Innovations in Muscimol Hydrogel Delivery Systems
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Hydrogel Background and Objectives
Muscimol, a potent GABA-A receptor agonist, has garnered significant attention in the field of neuropharmacology due to its potential therapeutic applications. The development of innovative muscimol hydrogel delivery systems represents a crucial advancement in addressing the challenges associated with traditional drug administration methods. This technological evolution aims to enhance the efficacy, safety, and precision of muscimol delivery for various neurological disorders.
The primary objective of this research is to explore and evaluate cutting-edge innovations in muscimol hydrogel delivery systems. These advancements seek to overcome limitations such as poor bioavailability, rapid clearance, and off-target effects associated with conventional delivery methods. By leveraging the unique properties of hydrogels, researchers aim to achieve controlled release, targeted delivery, and improved pharmacokinetics of muscimol.
Historically, muscimol research has focused on its potential as a tool for studying GABAergic neurotransmission and its role in various neurological processes. However, recent years have witnessed a shift towards exploring its therapeutic potential in conditions such as epilepsy, anxiety disorders, and neurodegenerative diseases. This paradigm shift has necessitated the development of more sophisticated delivery systems to maximize the therapeutic benefits while minimizing side effects.
The evolution of muscimol hydrogel delivery systems can be traced through several key milestones. Initial efforts focused on simple encapsulation techniques, which gradually gave way to more advanced formulations incorporating stimuli-responsive materials and nanotechnology. Current research trends indicate a move towards smart hydrogels capable of responding to physiological cues, enabling on-demand drug release and personalized treatment approaches.
One of the primary goals in this field is to achieve precise control over muscimol release kinetics. This involves developing hydrogel formulations that can maintain therapeutic concentrations of muscimol over extended periods, reducing the frequency of administration and improving patient compliance. Additionally, researchers are exploring ways to enhance the targeting capabilities of these delivery systems, allowing for localized drug delivery to specific brain regions while minimizing systemic exposure.
Another critical objective is to improve the stability and biocompatibility of muscimol hydrogel formulations. This includes investigating novel crosslinking methods, incorporating bioactive molecules to enhance tissue integration, and developing strategies to prevent premature degradation of the hydrogel matrix. These advancements aim to prolong the therapeutic window and reduce the risk of adverse reactions.
As the field progresses, there is a growing emphasis on translational research, bridging the gap between laboratory discoveries and clinical applications. This involves addressing challenges related to scalability, manufacturing processes, and regulatory compliance. The ultimate goal is to develop muscimol hydrogel delivery systems that are not only effective but also commercially viable and readily adaptable to clinical settings.
The primary objective of this research is to explore and evaluate cutting-edge innovations in muscimol hydrogel delivery systems. These advancements seek to overcome limitations such as poor bioavailability, rapid clearance, and off-target effects associated with conventional delivery methods. By leveraging the unique properties of hydrogels, researchers aim to achieve controlled release, targeted delivery, and improved pharmacokinetics of muscimol.
Historically, muscimol research has focused on its potential as a tool for studying GABAergic neurotransmission and its role in various neurological processes. However, recent years have witnessed a shift towards exploring its therapeutic potential in conditions such as epilepsy, anxiety disorders, and neurodegenerative diseases. This paradigm shift has necessitated the development of more sophisticated delivery systems to maximize the therapeutic benefits while minimizing side effects.
The evolution of muscimol hydrogel delivery systems can be traced through several key milestones. Initial efforts focused on simple encapsulation techniques, which gradually gave way to more advanced formulations incorporating stimuli-responsive materials and nanotechnology. Current research trends indicate a move towards smart hydrogels capable of responding to physiological cues, enabling on-demand drug release and personalized treatment approaches.
One of the primary goals in this field is to achieve precise control over muscimol release kinetics. This involves developing hydrogel formulations that can maintain therapeutic concentrations of muscimol over extended periods, reducing the frequency of administration and improving patient compliance. Additionally, researchers are exploring ways to enhance the targeting capabilities of these delivery systems, allowing for localized drug delivery to specific brain regions while minimizing systemic exposure.
