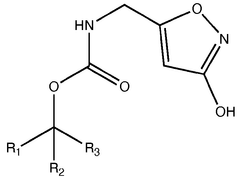
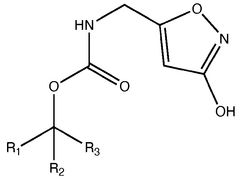
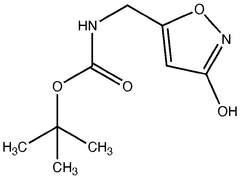
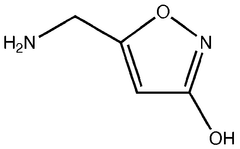
Legal Considerations in the Use of Muscimol Supplements
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Supplement Background and Objectives
Muscimol, a psychoactive compound found naturally in certain mushroom species, has gained increasing attention in recent years as a potential supplement for various health and cognitive benefits. This report aims to provide a comprehensive overview of the background and objectives related to muscimol supplements, focusing on the legal considerations surrounding their use.
Historically, muscimol has been used in traditional practices by indigenous cultures, particularly in Siberia and North America, for its psychoactive properties. In modern times, scientific interest in muscimol has grown due to its unique pharmacological profile and potential therapeutic applications. As a potent GABA-A receptor agonist, muscimol exhibits sedative, hypnotic, and anxiolytic effects, which have sparked research into its potential use for treating anxiety disorders, sleep disturbances, and neurological conditions.
The current landscape of muscimol supplements is characterized by a complex interplay of scientific research, regulatory frameworks, and market demand. While the compound shows promise in various therapeutic areas, its legal status remains a subject of debate and varies significantly across jurisdictions. This variability in legal status presents both challenges and opportunities for researchers, manufacturers, and consumers interested in muscimol-based products.
One of the primary objectives of this technical research is to elucidate the current legal considerations surrounding muscimol supplements. This includes examining the regulatory status of muscimol in different countries, understanding the legal distinctions between natural sources and synthetic analogues, and exploring the implications of these regulations on research, production, and distribution of muscimol-containing products.
Another key objective is to assess the potential for muscimol supplements to gain wider acceptance within the legal and regulatory frameworks governing dietary supplements and pharmaceutical products. This involves analyzing existing precedents for similar compounds, identifying potential pathways for legal approval, and evaluating the scientific evidence supporting the safety and efficacy of muscimol supplements.
Furthermore, this research aims to explore the technological aspects of muscimol supplement production, including extraction methods, synthesis techniques, and formulation strategies that comply with existing legal and quality standards. Understanding these technical challenges is crucial for developing legally compliant and commercially viable muscimol-based products.
By examining the intersection of legal considerations, scientific research, and market dynamics, this report seeks to provide a foundation for informed decision-making regarding the development and use of muscimol supplements. The insights gained from this analysis will be valuable for stakeholders across the pharmaceutical, nutraceutical, and regulatory sectors as they navigate the complex landscape of novel psychoactive compounds in the supplement market.
Historically, muscimol has been used in traditional practices by indigenous cultures, particularly in Siberia and North America, for its psychoactive properties. In modern times, scientific interest in muscimol has grown due to its unique pharmacological profile and potential therapeutic applications. As a potent GABA-A receptor agonist, muscimol exhibits sedative, hypnotic, and anxiolytic effects, which have sparked research into its potential use for treating anxiety disorders, sleep disturbances, and neurological conditions.
The current landscape of muscimol supplements is characterized by a complex interplay of scientific research, regulatory frameworks, and market demand. While the compound shows promise in various therapeutic areas, its legal status remains a subject of debate and varies significantly across jurisdictions. This variability in legal status presents both challenges and opportunities for researchers, manufacturers, and consumers interested in muscimol-based products.
One of the primary objectives of this technical research is to elucidate the current legal considerations surrounding muscimol supplements. This includes examining the regulatory status of muscimol in different countries, understanding the legal distinctions between natural sources and synthetic analogues, and exploring the implications of these regulations on research, production, and distribution of muscimol-containing products.
