Innovative Uses of Dimethyl Ether in Chemical Industries
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DME Background and Objectives
Dimethyl ether (DME) has emerged as a versatile compound with significant potential in various chemical industries. Its journey began in the early 20th century when it was first synthesized, but it wasn't until recent decades that its full potential started to be realized. DME's unique properties, including its clean-burning characteristics and ease of liquefaction, have positioned it as a promising alternative fuel and chemical feedstock.
The evolution of DME technology has been driven by the increasing demand for cleaner energy sources and more efficient chemical processes. Initially used primarily as a propellant in aerosol products, DME has since found applications in a wide range of industries, from energy and transportation to pharmaceuticals and materials science. This expansion of use cases has led to a surge in research and development efforts aimed at optimizing DME production and exploring novel applications.
In the context of innovative uses in chemical industries, DME presents several compelling advantages. Its molecular structure, consisting of two methyl groups bonded to an oxygen atom, allows for diverse chemical reactions and transformations. This versatility opens up possibilities for DME to serve as a building block in the synthesis of various chemical products, potentially replacing more environmentally harmful or expensive alternatives.
The primary objectives of current DME research and development in chemical industries are multifaceted. Firstly, there is a focus on enhancing the efficiency and cost-effectiveness of DME production processes. This includes exploring new catalysts and reaction conditions to improve yields and reduce energy consumption. Secondly, researchers are investigating the potential of DME as a green solvent, leveraging its low toxicity and favorable physical properties.
Another key objective is the development of DME-based materials with unique properties. For instance, DME's ability to form clathrate hydrates has sparked interest in its use for gas storage and separation technologies. Additionally, the pharmaceutical industry is exploring DME as a potential excipient or reaction medium in drug formulation and synthesis.
The push towards sustainability and circular economy principles has also influenced DME research objectives. There is growing interest in producing DME from renewable sources, such as biomass or captured carbon dioxide, aligning with global efforts to reduce greenhouse gas emissions and dependence on fossil fuels. This bio-based DME production not only addresses environmental concerns but also opens up new avenues for value creation in the chemical industry.
As we look to the future, the trajectory of DME technology in chemical industries is poised for significant growth and innovation. The ongoing research aims to unlock new applications, improve existing processes, and overcome current limitations in DME utilization. By addressing these challenges and capitalizing on DME's unique properties, the chemical industry stands to benefit from more sustainable, efficient, and versatile processes and products.
The evolution of DME technology has been driven by the increasing demand for cleaner energy sources and more efficient chemical processes. Initially used primarily as a propellant in aerosol products, DME has since found applications in a wide range of industries, from energy and transportation to pharmaceuticals and materials science. This expansion of use cases has led to a surge in research and development efforts aimed at optimizing DME production and exploring novel applications.
In the context of innovative uses in chemical industries, DME presents several compelling advantages. Its molecular structure, consisting of two methyl groups bonded to an oxygen atom, allows for diverse chemical reactions and transformations. This versatility opens up possibilities for DME to serve as a building block in the synthesis of various chemical products, potentially replacing more environmentally harmful or expensive alternatives.
The primary objectives of current DME research and development in chemical industries are multifaceted. Firstly, there is a focus on enhancing the efficiency and cost-effectiveness of DME production processes. This includes exploring new catalysts and reaction conditions to improve yields and reduce energy consumption. Secondly, researchers are investigating the potential of DME as a green solvent, leveraging its low toxicity and favorable physical properties.
Another key objective is the development of DME-based materials with unique properties. For instance, DME's ability to form clathrate hydrates has sparked interest in its use for gas storage and separation technologies. Additionally, the pharmaceutical industry is exploring DME as a potential excipient or reaction medium in drug formulation and synthesis.
The push towards sustainability and circular economy principles has also influenced DME research objectives. There is growing interest in producing DME from renewable sources, such as biomass or captured carbon dioxide, aligning with global efforts to reduce greenhouse gas emissions and dependence on fossil fuels. This bio-based DME production not only addresses environmental concerns but also opens up new avenues for value creation in the chemical industry.