Another critical objective is to improve the stability and biocompatibility of muscimol hydrogel formulations. This includes investigating novel crosslinking methods, incorporating bioactive molecules to enhance tissue integration, and developing strategies to prevent premature degradation of the hydrogel matrix. These advancements aim to prolong the therapeutic window and reduce the risk of adverse reactions.
As the field progresses, there is a growing emphasis on translational research, bridging the gap between laboratory discoveries and clinical applications. This involves addressing challenges related to scalability, manufacturing processes, and regulatory compliance. The ultimate goal is to develop muscimol hydrogel delivery systems that are not only effective but also commercially viable and readily adaptable to clinical settings.
Market Analysis for Muscimol Delivery Systems
The market for muscimol delivery systems, particularly those utilizing hydrogel technology, is experiencing significant growth and transformation. This emerging sector sits at the intersection of neuropharmacology, materials science, and drug delivery innovation, presenting a unique opportunity for pharmaceutical companies and biotechnology firms.
The global market for advanced drug delivery systems is projected to expand rapidly in the coming years, with hydrogel-based technologies playing an increasingly important role. Muscimol, a potent GABA receptor agonist, has shown promise in treating various neurological disorders, including epilepsy, anxiety, and certain types of pain. The development of innovative delivery systems for this compound is driven by the need for more targeted, controlled, and efficient drug administration methods.
Current market trends indicate a growing demand for localized and sustained-release drug delivery systems, which aligns well with the potential benefits of muscimol hydrogel formulations. These systems offer the advantage of prolonged drug release, reduced systemic side effects, and improved patient compliance compared to traditional oral or injectable formulations.
The pharmaceutical industry's increasing focus on personalized medicine and precision drug delivery is expected to further boost the market for advanced muscimol delivery systems. As research continues to uncover the therapeutic potential of muscimol in various neurological conditions, the demand for sophisticated delivery mechanisms is likely to grow correspondingly.
Key market drivers include the rising prevalence of neurological disorders, an aging global population, and the increasing adoption of minimally invasive treatment options. Additionally, the ongoing shift towards value-based healthcare models is encouraging the development of more effective and efficient drug delivery systems that can improve patient outcomes while potentially reducing overall treatment costs.
However, the market also faces several challenges. Regulatory hurdles for novel drug delivery systems, particularly those involving controlled substances like muscimol, can be significant. The high costs associated with research and development of these advanced technologies may also limit market growth, especially in developing economies.
Despite these challenges, the market outlook for muscimol hydrogel delivery systems remains positive. Collaborations between pharmaceutical companies, academic institutions, and biotechnology firms are likely to accelerate innovation in this field. As the technology matures and clinical evidence accumulates, we can expect to see increased investment and market penetration of these advanced delivery systems.
In conclusion, the market for muscimol hydrogel delivery systems represents a promising niche within the broader pharmaceutical and biotechnology sectors. With ongoing advancements in materials science and drug delivery technologies, this market segment is poised for substantial growth in the coming years, offering significant opportunities for companies at the forefront of innovation in this field.
The global market for advanced drug delivery systems is projected to expand rapidly in the coming years, with hydrogel-based technologies playing an increasingly important role. Muscimol, a potent GABA receptor agonist, has shown promise in treating various neurological disorders, including epilepsy, anxiety, and certain types of pain. The development of innovative delivery systems for this compound is driven by the need for more targeted, controlled, and efficient drug administration methods.
Current market trends indicate a growing demand for localized and sustained-release drug delivery systems, which aligns well with the potential benefits of muscimol hydrogel formulations. These systems offer the advantage of prolonged drug release, reduced systemic side effects, and improved patient compliance compared to traditional oral or injectable formulations.
The pharmaceutical industry's increasing focus on personalized medicine and precision drug delivery is expected to further boost the market for advanced muscimol delivery systems. As research continues to uncover the therapeutic potential of muscimol in various neurological conditions, the demand for sophisticated delivery mechanisms is likely to grow correspondingly.