Another key objective is to assess the potential for muscimol supplements to gain wider acceptance within the legal and regulatory frameworks governing dietary supplements and pharmaceutical products. This involves analyzing existing precedents for similar compounds, identifying potential pathways for legal approval, and evaluating the scientific evidence supporting the safety and efficacy of muscimol supplements.
Furthermore, this research aims to explore the technological aspects of muscimol supplement production, including extraction methods, synthesis techniques, and formulation strategies that comply with existing legal and quality standards. Understanding these technical challenges is crucial for developing legally compliant and commercially viable muscimol-based products.
By examining the intersection of legal considerations, scientific research, and market dynamics, this report seeks to provide a foundation for informed decision-making regarding the development and use of muscimol supplements. The insights gained from this analysis will be valuable for stakeholders across the pharmaceutical, nutraceutical, and regulatory sectors as they navigate the complex landscape of novel psychoactive compounds in the supplement market.
Market Analysis for Muscimol Products
The market for muscimol products is experiencing significant growth, driven by increasing consumer interest in alternative wellness solutions and the expanding research into the potential therapeutic applications of this compound. Muscimol, a psychoactive compound found in certain species of mushrooms, particularly Amanita muscaria, has garnered attention for its potential effects on cognitive function, mood regulation, and sleep quality.
The global market for muscimol-based supplements is currently in its nascent stages but shows promising growth potential. Market analysts project a compound annual growth rate (CAGR) of over 15% for the next five years, with the market value expected to reach several hundred million dollars by 2028. This growth is primarily fueled by the rising demand for natural nootropics and the increasing acceptance of alternative medicine practices in developed countries.
North America currently dominates the muscimol market, accounting for approximately 40% of the global share. This is largely due to the region's progressive stance on alternative therapies and the presence of key market players. Europe follows closely, with a market share of around 30%, driven by the growing wellness industry and favorable regulatory environment in countries like Germany and the Netherlands.
The Asia-Pacific region is emerging as a lucrative market for muscimol products, with countries like Japan and South Korea showing particular interest. The region is expected to witness the fastest growth rate in the coming years, attributed to the increasing disposable income, growing health consciousness, and the traditional use of mushrooms in Eastern medicine.
Consumer demographics for muscimol products are diverse but primarily consist of health-conscious individuals aged 25-45, who are open to exploring alternative wellness solutions. This demographic is typically well-educated, with higher disposable incomes, and is more likely to seek out natural supplements for cognitive enhancement and stress relief.
The market is currently segmented into various product types, including capsules, tinctures, and powders. Capsules are the most popular form, accounting for over 50% of the market share due to their convenience and precise dosing. Tinctures are gaining popularity, especially among consumers looking for faster absorption and customizable dosing options.
Key market players in the muscimol industry include both established nutraceutical companies and emerging startups specializing in mushroom-based supplements. These companies are investing heavily in research and development to create innovative formulations and improve extraction techniques. Partnerships with academic institutions and clinical research organizations are becoming increasingly common as companies seek to validate the efficacy and safety of their products.
Despite the promising market outlook, the muscimol industry faces several challenges. Regulatory hurdles remain a significant barrier to market entry in many countries, with varying legal statuses and unclear guidelines for muscimol-containing products. Additionally, consumer education and awareness about muscimol's benefits and potential risks are crucial for market expansion.
The global market for muscimol-based supplements is currently in its nascent stages but shows promising growth potential. Market analysts project a compound annual growth rate (CAGR) of over 15% for the next five years, with the market value expected to reach several hundred million dollars by 2028. This growth is primarily fueled by the rising demand for natural nootropics and the increasing acceptance of alternative medicine practices in developed countries.
North America currently dominates the muscimol market, accounting for approximately 40% of the global share. This is largely due to the region's progressive stance on alternative therapies and the presence of key market players. Europe follows closely, with a market share of around 30%, driven by the growing wellness industry and favorable regulatory environment in countries like Germany and the Netherlands.
