Invasive Brain-Computer Interfaces safety considerations in long-term deployment
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Invasive BCI Evolution and Safety Objectives
Invasive Brain-Computer Interfaces (BCIs) have evolved significantly over the past decades, transitioning from experimental laboratory setups to clinically viable solutions for various neurological conditions. The journey began in the 1970s with early animal experiments demonstrating the feasibility of neural signal recording and interpretation, followed by the first human implants in the late 1990s primarily focused on motor function restoration for paralyzed patients.
The technological evolution of invasive BCIs has been marked by several key milestones. Early systems utilized single microelectrodes, progressing to multi-electrode arrays capable of recording from dozens to hundreds of neurons simultaneously. Recent advancements include flexible electrode materials, wireless transmission capabilities, and miniaturized implantable components that significantly reduce tissue damage and infection risks.
Safety considerations have become increasingly paramount as these devices transition from short-term clinical trials to long-term therapeutic solutions. The primary safety objectives in long-term invasive BCI deployment encompass multiple dimensions: biocompatibility of materials to minimize foreign body responses; mechanical stability to prevent electrode migration; infection prevention through improved surgical techniques and antimicrobial coatings; and long-term signal stability to maintain device efficacy.
The field has witnessed a paradigm shift in safety priorities. Initially, research focused predominantly on achieving functional outcomes, with safety as a secondary consideration. Contemporary approaches now integrate safety parameters into the fundamental design process, recognizing that long-term viability depends critically on minimizing adverse physiological responses and maintaining neural interface integrity.
Regulatory frameworks have evolved alongside technological developments. The FDA has established specific guidance for BCI devices, emphasizing rigorous pre-clinical testing, comprehensive risk assessment protocols, and extended monitoring requirements for chronic implants. International standards organizations have similarly developed specialized protocols for evaluating the long-term safety profile of neural interfaces.
Current research trends indicate a growing emphasis on adaptive systems that can respond to changing neural environments, self-diagnosing capabilities to detect potential safety issues before they become critical, and biologically inspired designs that better mimic natural tissue properties to reduce foreign body responses.
The ultimate objective for invasive BCI technology is to achieve seamless integration with neural tissue, maintaining stable recording and stimulation capabilities over decades without significant degradation or adverse effects. This goal necessitates interdisciplinary collaboration between neuroscientists, materials engineers, computer scientists, and clinicians to address the complex challenges of long-term neural interfacing while prioritizing patient safety above all other considerations.
The technological evolution of invasive BCIs has been marked by several key milestones. Early systems utilized single microelectrodes, progressing to multi-electrode arrays capable of recording from dozens to hundreds of neurons simultaneously. Recent advancements include flexible electrode materials, wireless transmission capabilities, and miniaturized implantable components that significantly reduce tissue damage and infection risks.
Safety considerations have become increasingly paramount as these devices transition from short-term clinical trials to long-term therapeutic solutions. The primary safety objectives in long-term invasive BCI deployment encompass multiple dimensions: biocompatibility of materials to minimize foreign body responses; mechanical stability to prevent electrode migration; infection prevention through improved surgical techniques and antimicrobial coatings; and long-term signal stability to maintain device efficacy.
The field has witnessed a paradigm shift in safety priorities. Initially, research focused predominantly on achieving functional outcomes, with safety as a secondary consideration. Contemporary approaches now integrate safety parameters into the fundamental design process, recognizing that long-term viability depends critically on minimizing adverse physiological responses and maintaining neural interface integrity.
Regulatory frameworks have evolved alongside technological developments. The FDA has established specific guidance for BCI devices, emphasizing rigorous pre-clinical testing, comprehensive risk assessment protocols, and extended monitoring requirements for chronic implants. International standards organizations have similarly developed specialized protocols for evaluating the long-term safety profile of neural interfaces.
Current research trends indicate a growing emphasis on adaptive systems that can respond to changing neural environments, self-diagnosing capabilities to detect potential safety issues before they become critical, and biologically inspired designs that better mimic natural tissue properties to reduce foreign body responses.
The ultimate objective for invasive BCI technology is to achieve seamless integration with neural tissue, maintaining stable recording and stimulation capabilities over decades without significant degradation or adverse effects. This goal necessitates interdisciplinary collaboration between neuroscientists, materials engineers, computer scientists, and clinicians to address the complex challenges of long-term neural interfacing while prioritizing patient safety above all other considerations.