Key market drivers include the rising prevalence of neurological disorders, an aging global population, and the increasing adoption of minimally invasive treatment options. Additionally, the ongoing shift towards value-based healthcare models is encouraging the development of more effective and efficient drug delivery systems that can improve patient outcomes while potentially reducing overall treatment costs.
However, the market also faces several challenges. Regulatory hurdles for novel drug delivery systems, particularly those involving controlled substances like muscimol, can be significant. The high costs associated with research and development of these advanced technologies may also limit market growth, especially in developing economies.
Despite these challenges, the market outlook for muscimol hydrogel delivery systems remains positive. Collaborations between pharmaceutical companies, academic institutions, and biotechnology firms are likely to accelerate innovation in this field. As the technology matures and clinical evidence accumulates, we can expect to see increased investment and market penetration of these advanced delivery systems.
In conclusion, the market for muscimol hydrogel delivery systems represents a promising niche within the broader pharmaceutical and biotechnology sectors. With ongoing advancements in materials science and drug delivery technologies, this market segment is poised for substantial growth in the coming years, offering significant opportunities for companies at the forefront of innovation in this field.
Current Challenges in Muscimol Hydrogel Technology
Despite the promising potential of muscimol hydrogel delivery systems, several significant challenges currently hinder their widespread adoption and clinical application. One of the primary obstacles is achieving precise control over the release kinetics of muscimol from the hydrogel matrix. The complex interplay between the hydrogel's physical properties and the drug's molecular characteristics makes it difficult to maintain a consistent and predictable release profile over extended periods.
Another critical challenge lies in the stability of muscimol within the hydrogel environment. The drug's susceptibility to degradation under certain pH conditions or in the presence of specific enzymes can compromise its therapeutic efficacy. Researchers are grappling with the task of developing hydrogel formulations that not only encapsulate muscimol effectively but also protect it from premature breakdown or inactivation.
The biocompatibility and biodegradability of the hydrogel materials pose additional hurdles. While many hydrogels demonstrate good biocompatibility in vitro, translating these results to in vivo applications often reveals unforeseen complications. Some hydrogels may trigger inflammatory responses or form undesirable residues upon degradation, potentially leading to adverse effects in the surrounding tissues.
Scaling up the production of muscimol-loaded hydrogels for clinical use presents its own set of challenges. Maintaining consistency in hydrogel properties and drug loading across different batches is crucial for ensuring reproducible therapeutic outcomes. However, the complex nature of hydrogel synthesis and drug incorporation processes makes this a formidable task.
The targeted delivery of muscimol-loaded hydrogels to specific brain regions remains a significant technical challenge. Current delivery methods often lack the precision required for localized administration, potentially leading to off-target effects or suboptimal therapeutic concentrations at the desired site of action.
Furthermore, the long-term stability of muscimol hydrogels under physiological conditions is a concern. The dynamic nature of the in vivo environment, including fluctuations in pH, temperature, and the presence of various biomolecules, can alter the hydrogel's structure and drug release properties over time. Developing hydrogel systems that maintain their integrity and functionality in these complex biological settings is an ongoing challenge.
Lastly, regulatory hurdles present a significant obstacle in the clinical translation of muscimol hydrogel delivery systems. The novel nature of these drug-device combinations often necessitates extensive safety and efficacy studies, prolonging the path to clinical approval and increasing development costs.
Another critical challenge lies in the stability of muscimol within the hydrogel environment. The drug's susceptibility to degradation under certain pH conditions or in the presence of specific enzymes can compromise its therapeutic efficacy. Researchers are grappling with the task of developing hydrogel formulations that not only encapsulate muscimol effectively but also protect it from premature breakdown or inactivation.
The biocompatibility and biodegradability of the hydrogel materials pose additional hurdles. While many hydrogels demonstrate good biocompatibility in vitro, translating these results to in vivo applications often reveals unforeseen complications. Some hydrogels may trigger inflammatory responses or form undesirable residues upon degradation, potentially leading to adverse effects in the surrounding tissues.