The Asia-Pacific region is emerging as a lucrative market for muscimol products, with countries like Japan and South Korea showing particular interest. The region is expected to witness the fastest growth rate in the coming years, attributed to the increasing disposable income, growing health consciousness, and the traditional use of mushrooms in Eastern medicine.
Consumer demographics for muscimol products are diverse but primarily consist of health-conscious individuals aged 25-45, who are open to exploring alternative wellness solutions. This demographic is typically well-educated, with higher disposable incomes, and is more likely to seek out natural supplements for cognitive enhancement and stress relief.
The market is currently segmented into various product types, including capsules, tinctures, and powders. Capsules are the most popular form, accounting for over 50% of the market share due to their convenience and precise dosing. Tinctures are gaining popularity, especially among consumers looking for faster absorption and customizable dosing options.
Key market players in the muscimol industry include both established nutraceutical companies and emerging startups specializing in mushroom-based supplements. These companies are investing heavily in research and development to create innovative formulations and improve extraction techniques. Partnerships with academic institutions and clinical research organizations are becoming increasingly common as companies seek to validate the efficacy and safety of their products.
Despite the promising market outlook, the muscimol industry faces several challenges. Regulatory hurdles remain a significant barrier to market entry in many countries, with varying legal statuses and unclear guidelines for muscimol-containing products. Additionally, consumer education and awareness about muscimol's benefits and potential risks are crucial for market expansion.
Regulatory Landscape and Challenges
The regulatory landscape surrounding muscimol supplements is complex and evolving, presenting significant challenges for manufacturers, distributors, and consumers. In the United States, the Food and Drug Administration (FDA) has not explicitly approved muscimol as a dietary supplement ingredient, placing it in a regulatory gray area. This ambiguity stems from the fact that muscimol is a naturally occurring compound found in certain mushroom species, but it also possesses psychoactive properties.
The Dietary Supplement Health and Education Act (DSHEA) of 1994 provides the primary regulatory framework for dietary supplements in the U.S. Under DSHEA, manufacturers are responsible for ensuring the safety of their products before marketing them. However, the FDA has the authority to take action against unsafe or misbranded supplements after they reach the market. This post-market regulatory approach creates challenges for both regulators and industry stakeholders in managing the risks associated with muscimol supplements.
Internationally, the regulatory status of muscimol varies widely. Some countries have classified it as a controlled substance due to its psychoactive effects, while others have not specifically addressed its legal status. This lack of global consensus complicates international trade and creates potential legal risks for companies operating across borders.
One of the primary regulatory challenges is the lack of standardized testing methods for muscimol content in supplements. This absence of standardization makes it difficult for manufacturers to ensure consistent product quality and for regulators to enforce safety standards. Additionally, the potential for misuse or abuse of muscimol supplements raises concerns about public health and safety, prompting calls for stricter regulation.
The intersection of muscimol supplements with existing drug laws presents another layer of complexity. In some jurisdictions, the line between dietary supplements and drugs becomes blurred when dealing with compounds that have potential therapeutic effects. This ambiguity can lead to legal challenges and regulatory enforcement actions against manufacturers and distributors.
Labeling and marketing claims for muscimol supplements are subject to scrutiny by regulatory bodies. The FDA prohibits dietary supplement manufacturers from making disease claims, limiting them to structure/function claims. However, the psychoactive nature of muscimol makes it challenging to navigate these restrictions without running afoul of regulations.
As research into the potential therapeutic applications of muscimol continues, there is growing pressure on regulatory agencies to provide clearer guidance. Some stakeholders advocate for a more nuanced approach that acknowledges the potential benefits of muscimol while implementing appropriate safeguards. Others argue for stricter controls, citing concerns about safety and potential for abuse.
The Dietary Supplement Health and Education Act (DSHEA) of 1994 provides the primary regulatory framework for dietary supplements in the U.S. Under DSHEA, manufacturers are responsible for ensuring the safety of their products before marketing them. However, the FDA has the authority to take action against unsafe or misbranded supplements after they reach the market. This post-market regulatory approach creates challenges for both regulators and industry stakeholders in managing the risks associated with muscimol supplements.