Market Analysis for Long-term Neural Implants
The global market for long-term neural implants is experiencing significant growth, driven by advancements in invasive Brain-Computer Interface (BCI) technologies and increasing applications in treating neurological disorders. Current market valuations indicate that the neural implant sector reached approximately $2.4 billion in 2022, with projections suggesting a compound annual growth rate of 12.7% through 2030.
The primary market segments for long-term neural implants include medical applications (particularly for conditions such as epilepsy, Parkinson's disease, and paralysis), research institutions, and emerging consumer applications. The medical segment currently dominates, accounting for over 70% of market share, with therapeutic applications representing the largest subsegment.
Geographically, North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The United States specifically maintains market dominance due to substantial research funding, presence of key industry players, and favorable regulatory pathways for neurotechnology. However, China is demonstrating the fastest growth rate, investing heavily in neural technology research and development.
Key market drivers include the rising prevalence of neurological disorders, increasing geriatric population, technological advancements in miniaturization and biocompatibility, and growing acceptance of implantable medical devices. The aging global population particularly represents a significant market opportunity, as neurological conditions become more prevalent with age.
Market restraints primarily revolve around safety considerations for long-term deployment. These include concerns about tissue damage, infection risks, device degradation over time, and potential immune responses to foreign materials. Additionally, high costs associated with implantation procedures and devices (ranging from $35,000 to $150,000 depending on complexity) limit market penetration, particularly in developing economies.
Consumer acceptance represents another significant market challenge. Public surveys indicate that approximately 65% of potential patients express concerns about invasive brain technologies, with safety and privacy being the primary considerations. This hesitation creates a market barrier that companies must address through transparent safety protocols and long-term efficacy data.
Reimbursement policies also significantly impact market growth. Currently, insurance coverage for neural implants varies widely by country and condition, with established therapeutic applications receiving better coverage than experimental or enhancement-focused applications. This inconsistency creates market fragmentation and affects adoption rates across different regions.
The primary market segments for long-term neural implants include medical applications (particularly for conditions such as epilepsy, Parkinson's disease, and paralysis), research institutions, and emerging consumer applications. The medical segment currently dominates, accounting for over 70% of market share, with therapeutic applications representing the largest subsegment.
Geographically, North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The United States specifically maintains market dominance due to substantial research funding, presence of key industry players, and favorable regulatory pathways for neurotechnology. However, China is demonstrating the fastest growth rate, investing heavily in neural technology research and development.
Key market drivers include the rising prevalence of neurological disorders, increasing geriatric population, technological advancements in miniaturization and biocompatibility, and growing acceptance of implantable medical devices. The aging global population particularly represents a significant market opportunity, as neurological conditions become more prevalent with age.
Market restraints primarily revolve around safety considerations for long-term deployment. These include concerns about tissue damage, infection risks, device degradation over time, and potential immune responses to foreign materials. Additionally, high costs associated with implantation procedures and devices (ranging from $35,000 to $150,000 depending on complexity) limit market penetration, particularly in developing economies.
Consumer acceptance represents another significant market challenge. Public surveys indicate that approximately 65% of potential patients express concerns about invasive brain technologies, with safety and privacy being the primary considerations. This hesitation creates a market barrier that companies must address through transparent safety protocols and long-term efficacy data.
Reimbursement policies also significantly impact market growth. Currently, insurance coverage for neural implants varies widely by country and condition, with established therapeutic applications receiving better coverage than experimental or enhancement-focused applications. This inconsistency creates market fragmentation and affects adoption rates across different regions.
Current Challenges in Chronic Neural Interface Biocompatibility
The biocompatibility of chronic neural interfaces represents one of the most significant challenges in the long-term deployment of invasive Brain-Computer Interfaces (BCIs). Current neural implants trigger complex biological responses when introduced into brain tissue, leading to a cascade of inflammatory reactions that ultimately compromise device functionality and patient safety.
The foreign body response remains a primary obstacle, characterized by microglial activation and astrocyte encapsulation around implanted electrodes. This cellular encapsulation forms a glial scar that increases electrical impedance and creates a physical barrier between neurons and recording sites. Studies indicate that this response typically begins within hours of implantation and can continue for months, progressively degrading signal quality.