Scaling up the production of muscimol-loaded hydrogels for clinical use presents its own set of challenges. Maintaining consistency in hydrogel properties and drug loading across different batches is crucial for ensuring reproducible therapeutic outcomes. However, the complex nature of hydrogel synthesis and drug incorporation processes makes this a formidable task.
The targeted delivery of muscimol-loaded hydrogels to specific brain regions remains a significant technical challenge. Current delivery methods often lack the precision required for localized administration, potentially leading to off-target effects or suboptimal therapeutic concentrations at the desired site of action.
Furthermore, the long-term stability of muscimol hydrogels under physiological conditions is a concern. The dynamic nature of the in vivo environment, including fluctuations in pH, temperature, and the presence of various biomolecules, can alter the hydrogel's structure and drug release properties over time. Developing hydrogel systems that maintain their integrity and functionality in these complex biological settings is an ongoing challenge.
Lastly, regulatory hurdles present a significant obstacle in the clinical translation of muscimol hydrogel delivery systems. The novel nature of these drug-device combinations often necessitates extensive safety and efficacy studies, prolonging the path to clinical approval and increasing development costs.
Existing Muscimol Hydrogel Delivery Methods
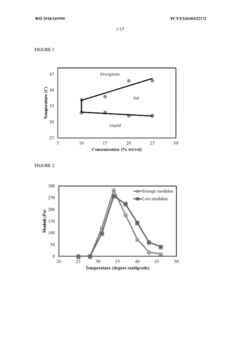
01 Hydrogel-based delivery systems for muscimol
Hydrogels are used as a delivery system for muscimol, providing controlled release and improved bioavailability. These systems can be designed to respond to various stimuli, such as pH or temperature, allowing for targeted drug delivery. The hydrogel matrix can protect the muscimol from degradation and enhance its stability.- Hydrogel-based drug delivery systems: Hydrogels are used as carriers for controlled release of muscimol. These systems can be designed to provide sustained or targeted delivery of the drug, improving its therapeutic efficacy and reducing side effects. The hydrogel matrix can be tailored to respond to specific stimuli or environmental conditions, allowing for precise control over drug release.
- Muscimol formulations for transdermal delivery: Transdermal delivery systems for muscimol are developed using various formulations and technologies. These may include patches, gels, or other topical preparations that allow the drug to be absorbed through the skin. This route of administration can provide advantages such as avoiding first-pass metabolism and offering a more convenient dosing method for patients.
- Nanoparticle-based muscimol delivery: Nanoparticles are utilized to enhance the delivery of muscimol. These nano-sized carriers can improve the drug's solubility, stability, and ability to cross biological barriers. Various types of nanoparticles, such as polymeric nanoparticles or lipid-based nanocarriers, may be employed to optimize muscimol delivery to specific target sites.
- Combination therapies involving muscimol: Muscimol is incorporated into combination therapies with other active ingredients or treatment modalities. These combinations may aim to enhance therapeutic effects, reduce side effects, or target multiple aspects of a condition simultaneously. The delivery systems are designed to accommodate the co-delivery of muscimol with other compounds in a controlled and effective manner.
- Novel muscimol analogs and prodrugs: Research focuses on developing novel muscimol analogs or prodrugs to improve its pharmacokinetic properties and delivery. These modified forms of muscimol may offer advantages such as enhanced stability, improved bioavailability, or targeted action. The delivery systems are tailored to accommodate the specific properties of these new compounds.