Internationally, the regulatory status of muscimol varies widely. Some countries have classified it as a controlled substance due to its psychoactive effects, while others have not specifically addressed its legal status. This lack of global consensus complicates international trade and creates potential legal risks for companies operating across borders.
One of the primary regulatory challenges is the lack of standardized testing methods for muscimol content in supplements. This absence of standardization makes it difficult for manufacturers to ensure consistent product quality and for regulators to enforce safety standards. Additionally, the potential for misuse or abuse of muscimol supplements raises concerns about public health and safety, prompting calls for stricter regulation.
The intersection of muscimol supplements with existing drug laws presents another layer of complexity. In some jurisdictions, the line between dietary supplements and drugs becomes blurred when dealing with compounds that have potential therapeutic effects. This ambiguity can lead to legal challenges and regulatory enforcement actions against manufacturers and distributors.
Labeling and marketing claims for muscimol supplements are subject to scrutiny by regulatory bodies. The FDA prohibits dietary supplement manufacturers from making disease claims, limiting them to structure/function claims. However, the psychoactive nature of muscimol makes it challenging to navigate these restrictions without running afoul of regulations.
As research into the potential therapeutic applications of muscimol continues, there is growing pressure on regulatory agencies to provide clearer guidance. Some stakeholders advocate for a more nuanced approach that acknowledges the potential benefits of muscimol while implementing appropriate safeguards. Others argue for stricter controls, citing concerns about safety and potential for abuse.
Current Legal Framework for Muscimol Use
01 Muscimol-based pharmaceutical compositions
Pharmaceutical compositions containing muscimol or its derivatives are developed for various therapeutic applications. These formulations may include specific dosage forms, delivery methods, or combinations with other active ingredients to enhance efficacy or reduce side effects.- Muscimol-based pharmaceutical compositions: Pharmaceutical compositions containing muscimol or its derivatives are being developed for various therapeutic applications. These formulations may include controlled-release mechanisms, specific dosage forms, or combinations with other active ingredients to enhance efficacy or reduce side effects.
- Muscimol extraction and purification methods: Various techniques are being developed to extract and purify muscimol from natural sources or to synthesize it artificially. These methods aim to improve the yield, purity, and cost-effectiveness of muscimol production for use in supplements or pharmaceutical applications.
- Muscimol-containing dietary supplements: Dietary supplements containing muscimol or muscimol-rich extracts are being formulated for potential cognitive enhancement, stress relief, or sleep improvement. These supplements may combine muscimol with other natural ingredients to create synergistic effects.
- Muscimol delivery systems: Novel delivery systems are being developed to improve the bioavailability and targeted delivery of muscimol. These may include nanoparticle formulations, transdermal patches, or other innovative approaches to enhance the effectiveness of muscimol-based supplements or medications.
- Safety and efficacy studies of muscimol supplements: Research is being conducted to evaluate the safety profile and therapeutic efficacy of muscimol supplements. These studies aim to determine optimal dosages, potential side effects, and long-term impacts of muscimol consumption in various populations and for different health conditions.
