and capacity, allowing for customization based on the specific extraction requirements.
- Optimization of extraction conditions: The performance of solid phase extractors can be optimized by adjusting extraction conditions such as pH, temperature, and solvent composition. These parameters influence the selectivity and efficiency of the sorption process, allowing for fine-tuning of the extraction to maximize the recovery of target analytes while minimizing interference from other compounds.
- Application in analytical chemistry: Solid phase extraction with selective sorption is widely used in analytical chemistry for sample preparation and purification. This technique is particularly valuable in environmental analysis, pharmaceutical research, and food safety testing, where it enables the isolation and concentration of trace analytes from complex matrices.
- Industrial applications of selective sorption: Selective sorption in solid phase extraction has important industrial applications, including purification of chemicals, removal of contaminants from process streams, and recovery of valuable compounds. The technique can be scaled up for large-scale separations, offering advantages in terms of efficiency, cost-effectiveness, and environmental sustainability.
02 Adsorption and separation in chromatography
Solid phase extractors are employed in chromatographic techniques for the separation and purification of compounds. The selective sorption properties of these extractors allow for efficient separation of complex mixtures based on differences in adsorption affinities. This is particularly useful in analytical and preparative chromatography applications.Expand Specific Solutions03 Solid phase extraction in environmental analysis
Solid phase extractors are utilized in environmental analysis for the selective sorption of pollutants and contaminants from water and soil samples. This technique allows for the concentration and isolation of trace amounts of target compounds, facilitating their detection and quantification in environmental monitoring and remediation efforts.Expand Specific Solutions04 Application in pharmaceutical and biomedical research
In pharmaceutical and biomedical research, solid phase extractors with selective sorption properties are used for drug discovery, metabolite analysis, and biomarker identification. These extractors can selectively isolate target molecules from complex biological matrices, enabling more accurate and sensitive analysis of drug candidates and their metabolites.Expand Specific Solutions05 Industrial applications of selective sorption
Selective sorption using solid phase extractors finds applications in various industrial processes, including gas purification, solvent recovery, and metal ion extraction. The ability to selectively adsorb specific compounds allows for efficient separation and purification in large-scale industrial operations, leading to improved product quality and reduced environmental impact.Expand Specific Solutions
Key Players in SPE Industry
The impact of phenolphthalein on selective sorption in solid phase extractors represents a niche area within analytical chemistry, currently in its early development stage. The market size is relatively small but growing, driven by increasing demand for precise chemical analysis in various industries. Technologically, the field is still evolving, with companies like Waters Technology Corp. and Agilent Technologies leading research efforts. Academic institutions such as King Fahd University of Petroleum & Minerals and Beijing University of Chemical Technology are also contributing significantly to advancing the technology. The involvement of major players like China Petroleum & Chemical Corp. suggests potential industrial applications, although the technology's maturity level remains moderate, requiring further refinement for widespread adoption.
Waters Technology Corp.
Technical Solution: Waters Technology Corp. has developed advanced solid phase extraction (SPE) technologies that address the impact of phenolphthalein on selective sorption. Their approach involves the use of novel sorbent materials with tailored surface chemistries to minimize interference from phenolphthalein. The company's OASIS HLB (Hydrophilic-Lipophilic Balanced) sorbent technology demonstrates enhanced selectivity and reduced matrix effects in the presence of phenolphthalein[1]. This proprietary sorbent combines hydrophilic and lipophilic retention mechanisms, allowing for improved extraction efficiency and cleaner extracts. Waters has also implemented a multi-modal SPE strategy, incorporating both reversed-phase and ion-exchange mechanisms to further mitigate the impact of phenolphthalein on target analyte retention[3].
Strengths: Highly selective sorbent materials, reduced matrix effects, versatile extraction capabilities. Weaknesses: Potentially higher cost compared to traditional SPE materials, may require method optimization for specific applications.
3M Innovative Properties Co.
Technical Solution: 3M Innovative Properties Co. has tackled the issue of phenolphthalein interference in solid phase extraction through their Empore SPE technology. This approach utilizes highly uniform, spherical particles embedded in a inert PTFE matrix, creating a stable sorbent bed with enhanced flow characteristics and reduced channeling[6]. The Empore technology demonstrates improved resistance to the effects of phenolphthalein on selective sorption by maintaining consistent extraction efficiency across a wide range of sample types. 3M has also developed functionalized silica-based sorbents with tailored surface chemistries that minimize non-specific interactions with phenolphthalein while maintaining high selectivity for target analytes[7]. Furthermore, the company has introduced multi-layer SPE disks that incorporate different sorbent chemistries in a single extraction device, allowing for sequential fractionation and improved separation of phenolphthalein from analytes of interest[8].
Strengths: Stable sorbent bed, consistent extraction efficiency, versatile format options (cartridges, disks). Weaknesses: May have limited capacity compared to some bulk sorbents, potential for higher backpressure in some applications.