As we look to the future, the trajectory of DME technology in chemical industries is poised for significant growth and innovation. The ongoing research aims to unlock new applications, improve existing processes, and overcome current limitations in DME utilization. By addressing these challenges and capitalizing on DME's unique properties, the chemical industry stands to benefit from more sustainable, efficient, and versatile processes and products.
Market Demand Analysis
The market demand for innovative uses of dimethyl ether (DME) in chemical industries has been steadily growing, driven by its versatile properties and potential as a clean energy source. DME's unique characteristics, including its high cetane number, low emissions, and ease of handling, have positioned it as a promising alternative fuel and chemical feedstock.
In the energy sector, DME has gained significant traction as a substitute for liquefied petroleum gas (LPG) and diesel fuel. The global DME market size was valued at over $5 billion in 2020, with projections indicating a compound annual growth rate (CAGR) of around 10% from 2021 to 2028. This growth is primarily attributed to the increasing demand for clean-burning fuels and the stringent environmental regulations imposed by various governments worldwide.
The chemical industry has shown particular interest in DME as a raw material for the production of olefins, such as ethylene and propylene. These basic building blocks are essential in the manufacture of plastics, resins, and other petrochemical products. The demand for DME in this application is expected to rise significantly, driven by the growing need for sustainable and cost-effective feedstock alternatives.
In the automotive sector, DME has emerged as a potential replacement for conventional diesel fuel. Several major automotive manufacturers have conducted trials and demonstrations of DME-powered vehicles, showcasing its potential to reduce emissions and improve engine efficiency. The market for DME as an automotive fuel is still in its nascent stages but is expected to grow as more countries adopt stricter emission standards.
The pharmaceutical and personal care industries have also shown interest in DME as a propellant and solvent. Its low toxicity and favorable environmental profile make it an attractive option for aerosol applications, replacing traditional hydrocarbon propellants. This segment of the market is anticipated to experience steady growth, driven by consumer demand for eco-friendly products.
Geographically, Asia-Pacific dominates the DME market, with China being the largest producer and consumer. The region's rapid industrialization, coupled with government initiatives to promote clean energy, has been a key driver for DME adoption. North America and Europe are also witnessing increased demand, particularly in the transportation and chemical sectors.
Despite the positive market outlook, challenges remain in scaling up DME production and distribution infrastructure. The relatively high production costs compared to conventional fuels and the need for specialized handling equipment have been identified as potential barriers to widespread adoption. However, ongoing research and development efforts are focused on improving production efficiency and reducing costs, which is expected to further stimulate market growth in the coming years.
In the energy sector, DME has gained significant traction as a substitute for liquefied petroleum gas (LPG) and diesel fuel. The global DME market size was valued at over $5 billion in 2020, with projections indicating a compound annual growth rate (CAGR) of around 10% from 2021 to 2028. This growth is primarily attributed to the increasing demand for clean-burning fuels and the stringent environmental regulations imposed by various governments worldwide.
The chemical industry has shown particular interest in DME as a raw material for the production of olefins, such as ethylene and propylene. These basic building blocks are essential in the manufacture of plastics, resins, and other petrochemical products. The demand for DME in this application is expected to rise significantly, driven by the growing need for sustainable and cost-effective feedstock alternatives.
In the automotive sector, DME has emerged as a potential replacement for conventional diesel fuel. Several major automotive manufacturers have conducted trials and demonstrations of DME-powered vehicles, showcasing its potential to reduce emissions and improve engine efficiency. The market for DME as an automotive fuel is still in its nascent stages but is expected to grow as more countries adopt stricter emission standards.
The pharmaceutical and personal care industries have also shown interest in DME as a propellant and solvent. Its low toxicity and favorable environmental profile make it an attractive option for aerosol applications, replacing traditional hydrocarbon propellants. This segment of the market is anticipated to experience steady growth, driven by consumer demand for eco-friendly products.
Geographically, Asia-Pacific dominates the DME market, with China being the largest producer and consumer. The region's rapid industrialization, coupled with government initiatives to promote clean energy, has been a key driver for DME adoption. North America and Europe are also witnessing increased demand, particularly in the transportation and chemical sectors.
Despite the positive market outlook, challenges remain in scaling up DME production and distribution infrastructure. The relatively high production costs compared to conventional fuels and the need for specialized handling equipment have been identified as potential barriers to widespread adoption. However, ongoing research and development efforts are focused on improving production efficiency and reducing costs, which is expected to further stimulate market growth in the coming years.