02 Muscimol extraction and purification methods
Various techniques are employed to extract and purify muscimol from natural sources or to synthesize it artificially. These methods aim to improve the yield, purity, and cost-effectiveness of muscimol production for use in supplements or pharmaceutical applications.Expand Specific Solutions03 Muscimol-containing dietary supplements
Dietary supplements incorporating muscimol or its precursors are formulated for potential cognitive enhancement, stress relief, or other health benefits. These supplements may combine muscimol with other natural ingredients or nutrients to create unique product offerings.Expand Specific Solutions04 Muscimol delivery systems
Novel delivery systems are developed to improve the bioavailability and targeted delivery of muscimol in the body. These may include specialized encapsulation techniques, transdermal patches, or other innovative methods to enhance the effectiveness of muscimol supplements.Expand Specific Solutions05 Safety and quality control of muscimol supplements
Methods and systems are implemented to ensure the safety, purity, and consistent potency of muscimol-containing supplements. This includes analytical techniques for detecting contaminants, standardizing dosages, and monitoring the stability of muscimol in various formulations.Expand Specific Solutions
Key Players in Muscimol Industry
The legal landscape surrounding muscimol supplements is evolving, reflecting the industry's early developmental stage. The market size remains relatively small but is growing as research progresses. Technologically, muscimol supplements are still in the early stages of development, with varying levels of maturity across companies. Established pharmaceutical giants like Novartis AG and Allergan, Inc. possess advanced research capabilities, while newer entrants such as Psyched Wellness Ltd. and Karallief, Inc. are exploring innovative applications. Academic institutions, including the University of California and University of South Florida, contribute significantly to the fundamental research. The regulatory environment is complex, with companies like ACADIA Pharmaceuticals and Revive Therapeutics navigating the intersection of pharmaceutical regulations and supplement guidelines.
Allergan, Inc.
Technical Solution: Allergan, Inc. has been investigating muscimol and related GABA-A receptor modulators for potential use in treating various neurological disorders. Their approach includes developing synthetic analogs of muscimol with enhanced selectivity and reduced side effects[10]. Allergan is addressing legal considerations by pursuing a traditional pharmaceutical development pathway, including extensive preclinical studies, clinical trials, and regulatory submissions[11]. The company is leveraging its expertise in neuroscience and its established regulatory affairs team to navigate the complex legal landscape surrounding the development of drugs derived from or inspired by psychoactive compounds[12].
Strengths: Extensive experience in drug development and regulatory processes, strong financial resources. Weaknesses: Potential for long development timelines, high costs associated with bringing new CNS drugs to market.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals, Inc. is exploring muscimol and related GABA-A receptor agonists for potential therapeutic use in neurological and psychiatric disorders. Their approach involves developing novel compounds that modulate GABA-A receptors, including muscimol derivatives, with improved pharmacological properties[7]. ACADIA is addressing legal considerations by focusing on prescription drug development, conducting rigorous clinical trials, and working closely with regulatory agencies such as the FDA[8]. The company's strategy includes patenting novel muscimol-related compounds and formulations to ensure intellectual property protection and compliance with pharmaceutical regulations[9].
Strengths: Strong expertise in CNS drug development, established relationships with regulatory agencies. Weaknesses: High competition in the pharmaceutical industry, potential off-target effects of GABA-A modulators.
Key Legal Precedents and Rulings
Pharmaceutical intermediates and methods for preparing the same in the synthesis of muscimol and congeners and derivatives thereof
PatentWO2025128106A1
Innovation
- A novel method for preparing muscimol mono-BOC and muscimol hydrochloride that avoids the use of ion exchange chromatography by modifying the original synthetic route to include a flow reactor for the cyclization step and using BOC anhydride to purify the muscimol, thereby stabilizing the product and improving yields.
Processes for extracting muscimol from amanita muscaria
PatentWO2022187974A1
Innovation
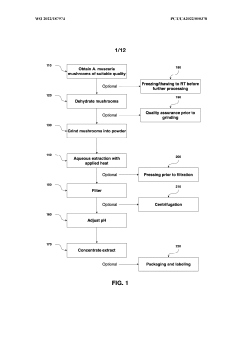
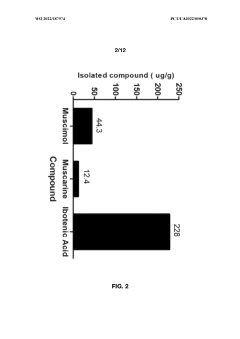
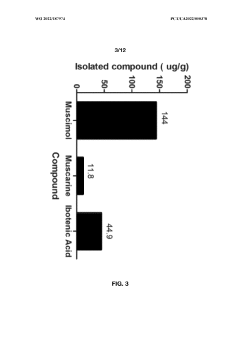
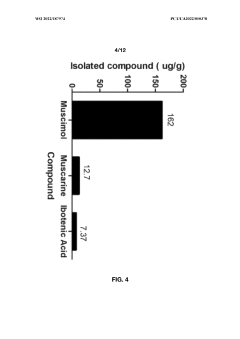
- Aqueous extraction methods involving heat, pH reduction, and concentration techniques such as distillation and refluxing are employed to decrease ibotenic acid content and increase muscimol content in the extract, including steps like grinding the mushroom biomass, filtering, and acidification to facilitate decarboxylation of ibotenic acid to muscimol.