Material compatibility issues further complicate long-term viability. Traditional electrode materials like silicon, platinum, and various metal alloys exhibit mechanical property mismatches with brain tissue. The brain's soft tissue environment (Young's modulus ~3-5 kPa) contrasts sharply with rigid implant materials (Young's modulus in GPa range), resulting in micromotion that exacerbates tissue damage and inflammatory responses during normal brain movements.
Infection risks present another critical concern. Despite sterilization protocols, the transcutaneous components of many current BCI systems create potential pathways for microbial invasion. Clinical data shows infection rates ranging from 4-12% for implanted neural devices, with consequences ranging from localized inflammation to life-threatening conditions requiring device removal.
Signal stability degradation over time remains poorly addressed by current technologies. Recording quality typically deteriorates within months to a few years post-implantation due to biofouling, electrode corrosion, and the aforementioned biological responses. This progressive decline severely limits the clinical utility of chronic neural interfaces for patients requiring lifetime support.
Biocompatibility challenges extend to power and data transmission components. Batteries and transmission hardware generate heat that can damage surrounding neural tissue, with studies showing that even 1-2°C temperature increases can alter neural activity and potentially cause tissue necrosis in chronic settings.
The cumulative effect of these biocompatibility issues manifests as shortened device lifespan, with most current invasive BCIs maintaining optimal functionality for only 1-5 years before requiring replacement or adjustment. This limitation presents significant barriers to widespread clinical adoption, particularly for applications targeting conditions requiring decades of support.
The foreign body response remains a primary obstacle, characterized by microglial activation and astrocyte encapsulation around implanted electrodes. This cellular encapsulation forms a glial scar that increases electrical impedance and creates a physical barrier between neurons and recording sites. Studies indicate that this response typically begins within hours of implantation and can continue for months, progressively degrading signal quality.
Material compatibility issues further complicate long-term viability. Traditional electrode materials like silicon, platinum, and various metal alloys exhibit mechanical property mismatches with brain tissue. The brain's soft tissue environment (Young's modulus ~3-5 kPa) contrasts sharply with rigid implant materials (Young's modulus in GPa range), resulting in micromotion that exacerbates tissue damage and inflammatory responses during normal brain movements.
Infection risks present another critical concern. Despite sterilization protocols, the transcutaneous components of many current BCI systems create potential pathways for microbial invasion. Clinical data shows infection rates ranging from 4-12% for implanted neural devices, with consequences ranging from localized inflammation to life-threatening conditions requiring device removal.
Signal stability degradation over time remains poorly addressed by current technologies. Recording quality typically deteriorates within months to a few years post-implantation due to biofouling, electrode corrosion, and the aforementioned biological responses. This progressive decline severely limits the clinical utility of chronic neural interfaces for patients requiring lifetime support.
Biocompatibility challenges extend to power and data transmission components. Batteries and transmission hardware generate heat that can damage surrounding neural tissue, with studies showing that even 1-2°C temperature increases can alter neural activity and potentially cause tissue necrosis in chronic settings.
The cumulative effect of these biocompatibility issues manifests as shortened device lifespan, with most current invasive BCIs maintaining optimal functionality for only 1-5 years before requiring replacement or adjustment. This limitation presents significant barriers to widespread clinical adoption, particularly for applications targeting conditions requiring decades of support.
Existing Safety Mechanisms for Chronic Neural Implants
01 Biocompatible materials and coatings for neural interfaces
The use of biocompatible materials and specialized coatings in invasive brain-computer interfaces helps minimize tissue damage and immune responses. These materials are designed to reduce inflammation, prevent electrode degradation, and enhance long-term stability of the implanted devices. Advanced coatings can also incorporate anti-inflammatory agents or growth factors to promote healing at the implant site, thereby improving the safety profile of neural interfaces for extended periods.- Biocompatible materials and coatings for neural implants: The use of biocompatible materials and specialized coatings for neural implants is crucial for reducing tissue inflammation and rejection responses. These materials help minimize scarring around the implant site and extend the functional lifespan of invasive brain-computer interfaces. Advanced coatings can also incorporate anti-inflammatory agents or growth factors that promote better integration with neural tissue while reducing the risk of infection and immune responses that could compromise both safety and device performance.