DME Technology Status
Dimethyl ether (DME) has emerged as a versatile compound with significant potential in various chemical industries. The current technology status of DME showcases its growing importance and diverse applications. As a clean-burning, non-toxic, and easily handled fuel, DME has gained traction in the energy sector as a potential alternative to conventional fossil fuels.
In the chemical industry, DME serves as a crucial intermediate in the production of various chemicals. It is widely used as a propellant in aerosol products, replacing chlorofluorocarbons (CFCs) due to its environmentally friendly nature. The pharmaceutical sector has also embraced DME as a solvent and extraction agent, particularly in the production of certain medications and cosmetics.
Recent advancements in DME production technology have led to more efficient and cost-effective manufacturing processes. The traditional method of DME production involves the dehydration of methanol, but newer technologies focus on direct synthesis from syngas, offering improved yields and reduced production costs. This development has significantly enhanced the economic viability of DME in various applications.
The automotive industry has shown increasing interest in DME as a potential fuel for diesel engines. Its high cetane number and clean-burning properties make it an attractive alternative to conventional diesel fuel. Several major automotive manufacturers have conducted extensive research and trials on DME-powered vehicles, demonstrating promising results in terms of performance and emissions reduction.
In the realm of energy storage and transportation, DME has gained attention as a potential carrier for hydrogen. Its ability to be easily liquefied and stored at moderate pressures makes it an attractive option for hydrogen storage and distribution, addressing some of the challenges associated with the hydrogen economy.
The petrochemical industry has also found innovative uses for DME. It serves as a feedstock for the production of olefins, particularly in regions where traditional feedstocks like naphtha are less available or more expensive. This application has opened up new possibilities for diversifying petrochemical production and reducing dependence on conventional raw materials.
Environmental considerations have further propelled the development of DME technology. As a sulfur-free and low-emission fuel, DME aligns well with increasingly stringent environmental regulations. This has led to increased research and development efforts to optimize DME production and utilization processes, focusing on improving energy efficiency and reducing carbon footprint.
In the chemical industry, DME serves as a crucial intermediate in the production of various chemicals. It is widely used as a propellant in aerosol products, replacing chlorofluorocarbons (CFCs) due to its environmentally friendly nature. The pharmaceutical sector has also embraced DME as a solvent and extraction agent, particularly in the production of certain medications and cosmetics.
Recent advancements in DME production technology have led to more efficient and cost-effective manufacturing processes. The traditional method of DME production involves the dehydration of methanol, but newer technologies focus on direct synthesis from syngas, offering improved yields and reduced production costs. This development has significantly enhanced the economic viability of DME in various applications.
The automotive industry has shown increasing interest in DME as a potential fuel for diesel engines. Its high cetane number and clean-burning properties make it an attractive alternative to conventional diesel fuel. Several major automotive manufacturers have conducted extensive research and trials on DME-powered vehicles, demonstrating promising results in terms of performance and emissions reduction.
In the realm of energy storage and transportation, DME has gained attention as a potential carrier for hydrogen. Its ability to be easily liquefied and stored at moderate pressures makes it an attractive option for hydrogen storage and distribution, addressing some of the challenges associated with the hydrogen economy.
The petrochemical industry has also found innovative uses for DME. It serves as a feedstock for the production of olefins, particularly in regions where traditional feedstocks like naphtha are less available or more expensive. This application has opened up new possibilities for diversifying petrochemical production and reducing dependence on conventional raw materials.
Environmental considerations have further propelled the development of DME technology. As a sulfur-free and low-emission fuel, DME aligns well with increasingly stringent environmental regulations. This has led to increased research and development efforts to optimize DME production and utilization processes, focusing on improving energy efficiency and reducing carbon footprint.
Current DME Applications
01 Production of dimethyl ether
Various methods for producing dimethyl ether are described, including catalytic dehydration of methanol, direct synthesis from syngas, and conversion of other hydrocarbons. These processes often involve specific catalysts and reaction conditions to optimize yield and selectivity.- Production of dimethyl ether: Various methods for producing dimethyl ether are described, including catalytic dehydration of methanol, direct synthesis from syngas, and conversion of other hydrocarbons. These processes often involve specific catalysts and reaction conditions to optimize yield and selectivity.