Safety and Efficacy Considerations
The safety and efficacy of muscimol supplements are critical considerations in their legal use. Muscimol, a psychoactive compound found in certain mushroom species, has gained attention for its potential therapeutic applications. However, its use as a supplement raises significant safety concerns due to its potent effects on the central nervous system.
From a safety perspective, muscimol acts as a selective GABA-A receptor agonist, which can lead to sedation, altered perception, and potential cognitive impairment. The dosage and purity of muscimol supplements are crucial factors in determining their safety profile. Standardization of production processes and rigorous quality control measures are essential to ensure consistent potency and minimize the risk of contamination.
Regulatory bodies, such as the FDA, have not approved muscimol for use in dietary supplements, primarily due to the lack of comprehensive safety data. This regulatory status presents a significant challenge for companies seeking to market muscimol-based products. Extensive toxicology studies and clinical trials would be necessary to establish a safety profile acceptable for legal use as a supplement.
Efficacy considerations for muscimol supplements are equally complex. While preliminary research suggests potential benefits in areas such as anxiety reduction and sleep improvement, the evidence base remains limited. Controlled clinical trials are needed to validate these potential therapeutic effects and determine optimal dosing regimens.
The pharmacokinetics of muscimol, including its absorption, distribution, metabolism, and excretion, must be thoroughly understood to assess both safety and efficacy. This information is crucial for developing appropriate dosing guidelines and identifying potential drug interactions or contraindications.
Long-term effects of muscimol supplementation are largely unknown, necessitating careful monitoring and longitudinal studies. The potential for tolerance, dependence, or withdrawal symptoms must be evaluated to inform responsible use guidelines and potential restrictions.
Given the psychoactive nature of muscimol, there are concerns about its potential for misuse or abuse. Implementing strict controls on production, distribution, and sale would be necessary to mitigate these risks. Additionally, clear labeling and consumer education initiatives would be essential to ensure informed use and minimize the potential for adverse effects.
In conclusion, the legal use of muscimol supplements hinges on addressing these critical safety and efficacy considerations. Comprehensive research, rigorous testing, and careful regulatory oversight are prerequisites for establishing a framework that balances potential therapeutic benefits with public safety concerns.
From a safety perspective, muscimol acts as a selective GABA-A receptor agonist, which can lead to sedation, altered perception, and potential cognitive impairment. The dosage and purity of muscimol supplements are crucial factors in determining their safety profile. Standardization of production processes and rigorous quality control measures are essential to ensure consistent potency and minimize the risk of contamination.
Regulatory bodies, such as the FDA, have not approved muscimol for use in dietary supplements, primarily due to the lack of comprehensive safety data. This regulatory status presents a significant challenge for companies seeking to market muscimol-based products. Extensive toxicology studies and clinical trials would be necessary to establish a safety profile acceptable for legal use as a supplement.
Efficacy considerations for muscimol supplements are equally complex. While preliminary research suggests potential benefits in areas such as anxiety reduction and sleep improvement, the evidence base remains limited. Controlled clinical trials are needed to validate these potential therapeutic effects and determine optimal dosing regimens.
The pharmacokinetics of muscimol, including its absorption, distribution, metabolism, and excretion, must be thoroughly understood to assess both safety and efficacy. This information is crucial for developing appropriate dosing guidelines and identifying potential drug interactions or contraindications.
Long-term effects of muscimol supplementation are largely unknown, necessitating careful monitoring and longitudinal studies. The potential for tolerance, dependence, or withdrawal symptoms must be evaluated to inform responsible use guidelines and potential restrictions.