- Minimally invasive implantation techniques: Development of minimally invasive surgical techniques for implanting brain-computer interfaces helps reduce trauma to brain tissue during device placement. These approaches include micro-needle arrays, endovascular delivery systems, and robotic-assisted implantation that can navigate to target brain regions with precision. By minimizing the surgical footprint, these techniques reduce the risk of bleeding, infection, and long-term neural damage while still allowing effective placement of electrodes in specific brain regions for signal acquisition.
- Wireless power and data transmission systems: Wireless power and data transmission systems eliminate the need for transcranial wires that can serve as infection pathways and cause mechanical stress on brain tissue. These systems use technologies such as radiofrequency, ultrasound, or infrared light to power implanted devices and transmit neural data externally. This approach significantly reduces infection risks associated with percutaneous connections while also allowing for greater patient mobility and comfort during long-term use of brain-computer interfaces.
- Real-time monitoring and safety shutdown mechanisms: Advanced brain-computer interfaces incorporate real-time monitoring systems that continuously assess device performance and neural tissue health. These systems can detect abnormal neural activity patterns, temperature changes, or electrical anomalies that might indicate potential harm. Integrated safety shutdown mechanisms can automatically deactivate or modify device operation if dangerous conditions are detected, preventing tissue damage from electrical overstimulation or device malfunction and providing an essential safety layer for invasive neural interfaces.
- Biodegradable and removable interface components: The development of partially biodegradable or easily removable components for brain-computer interfaces addresses concerns about long-term implantation risks. These designs allow for the non-functional or potentially problematic parts of the device to be naturally absorbed by the body or removed through minimally invasive procedures after they have served their purpose. This approach reduces the long-term foreign body response and allows for device upgrades without requiring complete surgical extraction, significantly improving the safety profile for patients requiring extended use of neural interfaces.
02 Minimally invasive implantation techniques
Development of minimally invasive surgical techniques for implanting brain-computer interfaces reduces trauma to brain tissue and associated risks. These techniques include precision micro-surgical approaches, robotic-assisted implantation, and endovascular delivery methods that can access target brain regions with minimal disruption to surrounding tissues. By reducing the invasiveness of the implantation procedure, these methods help decrease complications such as bleeding, infection, and neurological deficits.Expand Specific Solutions03 Real-time monitoring and safety control systems
Integration of real-time monitoring systems in brain-computer interfaces allows for continuous assessment of neural activity, device performance, and potential adverse events. These systems can detect abnormal brain signals, tissue responses, or device malfunctions, triggering automatic safety protocols when necessary. Advanced algorithms can identify early warning signs of seizures, inflammation, or other complications, enabling timely intervention before serious adverse effects occur.Expand Specific Solutions04 Wireless power and data transmission technologies
Wireless technologies for power delivery and data transmission reduce the need for transcranial wires or connectors that can increase infection risk and mechanical stress on brain tissue. These systems use electromagnetic induction, ultrasound, or optical methods to power implanted devices and communicate with external components without physical connections through the skull. This approach minimizes infection pathways and allows for more flexible, safer designs that can adapt to brain movement and reduce chronic tissue damage.Expand Specific Solutions05 Biodegradable and removable neural interface components
Development of partially biodegradable or fully removable brain-computer interface components addresses long-term safety concerns associated with permanent implants. These designs incorporate materials that safely degrade over time or can be retrieved without significant tissue damage when no longer needed. Some approaches include dissolvable electrode arrays, magnetically retrievable microdevices, or hybrid systems with removable active components and permanent passive structures, providing safer options for both temporary and long-term neural monitoring applications.Expand Specific Solutions
Leading Organizations in Invasive BCI Development
The invasive Brain-Computer Interfaces (BCIs) safety landscape is currently in an early commercialization phase, with a growing market projected to reach $3.7 billion by 2027. Technical maturity varies significantly among key players. Neuralink leads with its minimally invasive implantable system, while Precision Neuroscience focuses on removable interfaces. NeuroPace has established clinical credibility with FDA-approved neurostimulators for epilepsy. Academic institutions (Johns Hopkins, MIT, Caltech) drive fundamental research, while companies like Cognixion bridge consumer applications. Long-term safety considerations remain the primary technical challenge, with research organizations like Battelle Memorial Institute and DURECT developing biocompatible materials and improved surgical techniques to address tissue inflammation, device degradation, and infection risks.