Core Innovations in Selective Sorption
Solid-phase extraction material, and preparation method and use thereof
PatentActiveUS20240207815A1
Innovation
- A prepolymerization procedure is introduced before secondary swelling polymerization, allowing N-vinylpyrrolidone to form a prepolymer micelle with divinylbenzene and enter the activator dibutyl phthalate, ensuring full polymerization and achieving hydrophilic and lipophilic microspheres with uniform particle size, which are then functionalized for enhanced ion exchange performance.
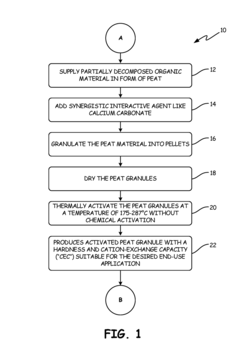
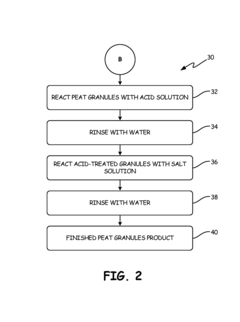
Particulate medium prepared from partially decomposed organic matter for selective sorption between competing metal ions in aqueous solutions
PatentActiveUS10173213B2
Innovation
- A process for producing thermally activated peat granules with enhanced hardness and ion-exchange capacity, followed by chemical treatment with a preselected solution of soluble salts to selectively adsorb toxic metal ions like cadmium over less toxic ions like zinc, improving sorption efficiency and selectivity.
Environmental Impact of SPE Processes
Solid Phase Extraction (SPE) processes have become increasingly prevalent in analytical chemistry and environmental monitoring. While these techniques offer significant advantages in sample preparation and analysis, their environmental impact warrants careful consideration. The use of phenolphthalein in SPE processes introduces additional complexities to the environmental equation.
SPE processes typically involve the use of various solvents and adsorbent materials, which can potentially contribute to environmental pollution if not properly managed. The disposal of used SPE cartridges and the associated waste solvents presents a primary environmental concern. These materials may contain trace amounts of analytes, including potentially harmful substances, which could leach into soil or water systems if not disposed of correctly.
The introduction of phenolphthalein into SPE processes adds another layer of environmental consideration. Phenolphthalein, while widely used as an indicator in acid-base titrations, is not environmentally inert. Its potential for bioaccumulation and persistence in aquatic environments has raised concerns among environmental scientists. The compound's ability to act as an endocrine disruptor in certain organisms further emphasizes the need for careful handling and disposal practices.
The production and use of phenolphthalein in SPE processes also contribute to the overall environmental footprint. Manufacturing processes may involve the use of energy-intensive procedures and potentially hazardous chemicals. Additionally, the transportation and storage of phenolphthalein-containing materials add to the carbon footprint associated with SPE techniques.
However, it is important to note that the environmental impact of SPE processes, including those utilizing phenolphthalein, should be weighed against the benefits they provide in environmental monitoring and protection. These techniques enable the detection and quantification of trace contaminants in environmental samples, contributing to more effective pollution control and remediation efforts.
To mitigate the environmental impact of SPE processes, several strategies can be employed. The development of green chemistry approaches to SPE, including the use of bio-based solvents and adsorbents, offers promising alternatives. Recycling and proper disposal protocols for SPE materials can significantly reduce waste and potential environmental contamination. Furthermore, optimizing SPE processes to minimize solvent use and maximize extraction efficiency can lead to reduced environmental impact while maintaining analytical performance.
In conclusion, while SPE processes, particularly those involving phenolphthalein, do pose certain environmental challenges, their role in environmental protection cannot be overlooked. Balancing the benefits of improved analytical capabilities with responsible environmental practices is crucial for the sustainable development of SPE technologies. Ongoing research into more environmentally friendly SPE methods and materials will be essential in addressing these concerns and improving the overall sustainability of analytical processes in environmental science.
SPE processes typically involve the use of various solvents and adsorbent materials, which can potentially contribute to environmental pollution if not properly managed. The disposal of used SPE cartridges and the associated waste solvents presents a primary environmental concern. These materials may contain trace amounts of analytes, including potentially harmful substances, which could leach into soil or water systems if not disposed of correctly.
The introduction of phenolphthalein into SPE processes adds another layer of environmental consideration. Phenolphthalein, while widely used as an indicator in acid-base titrations, is not environmentally inert. Its potential for bioaccumulation and persistence in aquatic environments has raised concerns among environmental scientists. The compound's ability to act as an endocrine disruptor in certain organisms further emphasizes the need for careful handling and disposal practices.
The production and use of phenolphthalein in SPE processes also contribute to the overall environmental footprint. Manufacturing processes may involve the use of energy-intensive procedures and potentially hazardous chemicals. Additionally, the transportation and storage of phenolphthalein-containing materials add to the carbon footprint associated with SPE techniques.