- Catalysts for dimethyl ether synthesis: Different types of catalysts are used in the production of dimethyl ether, including zeolites, metal oxides, and composite catalysts. The choice of catalyst can significantly affect the reaction efficiency, product selectivity, and overall process economics.
- Applications of dimethyl ether: Dimethyl ether has various applications, including use as a fuel additive, aerosol propellant, and refrigerant. It is also being explored as a potential alternative fuel for diesel engines due to its clean-burning properties and high cetane number.
- Purification and separation of dimethyl ether: Techniques for purifying and separating dimethyl ether from reaction mixtures or other compounds are described. These may include distillation, adsorption, and membrane separation processes to achieve high-purity dimethyl ether for various applications.
- Environmental and safety considerations: Research on the environmental impact and safety aspects of dimethyl ether production and use is ongoing. This includes studies on emissions reduction, handling procedures, and storage requirements to ensure safe and sustainable utilization of dimethyl ether in various applications.
02 Catalysts for dimethyl ether synthesis
Different types of catalysts are used in the production of dimethyl ether, including zeolites, metal oxides, and composite catalysts. The choice of catalyst can significantly affect the reaction efficiency, product selectivity, and overall process economics.Expand Specific Solutions03 Applications of dimethyl ether
Dimethyl ether has various applications, including use as a fuel substitute, aerosol propellant, and chemical intermediate. Its properties make it suitable for use in diesel engines, household products, and as a feedstock for other chemical processes.Expand Specific Solutions04 Purification and separation of dimethyl ether
Methods for purifying and separating dimethyl ether from reaction mixtures or other compounds are described. These processes may involve distillation, adsorption, or membrane separation techniques to obtain high-purity dimethyl ether.Expand Specific Solutions05 Environmental and safety considerations
Research on the environmental impact and safety aspects of dimethyl ether production and use is presented. This includes studies on emissions reduction, handling procedures, and potential hazards associated with its storage and transportation.Expand Specific Solutions
Key Industry Players
The innovative uses of Dimethyl Ether (DME) in chemical industries represent a growing market with significant potential. The industry is in a transitional phase, moving from research and development to commercial applications. Market size is expanding, driven by DME's versatility as a clean-burning fuel and chemical feedstock. Technologically, the field is maturing rapidly, with major players like China Petroleum & Chemical Corp., DuPont de Nemours, and SK Energy leading the way. Research institutions such as Shanghai Petrochemical Research Institute and Sinopec Research Institute of Petroleum Processing are contributing to technological advancements. The involvement of diverse companies across petrochemical, energy, and chemical sectors indicates a competitive and innovative landscape for DME applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed innovative processes for dimethyl ether (DME) production and utilization in chemical industries. Their technology focuses on the direct synthesis of DME from syngas, which is more efficient than traditional methanol-based routes[1]. Sinopec has implemented a large-scale DME production facility with a capacity of 1 million tons per year, utilizing coal-based syngas as feedstock[2]. The company has also explored DME as a clean alternative fuel for diesel engines, developing DME-powered vehicles and refueling infrastructure[3]. Additionally, Sinopec has researched the use of DME as a propellant in aerosol products, replacing harmful chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs)[4].
Strengths: Vertically integrated production from coal to DME, large-scale production capacity, diverse applications in fuel and chemical sectors. Weaknesses: Dependence on coal-based feedstock may face environmental challenges, competition from other alternative fuels in the transportation sector.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed innovative applications for dimethyl ether (DME) in the chemical industry, focusing on its use as a propellant and solvent. The company has patented a process for using DME as a blowing agent in the production of polyurethane foams, offering an environmentally friendly alternative to traditional hydrofluorocarbons (HFCs)[5]. DuPont has also explored DME's potential as a refrigerant, leveraging its low global warming potential and zero ozone depletion potential[6]. In the field of precision cleaning, DuPont has developed DME-based formulations for electronics and medical device manufacturing, providing effective cleaning without leaving residues[7]. Furthermore, the company has investigated DME's role in chemical synthesis, particularly as a methylating agent in organic reactions[8].