NeuroPace, Inc.
Technical Solution: NeuroPace has pioneered long-term invasive BCI safety with its RNS® System, an FDA-approved responsive neurostimulation device for treating epilepsy. The system has been implanted in patients for over 10 years, providing valuable long-term safety data. NeuroPace's approach focuses on a closed-loop system that monitors brain activity and delivers electrical stimulation only when abnormal activity is detected. For long-term safety, the RNS System incorporates: 1) A titanium hermetically sealed case with feedthroughs tested to maintain integrity for 8+ years; 2) Silicone-based lead technology with demonstrated stability over multiple years; 3) Low-power stimulation parameters designed to minimize tissue damage; 4) Regular remote monitoring capabilities that allow physicians to detect potential issues before they become serious; 5) Battery replacement procedures that don't require removing the electrodes, reducing surgical risks during device maintenance; and 6) Comprehensive adverse event tracking systems that have documented safety outcomes across hundreds of patient-years.
Strengths: Extensive long-term human implantation data (10+ years) demonstrating safety profile; FDA-approved technology with established clinical protocols; remote monitoring capabilities allow for early intervention; battery replacement without electrode disturbance reduces surgical risks. Weaknesses: Limited to specific brain regions and primarily focused on stimulation rather than high-bandwidth recording; current designs still require periodic battery replacement surgeries; system is optimized for therapeutic use rather than general-purpose BCI applications.
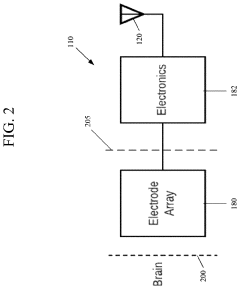
Neuralink Corp.
Technical Solution: Neuralink has developed an invasive BCI system called the N1 Link, which uses flexible polymer threads (about 1/10th the width of a human hair) containing electrodes that are surgically implanted into the brain by a precision robot. For long-term safety, Neuralink has implemented several key features: 1) A hermetically sealed device case to protect electronics from bodily fluids; 2) Biocompatible materials to minimize tissue reaction and inflammation; 3) Wireless power and data transmission to eliminate infection risks from percutaneous connectors; 4) Automated monitoring systems that continuously check device integrity and neural activity patterns; 5) Thermal management systems to prevent tissue damage from device heat; and 6) Removable implant design to allow for upgrades or complete extraction if necessary. The company has also developed specialized surgical robots for precise, minimally invasive implantation to reduce trauma and infection risk during the initial procedure.
Strengths: Advanced miniaturization and flexible electrode technology minimizes tissue damage; wireless design eliminates infection pathways; automated monitoring systems provide early warning of potential issues; removable design allows for future upgrades. Weaknesses: Limited long-term human data on biocompatibility; potential for mechanical failure of microelectrodes over time; challenges in maintaining stable neural recordings as glial scarring occurs around electrodes.
Critical Patents in Neural Tissue-Implant Interface Stability
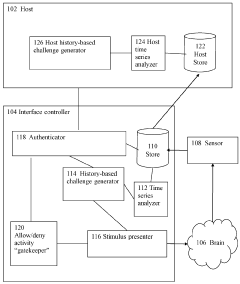
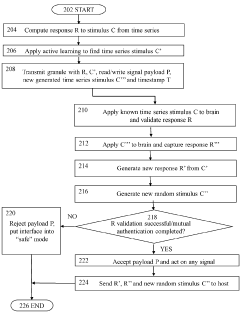
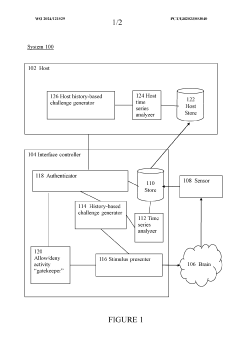
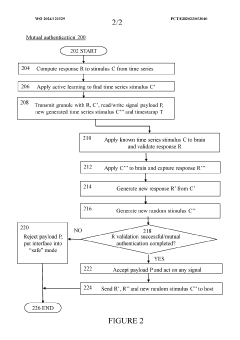
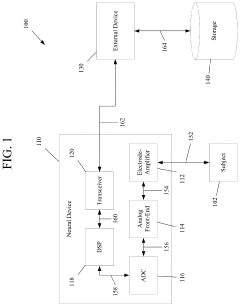
Brain-computer interface device, system and operating method
PatentWO2024121529A1
Innovation
- A time-series authentication system using a long-short-term memory (LSTM) neural network and autoencoders to generate and verify stimulus-response pairs, providing a firewall-like protection between the brain and external entities, ensuring that only valid signals are processed and preventing replay attacks by using temporal authentication and biometric proof of life.