02 Transdermal delivery of muscimol using hydrogels
Transdermal delivery systems incorporating hydrogels are developed for muscimol administration. These systems allow for non-invasive drug delivery through the skin, potentially improving patient compliance and reducing side effects associated with oral administration. The hydrogel formulation can be optimized to enhance skin permeation of muscimol.Expand Specific Solutions03 Nanoparticle-loaded hydrogels for muscimol delivery
Nanoparticles loaded with muscimol are incorporated into hydrogel matrices to create advanced drug delivery systems. This approach combines the benefits of nanoparticle encapsulation with the controlled release properties of hydrogels. The nanoparticles can protect muscimol from degradation and potentially enhance its cellular uptake.Expand Specific Solutions04 Injectable hydrogels for localized muscimol delivery
Injectable hydrogel formulations are developed for localized delivery of muscimol. These systems can be administered as liquids that form gels in situ, allowing for minimally invasive delivery to specific target sites. The hydrogel can provide sustained release of muscimol over extended periods, potentially reducing the frequency of administration.Expand Specific Solutions05 Mucoadhesive hydrogels for muscimol delivery
Mucoadhesive hydrogels are designed for muscimol delivery across mucosal surfaces, such as nasal or buccal routes. These systems can enhance drug absorption and bioavailability by prolonging the contact time with the mucosal surface. The hydrogel formulation can be tailored to optimize mucoadhesion and drug release properties.Expand Specific Solutions
Key Players in Muscimol Hydrogel Research
The field of muscimol hydrogel delivery systems is in an early stage of development, with significant potential for growth. The market size is currently limited but expected to expand as research progresses. Technological maturity is still evolving, with key players like Contraline, Inc. and T&R Biofab Co., Ltd leading innovation in hydrogel technologies. Academic institutions such as MIT, Johns Hopkins University, and Tsinghua University are contributing fundamental research. The competitive landscape is characterized by collaboration between industry and academia, with companies like Reckitt Benckiser (UK) Ltd. and GlobalFoundries U.S., Inc. exploring potential applications. As the technology advances, we can anticipate increased commercial interest and market expansion in this promising area.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered a cutting-edge muscimol hydrogel delivery system utilizing advanced materials science and nanotechnology. Their approach involves the development of self-assembling peptide nanofibers that form a hydrogel matrix capable of encapsulating and releasing muscimol[7]. The peptide sequences are designed to respond to specific enzymatic cleavage, allowing for precise control over the release kinetics. The hydrogel also incorporates graphene oxide nanosheets, which enhance the mechanical properties and provide an additional mechanism for muscimol binding and release[8]. MIT researchers have demonstrated that this system can achieve zero-order release kinetics for up to two months, maintaining consistent therapeutic levels of muscimol. Additionally, they have developed a non-invasive method for hydrogel administration using focused ultrasound to trigger gelation in deep brain tissues[9].
Strengths: Precise control over release kinetics, long-term stability, potential for non-invasive administration. Weaknesses: High cost of materials, potential long-term effects of nanomaterials in the brain.
Wisconsin Alumni Research Foundation
Technical Solution: The Wisconsin Alumni Research Foundation has developed a muscimol hydrogel delivery system based on a novel interpenetrating polymer network (IPN) approach. This system combines a thermosensitive poly(N-vinylcaprolactam) (PVCL) hydrogel with a pH-responsive chitosan network[10]. The dual-responsive nature of the IPN allows for both temperature-triggered gelation upon injection and pH-dependent release of muscimol in the brain microenvironment. The researchers have optimized the hydrogel composition to achieve a biphasic release profile, with an initial burst release followed by sustained delivery over several weeks[11]. They have also incorporated antioxidant moieties into the polymer backbone to protect muscimol from degradation and enhance its stability. Furthermore, the hydrogel has been designed to promote neuroregeneration by incorporating neural growth factors alongside muscimol[12].
Strengths: Dual-responsive system, enhanced drug stability, potential for promoting neural regeneration. Weaknesses: Complex synthesis process, potential for undesired interactions between multiple active components.
Breakthrough Muscimol Hydrogel Patents
Injectable hydrogel for drug delivery
PatentWO2024061673A1
Innovation
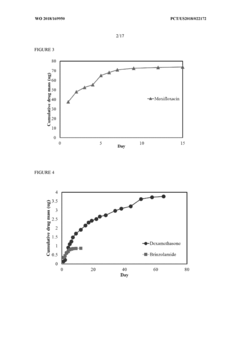
- Development of an injectable hydrogel based on AB and/or ABA block co-polymers crosslinked via disulphide bonds, allowing for biodegradable and minimally invasive delivery of dexamethasone through a 30G needle, with self-healing properties and controlled release over several months, facilitated by redox-sensitive degradation in the vitreous environment.