Strengths: Diverse applications across multiple industries, strong focus on environmentally friendly solutions, extensive patent portfolio. Weaknesses: May face competition from other green solvents and propellants, potential regulatory challenges in some applications.
DME Innovation Patents
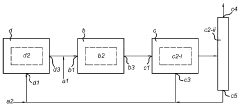
Process and system for producing dimethyl ether
PatentActiveUS11897839B2
Innovation
- A process combining conventional DME synthesis with a separation-enhanced reverse water gas shift reaction, allowing for flexible feedstock composition and reducing the need for CO2 recycles by converting CO2 to CO, thereby improving yield and reducing costs.
Dehydration of methanol to dimethyl ether using a catalyst based on zeolites supported on silicon carbide.
PatentInactiveJP2010511680A
Innovation
- Immobilizing zeolites on silicon carbide (SiC) supports with high specific surface areas, which enhances catalyst stability by rapid heat dissipation and reduces coking, maintaining activity for extended periods.
Environmental Impact
The environmental impact of dimethyl ether (DME) in chemical industries is a critical consideration as its innovative uses expand. DME offers several environmental advantages compared to traditional fossil fuels and chemical feedstocks. Its combustion produces lower emissions of particulate matter, nitrogen oxides, and sulfur oxides, contributing to improved air quality in industrial settings and surrounding areas.
One of the most significant environmental benefits of DME is its potential as a renewable fuel when produced from biomass or waste materials. This production method can significantly reduce greenhouse gas emissions compared to fossil fuel-based alternatives. The carbon footprint of DME can be further minimized through carbon capture and utilization technologies during its production process.
In the chemical industry, DME serves as a versatile and environmentally friendly solvent and propellant. It replaces more harmful substances like chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) in aerosol applications, contributing to the protection of the ozone layer. Additionally, DME's use as a refrigerant offers a lower global warming potential compared to traditional hydrofluorocarbons (HFCs).
The production of DME from methanol or syngas presents opportunities for industrial symbiosis, where waste streams from one process become feedstocks for another. This approach enhances resource efficiency and reduces overall environmental impact across chemical manufacturing processes. Furthermore, DME's potential as a hydrogen carrier for fuel cells opens avenues for cleaner energy storage and transportation solutions.
However, the environmental impact of DME is not without challenges. Large-scale production and use of DME require careful consideration of land use changes, especially when derived from biomass feedstocks. Sustainable sourcing practices are essential to prevent deforestation or competition with food crops. Additionally, the energy intensity of DME production processes needs ongoing optimization to maximize its environmental benefits.
Life cycle assessments of DME applications in chemical industries reveal varying degrees of environmental improvement depending on the specific use case and production pathway. Continuous research and development efforts focus on enhancing the efficiency of DME production and utilization to further reduce its environmental footprint. As regulatory frameworks evolve to address climate change and air quality concerns, DME's role in the chemical industry is likely to expand, driven by its favorable environmental profile.
One of the most significant environmental benefits of DME is its potential as a renewable fuel when produced from biomass or waste materials. This production method can significantly reduce greenhouse gas emissions compared to fossil fuel-based alternatives. The carbon footprint of DME can be further minimized through carbon capture and utilization technologies during its production process.
In the chemical industry, DME serves as a versatile and environmentally friendly solvent and propellant. It replaces more harmful substances like chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) in aerosol applications, contributing to the protection of the ozone layer. Additionally, DME's use as a refrigerant offers a lower global warming potential compared to traditional hydrofluorocarbons (HFCs).
The production of DME from methanol or syngas presents opportunities for industrial symbiosis, where waste streams from one process become feedstocks for another. This approach enhances resource efficiency and reduces overall environmental impact across chemical manufacturing processes. Furthermore, DME's potential as a hydrogen carrier for fuel cells opens avenues for cleaner energy storage and transportation solutions.
However, the environmental impact of DME is not without challenges. Large-scale production and use of DME require careful consideration of land use changes, especially when derived from biomass feedstocks. Sustainable sourcing practices are essential to prevent deforestation or competition with food crops. Additionally, the energy intensity of DME production processes needs ongoing optimization to maximize its environmental benefits.