Secure interfaces for neural devices
PatentPendingUS20240137348A1
Innovation
- Implementing a neural device system with end-to-end encryption protocols across communications interfaces, using advanced encryption standards such as AES, RSA, and ciphers, to secure data transfer between neural devices and external devices, including wired and wireless connections, ensuring the integrity of brain data handling and processing.
Regulatory Framework for Implantable Neural Devices
The regulatory landscape for implantable neural devices represents a complex and evolving framework that balances innovation with patient safety. Currently, the FDA's Center for Devices and Radiological Health (CDRH) serves as the primary regulatory body in the United States, classifying most invasive BCIs as Class III medical devices requiring premarket approval (PMA). This classification demands rigorous clinical trials demonstrating both safety and efficacy before market authorization, with particular emphasis on long-term biocompatibility and functional stability.
In the European Union, the Medical Device Regulation (MDR) implemented in 2021 has significantly strengthened requirements for neural implants, introducing more stringent clinical evidence standards and post-market surveillance obligations. The MDR specifically addresses active implantable medical devices through dedicated annexes that outline special requirements for devices interfacing directly with the central nervous system.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake Designation System to expedite approval for breakthrough medical technologies, including neural interfaces, while maintaining strict safety standards through their unique "stepwise approval" approach that enables conditional market access with mandatory continued monitoring.
International harmonization efforts are being led by the International Medical Device Regulators Forum (IMDRF), which has published specific guidance on software as a medical device (SaMD) that applies to the algorithmic components of BCIs. However, significant regulatory gaps remain regarding the unique challenges of long-term neural implants, particularly concerning neural tissue degradation, material biocompatibility over decades, and adaptive algorithm safety.
Emerging regulatory considerations specifically addressing long-term BCI deployment include requirements for "graceful degradation" protocols, ensuring devices maintain basic safety functions even as components deteriorate. Regulators are also developing frameworks for managing "adaptive learning" neural interfaces that modify their functionality based on brain activity patterns, raising novel questions about when software updates constitute new medical devices requiring re-approval.
The FDA's Digital Health Innovation Action Plan has begun addressing some of these challenges through its Pre-Cert program, which focuses on developer quality rather than product-by-product review, potentially offering a more flexible approach for iterative BCI technologies. Meanwhile, international standards organizations like ISO and IEC are developing specialized standards for neural interface technologies that will likely form the basis for future regulatory frameworks specifically tailored to long-term implantable BCIs.
In the European Union, the Medical Device Regulation (MDR) implemented in 2021 has significantly strengthened requirements for neural implants, introducing more stringent clinical evidence standards and post-market surveillance obligations. The MDR specifically addresses active implantable medical devices through dedicated annexes that outline special requirements for devices interfacing directly with the central nervous system.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake Designation System to expedite approval for breakthrough medical technologies, including neural interfaces, while maintaining strict safety standards through their unique "stepwise approval" approach that enables conditional market access with mandatory continued monitoring.
International harmonization efforts are being led by the International Medical Device Regulators Forum (IMDRF), which has published specific guidance on software as a medical device (SaMD) that applies to the algorithmic components of BCIs. However, significant regulatory gaps remain regarding the unique challenges of long-term neural implants, particularly concerning neural tissue degradation, material biocompatibility over decades, and adaptive algorithm safety.
Emerging regulatory considerations specifically addressing long-term BCI deployment include requirements for "graceful degradation" protocols, ensuring devices maintain basic safety functions even as components deteriorate. Regulators are also developing frameworks for managing "adaptive learning" neural interfaces that modify their functionality based on brain activity patterns, raising novel questions about when software updates constitute new medical devices requiring re-approval.