Given the psychoactive nature of muscimol, there are concerns about its potential for misuse or abuse. Implementing strict controls on production, distribution, and sale would be necessary to mitigate these risks. Additionally, clear labeling and consumer education initiatives would be essential to ensure informed use and minimize the potential for adverse effects.
In conclusion, the legal use of muscimol supplements hinges on addressing these critical safety and efficacy considerations. Comprehensive research, rigorous testing, and careful regulatory oversight are prerequisites for establishing a framework that balances potential therapeutic benefits with public safety concerns.
Ethical Implications of Muscimol Supplements
The use of muscimol supplements raises significant ethical concerns that warrant careful consideration. One primary issue is the potential for abuse and addiction, given muscimol's psychoactive properties. While it may offer therapeutic benefits, there is a risk of individuals using it recreationally or developing dependence, which could lead to negative health and social consequences.
Another ethical consideration is the impact on personal autonomy and decision-making capacity. Muscimol's effects on cognition and perception may impair an individual's ability to make informed choices, potentially compromising their autonomy. This raises questions about the ethical implications of using such substances, particularly in vulnerable populations or in situations where clear judgment is critical.
The principle of non-maleficence, or "do no harm," is also relevant in the context of muscimol supplements. While they may offer benefits, the long-term effects and potential risks are not fully understood. Ethical concerns arise regarding the balance between potential therapeutic benefits and unknown long-term consequences, especially given the limited research on prolonged use.
Equity and access present another ethical dimension. If muscimol supplements prove beneficial for certain conditions, ensuring fair and equitable access becomes an ethical imperative. However, this must be balanced against the need for responsible distribution and use, considering the potential for misuse or diversion.
The ethical implications extend to research practices as well. Conducting studies on muscimol supplements requires careful consideration of participant safety, informed consent, and the potential for adverse effects. Researchers must navigate the ethical challenges of studying a substance with psychoactive properties while ensuring scientific rigor and participant well-being.
Furthermore, the use of muscimol supplements raises questions about societal values and norms. As attitudes towards psychoactive substances evolve, there is a need to reconcile traditional views on drug use with emerging scientific understanding and potential therapeutic applications. This societal shift necessitates ongoing ethical dialogue and policy considerations.
Lastly, the environmental and sustainability aspects of muscimol production and use should not be overlooked. Ethical sourcing and sustainable practices in the cultivation and extraction of muscimol are important considerations, especially if demand for these supplements increases.
Another ethical consideration is the impact on personal autonomy and decision-making capacity. Muscimol's effects on cognition and perception may impair an individual's ability to make informed choices, potentially compromising their autonomy. This raises questions about the ethical implications of using such substances, particularly in vulnerable populations or in situations where clear judgment is critical.
The principle of non-maleficence, or "do no harm," is also relevant in the context of muscimol supplements. While they may offer benefits, the long-term effects and potential risks are not fully understood. Ethical concerns arise regarding the balance between potential therapeutic benefits and unknown long-term consequences, especially given the limited research on prolonged use.
Equity and access present another ethical dimension. If muscimol supplements prove beneficial for certain conditions, ensuring fair and equitable access becomes an ethical imperative. However, this must be balanced against the need for responsible distribution and use, considering the potential for misuse or diversion.
The ethical implications extend to research practices as well. Conducting studies on muscimol supplements requires careful consideration of participant safety, informed consent, and the potential for adverse effects. Researchers must navigate the ethical challenges of studying a substance with psychoactive properties while ensuring scientific rigor and participant well-being.
Furthermore, the use of muscimol supplements raises questions about societal values and norms. As attitudes towards psychoactive substances evolve, there is a need to reconcile traditional views on drug use with emerging scientific understanding and potential therapeutic applications. This societal shift necessitates ongoing ethical dialogue and policy considerations.
Lastly, the environmental and sustainability aspects of muscimol production and use should not be overlooked. Ethical sourcing and sustainable practices in the cultivation and extraction of muscimol are important considerations, especially if demand for these supplements increases.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!