Injectable multidrug delivery hydrogel and uses thereof
PatentWO2018169950A1
Innovation
- A thermosensitive hydrogel composition incorporating biocompatible and biodegradable triblock copolymers that forms a depot upon injection, allowing for the controlled release of multiple drug molecules, including antibiotics, corticosteroids, and ocular antihypertensives, for extended periods, thereby reducing the need for frequent topical applications.
Regulatory Framework for Muscimol-Based Therapeutics
The regulatory framework for muscimol-based therapeutics is a complex and evolving landscape that requires careful navigation by pharmaceutical companies and researchers. As muscimol, a psychoactive compound found in certain mushroom species, gains attention for its potential therapeutic applications, regulatory bodies worldwide are adapting their policies to address this emerging field.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development and approval of muscimol-based therapeutics. The FDA's regulatory approach for these compounds falls under the broader category of psychedelic medicine, which has seen increased interest in recent years. The agency has established specific guidelines for the conduct of clinical trials involving psychedelic substances, including muscimol, to ensure patient safety and scientific rigor.
The European Medicines Agency (EMA) has also recognized the potential of muscimol-based therapies and has developed a framework for their evaluation. The EMA's approach emphasizes the importance of quality control in the production of muscimol-containing products and the need for comprehensive preclinical and clinical data to support marketing authorization applications.
In Canada, Health Canada has implemented a regulatory pathway for psychedelic-assisted therapies, which includes provisions for muscimol-based treatments. This framework allows for controlled access to these therapies through special access programs and clinical trials, while maintaining strict oversight to mitigate potential risks.
Internationally, the United Nations Office on Drugs and Crime (UNODC) and the World Health Organization (WHO) play crucial roles in shaping global drug policies. These organizations are actively reviewing the status of psychedelic compounds, including muscimol, to inform international drug control conventions and public health recommendations.
Regulatory challenges specific to muscimol-based therapeutics include the need for standardized quality control measures for the extraction and synthesis of muscimol, as well as the development of appropriate dosing guidelines. Additionally, regulators must address the potential for abuse and diversion, necessitating robust risk management strategies and post-market surveillance programs.
As research in this field progresses, regulatory bodies are likely to refine their approaches, potentially leading to more streamlined pathways for the development and approval of muscimol-based therapeutics. This evolving regulatory landscape underscores the importance of ongoing dialogue between researchers, industry stakeholders, and regulatory authorities to ensure the safe and effective development of these innovative therapies.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development and approval of muscimol-based therapeutics. The FDA's regulatory approach for these compounds falls under the broader category of psychedelic medicine, which has seen increased interest in recent years. The agency has established specific guidelines for the conduct of clinical trials involving psychedelic substances, including muscimol, to ensure patient safety and scientific rigor.
The European Medicines Agency (EMA) has also recognized the potential of muscimol-based therapies and has developed a framework for their evaluation. The EMA's approach emphasizes the importance of quality control in the production of muscimol-containing products and the need for comprehensive preclinical and clinical data to support marketing authorization applications.
In Canada, Health Canada has implemented a regulatory pathway for psychedelic-assisted therapies, which includes provisions for muscimol-based treatments. This framework allows for controlled access to these therapies through special access programs and clinical trials, while maintaining strict oversight to mitigate potential risks.
Internationally, the United Nations Office on Drugs and Crime (UNODC) and the World Health Organization (WHO) play crucial roles in shaping global drug policies. These organizations are actively reviewing the status of psychedelic compounds, including muscimol, to inform international drug control conventions and public health recommendations.
Regulatory challenges specific to muscimol-based therapeutics include the need for standardized quality control measures for the extraction and synthesis of muscimol, as well as the development of appropriate dosing guidelines. Additionally, regulators must address the potential for abuse and diversion, necessitating robust risk management strategies and post-market surveillance programs.