The FDA's Digital Health Innovation Action Plan has begun addressing some of these challenges through its Pre-Cert program, which focuses on developer quality rather than product-by-product review, potentially offering a more flexible approach for iterative BCI technologies. Meanwhile, international standards organizations like ISO and IEC are developing specialized standards for neural interface technologies that will likely form the basis for future regulatory frameworks specifically tailored to long-term implantable BCIs.
Ethical Implications of Long-term Neural Monitoring
The long-term neural monitoring inherent in invasive Brain-Computer Interfaces (BCIs) raises profound ethical questions that extend beyond technical safety considerations. As these devices become more sophisticated and deployment durations increase from months to years or even decades, society must confront unprecedented ethical challenges regarding human autonomy, identity, and dignity.
Privacy concerns represent one of the most pressing ethical dimensions of long-term neural monitoring. Unlike conventional data, neural information provides direct insight into an individual's thoughts, emotions, and intentions—potentially the most intimate form of personal data. The continuous collection of such data creates significant risks regarding who can access this information and how it might be used beyond therapeutic purposes.
Informed consent becomes increasingly complex in the context of extended neural monitoring. Traditional models of consent may prove inadequate when dealing with technologies that could potentially alter a person's sense of self or agency over time. Questions arise about whether consent obtained initially remains valid years later, especially if the technology evolves or if the patient's cognitive capacity changes due to disease progression or the influence of the device itself.
The potential for neural data commodification presents another ethical frontier. As commercial interests in BCI technology grow, neural data represents an unprecedented form of valuable information. Without robust ethical frameworks, patients might face exploitation through the monetization of their neural patterns or be subjected to targeted neuromarketing based on their brain activity profiles.
Identity and agency considerations become particularly significant during long-term neural integration. As individuals adapt to and potentially become dependent on invasive BCIs, questions emerge about where the boundary lies between the technology and the person. This neural-technological integration may fundamentally alter how we conceptualize personhood and autonomy, especially when devices influence decision-making processes or emotional responses.
Distributive justice issues also demand attention, as expensive invasive BCI technologies may create or exacerbate existing healthcare disparities. Without careful consideration of equitable access, these potentially life-changing technologies could become available only to privileged populations, further widening social divides.
The potential for coercive applications represents perhaps the most concerning ethical dimension. As neural monitoring technologies advance, the possibility emerges for their application in contexts beyond voluntary medical treatment—potentially extending to surveillance, behavior modification, or even judicial applications that could fundamentally undermine human rights and dignity.
Privacy concerns represent one of the most pressing ethical dimensions of long-term neural monitoring. Unlike conventional data, neural information provides direct insight into an individual's thoughts, emotions, and intentions—potentially the most intimate form of personal data. The continuous collection of such data creates significant risks regarding who can access this information and how it might be used beyond therapeutic purposes.
Informed consent becomes increasingly complex in the context of extended neural monitoring. Traditional models of consent may prove inadequate when dealing with technologies that could potentially alter a person's sense of self or agency over time. Questions arise about whether consent obtained initially remains valid years later, especially if the technology evolves or if the patient's cognitive capacity changes due to disease progression or the influence of the device itself.
The potential for neural data commodification presents another ethical frontier. As commercial interests in BCI technology grow, neural data represents an unprecedented form of valuable information. Without robust ethical frameworks, patients might face exploitation through the monetization of their neural patterns or be subjected to targeted neuromarketing based on their brain activity profiles.
Identity and agency considerations become particularly significant during long-term neural integration. As individuals adapt to and potentially become dependent on invasive BCIs, questions emerge about where the boundary lies between the technology and the person. This neural-technological integration may fundamentally alter how we conceptualize personhood and autonomy, especially when devices influence decision-making processes or emotional responses.
Distributive justice issues also demand attention, as expensive invasive BCI technologies may create or exacerbate existing healthcare disparities. Without careful consideration of equitable access, these potentially life-changing technologies could become available only to privileged populations, further widening social divides.
The potential for coercive applications represents perhaps the most concerning ethical dimension. As neural monitoring technologies advance, the possibility emerges for their application in contexts beyond voluntary medical treatment—potentially extending to surveillance, behavior modification, or even judicial applications that could fundamentally undermine human rights and dignity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!