Life cycle assessments of DME applications in chemical industries reveal varying degrees of environmental improvement depending on the specific use case and production pathway. Continuous research and development efforts focus on enhancing the efficiency of DME production and utilization to further reduce its environmental footprint. As regulatory frameworks evolve to address climate change and air quality concerns, DME's role in the chemical industry is likely to expand, driven by its favorable environmental profile.
Safety Regulations
The use of dimethyl ether (DME) in chemical industries necessitates stringent safety regulations due to its flammable and potentially explosive nature. Regulatory bodies worldwide have established comprehensive guidelines to ensure the safe handling, storage, and transportation of DME. These regulations typically cover aspects such as proper containment, ventilation requirements, and fire prevention measures.
In the United States, the Occupational Safety and Health Administration (OSHA) has set specific standards for DME handling in industrial settings. These include requirements for personal protective equipment (PPE), emergency response procedures, and regular safety training for personnel working with DME. The Environmental Protection Agency (EPA) also regulates DME under the Clean Air Act, focusing on emissions control and environmental impact.
European regulations, governed by the European Chemicals Agency (ECHA), emphasize the implementation of REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) for DME. This framework ensures that manufacturers and importers assess and manage the risks associated with DME throughout its lifecycle. Additionally, the Classification, Labeling, and Packaging (CLP) Regulation mandates clear hazard communication for DME products.
Storage regulations for DME are particularly critical. Most jurisdictions require specialized storage tanks designed to withstand the pressure of DME in its liquid state. These tanks must be equipped with pressure relief valves, level indicators, and temperature monitoring systems. Regular inspections and maintenance of storage facilities are mandatory to prevent leaks and potential accidents.
Transportation of DME is subject to international agreements such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify requirements for container design, labeling, and documentation during transit. They also outline emergency procedures in case of accidents during transportation.
In chemical processing facilities, safety regulations often mandate the installation of gas detection systems to monitor DME levels in the air. Proper ventilation systems are crucial to prevent the accumulation of DME vapors, which can form explosive mixtures with air. Electrical equipment in areas where DME is present must be explosion-proof to minimize ignition risks.
As the innovative uses of DME in chemical industries continue to expand, regulatory bodies are adapting their guidelines to address new applications. This includes developing specific safety protocols for DME as a fuel additive, solvent, or refrigerant. The evolving regulatory landscape aims to balance the promotion of DME's potential benefits with the paramount importance of worker and environmental safety.
In the United States, the Occupational Safety and Health Administration (OSHA) has set specific standards for DME handling in industrial settings. These include requirements for personal protective equipment (PPE), emergency response procedures, and regular safety training for personnel working with DME. The Environmental Protection Agency (EPA) also regulates DME under the Clean Air Act, focusing on emissions control and environmental impact.
European regulations, governed by the European Chemicals Agency (ECHA), emphasize the implementation of REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) for DME. This framework ensures that manufacturers and importers assess and manage the risks associated with DME throughout its lifecycle. Additionally, the Classification, Labeling, and Packaging (CLP) Regulation mandates clear hazard communication for DME products.
Storage regulations for DME are particularly critical. Most jurisdictions require specialized storage tanks designed to withstand the pressure of DME in its liquid state. These tanks must be equipped with pressure relief valves, level indicators, and temperature monitoring systems. Regular inspections and maintenance of storage facilities are mandatory to prevent leaks and potential accidents.
Transportation of DME is subject to international agreements such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify requirements for container design, labeling, and documentation during transit. They also outline emergency procedures in case of accidents during transportation.
In chemical processing facilities, safety regulations often mandate the installation of gas detection systems to monitor DME levels in the air. Proper ventilation systems are crucial to prevent the accumulation of DME vapors, which can form explosive mixtures with air. Electrical equipment in areas where DME is present must be explosion-proof to minimize ignition risks.
As the innovative uses of DME in chemical industries continue to expand, regulatory bodies are adapting their guidelines to address new applications. This includes developing specific safety protocols for DME as a fuel additive, solvent, or refrigerant. The evolving regulatory landscape aims to balance the promotion of DME's potential benefits with the paramount importance of worker and environmental safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!