As research in this field progresses, regulatory bodies are likely to refine their approaches, potentially leading to more streamlined pathways for the development and approval of muscimol-based therapeutics. This evolving regulatory landscape underscores the importance of ongoing dialogue between researchers, industry stakeholders, and regulatory authorities to ensure the safe and effective development of these innovative therapies.
Safety and Efficacy Considerations
The safety and efficacy considerations for muscimol hydrogel delivery systems are paramount in the development and implementation of this innovative technology. The primary concern is ensuring that the hydrogel matrix can effectively encapsulate and release muscimol in a controlled manner, without compromising its pharmacological properties or introducing harmful byproducts.
One of the key safety aspects is the biocompatibility of the hydrogel materials used. These materials must be non-toxic, non-immunogenic, and biodegradable to prevent adverse reactions in the body. Extensive in vitro and in vivo studies are necessary to evaluate the long-term effects of the hydrogel components on surrounding tissues and organs.
The stability of muscimol within the hydrogel matrix is another critical factor. The delivery system must maintain the drug's chemical integrity throughout the preparation, storage, and release processes. This includes protection from degradation due to environmental factors such as light, temperature, and pH changes.
Efficacy considerations focus on the controlled release profile of muscimol from the hydrogel. The ideal system should provide a sustained and predictable release rate, maintaining therapeutic concentrations of muscimol at the target site over an extended period. This requires careful optimization of the hydrogel's physical properties, such as porosity, crosslinking density, and degradation rate.
The potential for off-target effects and systemic absorption of muscimol must also be thoroughly evaluated. While localized delivery via hydrogel systems aims to minimize systemic exposure, it is crucial to assess the risk of muscimol leakage into the bloodstream and its potential impact on non-target tissues.
Reproducibility and scalability of the hydrogel production process are essential for ensuring consistent safety and efficacy profiles across batches. Standardized manufacturing protocols and quality control measures must be established to maintain the desired characteristics of the delivery system.
Lastly, the long-term stability and shelf-life of the muscimol-loaded hydrogels need to be determined. This includes assessing the potential for microbial contamination, chemical degradation, and changes in physical properties over time, which could affect both safety and efficacy.
In conclusion, the development of muscimol hydrogel delivery systems requires a multifaceted approach to address safety and efficacy considerations. Rigorous testing, careful material selection, and precise control over release kinetics are essential for realizing the full potential of this innovative technology in clinical applications.
One of the key safety aspects is the biocompatibility of the hydrogel materials used. These materials must be non-toxic, non-immunogenic, and biodegradable to prevent adverse reactions in the body. Extensive in vitro and in vivo studies are necessary to evaluate the long-term effects of the hydrogel components on surrounding tissues and organs.
The stability of muscimol within the hydrogel matrix is another critical factor. The delivery system must maintain the drug's chemical integrity throughout the preparation, storage, and release processes. This includes protection from degradation due to environmental factors such as light, temperature, and pH changes.
Efficacy considerations focus on the controlled release profile of muscimol from the hydrogel. The ideal system should provide a sustained and predictable release rate, maintaining therapeutic concentrations of muscimol at the target site over an extended period. This requires careful optimization of the hydrogel's physical properties, such as porosity, crosslinking density, and degradation rate.
The potential for off-target effects and systemic absorption of muscimol must also be thoroughly evaluated. While localized delivery via hydrogel systems aims to minimize systemic exposure, it is crucial to assess the risk of muscimol leakage into the bloodstream and its potential impact on non-target tissues.
Reproducibility and scalability of the hydrogel production process are essential for ensuring consistent safety and efficacy profiles across batches. Standardized manufacturing protocols and quality control measures must be established to maintain the desired characteristics of the delivery system.
Lastly, the long-term stability and shelf-life of the muscimol-loaded hydrogels need to be determined. This includes assessing the potential for microbial contamination, chemical degradation, and changes in physical properties over time, which could affect both safety and efficacy.
In conclusion, the development of muscimol hydrogel delivery systems requires a multifaceted approach to address safety and efficacy considerations. Rigorous testing, careful material selection, and precise control over release kinetics are essential for realizing the full potential of this innovative technology in clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!