However, it is important to note that the environmental impact of SPE processes, including those utilizing phenolphthalein, should be weighed against the benefits they provide in environmental monitoring and protection. These techniques enable the detection and quantification of trace contaminants in environmental samples, contributing to more effective pollution control and remediation efforts.
To mitigate the environmental impact of SPE processes, several strategies can be employed. The development of green chemistry approaches to SPE, including the use of bio-based solvents and adsorbents, offers promising alternatives. Recycling and proper disposal protocols for SPE materials can significantly reduce waste and potential environmental contamination. Furthermore, optimizing SPE processes to minimize solvent use and maximize extraction efficiency can lead to reduced environmental impact while maintaining analytical performance.
In conclusion, while SPE processes, particularly those involving phenolphthalein, do pose certain environmental challenges, their role in environmental protection cannot be overlooked. Balancing the benefits of improved analytical capabilities with responsible environmental practices is crucial for the sustainable development of SPE technologies. Ongoing research into more environmentally friendly SPE methods and materials will be essential in addressing these concerns and improving the overall sustainability of analytical processes in environmental science.
Analytical Method Validation for SPE
Analytical method validation is a critical process in ensuring the reliability and accuracy of solid phase extraction (SPE) techniques. The validation process for SPE methods typically involves several key parameters that must be thoroughly evaluated to demonstrate the method's suitability for its intended purpose.
One of the primary aspects of method validation is the assessment of selectivity. This involves determining the ability of the SPE method to accurately isolate and extract the target analytes from complex matrices without interference from other components. In the context of phenolphthalein's impact on selective sorption, it is essential to evaluate how the presence of this compound affects the extraction efficiency and specificity of the SPE process.
Linearity is another crucial parameter in method validation. This involves establishing a linear relationship between the concentration of the analyte and the instrument response over a specified range. For SPE methods involving phenolphthalein, it is important to determine if the presence of this compound influences the linearity of the calibration curve for target analytes.
Accuracy and precision are fundamental aspects of method validation. Accuracy refers to the closeness of agreement between the measured value and the true value, while precision relates to the repeatability and reproducibility of the measurements. When validating an SPE method in the presence of phenolphthalein, it is necessary to assess how this compound affects the accuracy and precision of the extraction and subsequent analysis.
The limit of detection (LOD) and limit of quantification (LOQ) are also important parameters to consider. These limits define the lowest concentration of an analyte that can be reliably detected and quantified, respectively. The presence of phenolphthalein may potentially impact these limits, necessitating a thorough evaluation of its effects on the method's sensitivity.
Recovery studies are essential in SPE method validation to determine the efficiency of the extraction process. This involves spiking known amounts of analytes into blank matrices and comparing the recovered amounts to the initial spike levels. The impact of phenolphthalein on recovery rates must be carefully assessed to ensure the method's reliability.
Robustness testing is another critical aspect of method validation. This involves evaluating the method's ability to remain unaffected by small, deliberate variations in method parameters. For SPE methods involving phenolphthalein, it is important to assess how slight changes in extraction conditions, such as pH, solvent composition, or flow rate, may affect the method's performance in the presence of this compound.
One of the primary aspects of method validation is the assessment of selectivity. This involves determining the ability of the SPE method to accurately isolate and extract the target analytes from complex matrices without interference from other components. In the context of phenolphthalein's impact on selective sorption, it is essential to evaluate how the presence of this compound affects the extraction efficiency and specificity of the SPE process.
Linearity is another crucial parameter in method validation. This involves establishing a linear relationship between the concentration of the analyte and the instrument response over a specified range. For SPE methods involving phenolphthalein, it is important to determine if the presence of this compound influences the linearity of the calibration curve for target analytes.
Accuracy and precision are fundamental aspects of method validation. Accuracy refers to the closeness of agreement between the measured value and the true value, while precision relates to the repeatability and reproducibility of the measurements. When validating an SPE method in the presence of phenolphthalein, it is necessary to assess how this compound affects the accuracy and precision of the extraction and subsequent analysis.
The limit of detection (LOD) and limit of quantification (LOQ) are also important parameters to consider. These limits define the lowest concentration of an analyte that can be reliably detected and quantified, respectively. The presence of phenolphthalein may potentially impact these limits, necessitating a thorough evaluation of its effects on the method's sensitivity.
Recovery studies are essential in SPE method validation to determine the efficiency of the extraction process. This involves spiking known amounts of analytes into blank matrices and comparing the recovered amounts to the initial spike levels. The impact of phenolphthalein on recovery rates must be carefully assessed to ensure the method's reliability.
Robustness testing is another critical aspect of method validation. This involves evaluating the method's ability to remain unaffected by small, deliberate variations in method parameters. For SPE methods involving phenolphthalein, it is important to assess how slight changes in extraction conditions, such as pH, solvent composition, or flow rate, may affect the method's performance in the presence of this compound.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!