Long-term safety evaluation of Brain-Computer Interfaces implantable devices
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BCI Implant Safety Background and Objectives
Brain-Computer Interfaces (BCIs) represent a revolutionary technology that establishes direct communication pathways between the brain and external devices. The concept of BCIs dates back to the 1970s, but significant technological advancements in the past two decades have accelerated their development and potential applications. Implantable BCI devices, in particular, have shown remarkable progress in restoring sensory and motor functions for individuals with neurological disorders.
The evolution of implantable BCIs has been marked by several milestone achievements, including the development of multi-electrode arrays, wireless transmission capabilities, and miniaturized electronics. These advancements have enabled more sophisticated neural signal recording and stimulation, expanding the potential therapeutic applications of BCIs from motor function restoration to treatment of neuropsychiatric disorders.
Despite these promising developments, the long-term safety of implantable BCI devices remains a critical concern that requires comprehensive evaluation. The intimate interface between electronic components and neural tissue presents unique challenges that extend beyond traditional medical device safety considerations. These challenges include biocompatibility of materials, tissue response to chronic implantation, device degradation over time, and potential neurological effects of continuous electrical stimulation.
The primary objective of long-term safety evaluation for implantable BCIs is to establish robust protocols and standards that ensure these devices can function safely within the human brain for extended periods—potentially decades—without causing adverse effects. This includes assessing both immediate safety concerns, such as surgical risks and acute tissue reactions, and long-term considerations like material degradation, chronic inflammation, and potential neuroplastic changes induced by persistent neural interfaces.
Additionally, safety evaluations must address the unique ethical considerations associated with devices that directly interface with the brain, including cognitive liberty, identity, and autonomy. As BCI technology advances toward more complex applications beyond medical therapeutics, these ethical dimensions become increasingly important components of comprehensive safety frameworks.
The technological trajectory suggests that implantable BCIs will continue to become more sophisticated, with higher channel counts, improved longevity, and enhanced computational capabilities. This evolution necessitates parallel advancement in safety evaluation methodologies that can anticipate and address emerging risks while supporting responsible innovation in this transformative field.
The evolution of implantable BCIs has been marked by several milestone achievements, including the development of multi-electrode arrays, wireless transmission capabilities, and miniaturized electronics. These advancements have enabled more sophisticated neural signal recording and stimulation, expanding the potential therapeutic applications of BCIs from motor function restoration to treatment of neuropsychiatric disorders.
Despite these promising developments, the long-term safety of implantable BCI devices remains a critical concern that requires comprehensive evaluation. The intimate interface between electronic components and neural tissue presents unique challenges that extend beyond traditional medical device safety considerations. These challenges include biocompatibility of materials, tissue response to chronic implantation, device degradation over time, and potential neurological effects of continuous electrical stimulation.
The primary objective of long-term safety evaluation for implantable BCIs is to establish robust protocols and standards that ensure these devices can function safely within the human brain for extended periods—potentially decades—without causing adverse effects. This includes assessing both immediate safety concerns, such as surgical risks and acute tissue reactions, and long-term considerations like material degradation, chronic inflammation, and potential neuroplastic changes induced by persistent neural interfaces.
Additionally, safety evaluations must address the unique ethical considerations associated with devices that directly interface with the brain, including cognitive liberty, identity, and autonomy. As BCI technology advances toward more complex applications beyond medical therapeutics, these ethical dimensions become increasingly important components of comprehensive safety frameworks.
The technological trajectory suggests that implantable BCIs will continue to become more sophisticated, with higher channel counts, improved longevity, and enhanced computational capabilities. This evolution necessitates parallel advancement in safety evaluation methodologies that can anticipate and address emerging risks while supporting responsible innovation in this transformative field.
Market Analysis for Neural Interface Technologies
The neural interface technology market is experiencing significant growth, driven by advancements in brain-computer interface (BCI) implantable devices. Current market valuations estimate the global neural interface market at approximately $2.4 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 15.8% through 2030, potentially reaching $7.6 billion by the end of the decade.
Healthcare applications dominate the current market landscape, accounting for roughly 60% of market share. This segment is primarily focused on therapeutic applications for neurological disorders such as Parkinson's disease, epilepsy, and paralysis. The remaining market share is distributed across research institutions (25%), military applications (10%), and consumer applications (5%).
Regionally, North America leads with approximately 45% of the global market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). The United States specifically maintains dominance due to substantial research funding, presence of key industry players, and favorable regulatory pathways for neurotechnology.
Demand drivers for neural interface technologies include the rising prevalence of neurological disorders, increasing geriatric population, and growing acceptance of implantable medical devices. The global burden of neurological disorders affects over 1 billion people worldwide, creating substantial demand for innovative therapeutic solutions.
Investment in the sector has seen remarkable growth, with venture capital funding exceeding $1.5 billion in 2022 alone, representing a 35% increase compared to the previous year. Major technology corporations have also entered the space, with investments totaling over $3 billion in the past five years.
Customer segments for neural interface technologies are diversifying beyond traditional medical applications. While clinical applications remain primary, emerging segments include consumer wellness, gaming, productivity enhancement, and specialized professional applications in high-precision fields.
Pricing models vary significantly across the market. Medical-grade implantable BCI devices typically range from $15,000 to $150,000 depending on complexity and capabilities, while non-invasive consumer-oriented devices range from $300 to $3,000.
Market barriers include stringent regulatory requirements, particularly for implantable devices requiring long-term safety evaluations. The FDA and equivalent international bodies have established rigorous testing protocols for neural implants, with approval processes often extending 3-5 years. Reimbursement challenges also persist, as many insurance providers classify advanced neural interfaces as experimental technologies.
Healthcare applications dominate the current market landscape, accounting for roughly 60% of market share. This segment is primarily focused on therapeutic applications for neurological disorders such as Parkinson's disease, epilepsy, and paralysis. The remaining market share is distributed across research institutions (25%), military applications (10%), and consumer applications (5%).
Regionally, North America leads with approximately 45% of the global market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). The United States specifically maintains dominance due to substantial research funding, presence of key industry players, and favorable regulatory pathways for neurotechnology.
Demand drivers for neural interface technologies include the rising prevalence of neurological disorders, increasing geriatric population, and growing acceptance of implantable medical devices. The global burden of neurological disorders affects over 1 billion people worldwide, creating substantial demand for innovative therapeutic solutions.
Investment in the sector has seen remarkable growth, with venture capital funding exceeding $1.5 billion in 2022 alone, representing a 35% increase compared to the previous year. Major technology corporations have also entered the space, with investments totaling over $3 billion in the past five years.
Customer segments for neural interface technologies are diversifying beyond traditional medical applications. While clinical applications remain primary, emerging segments include consumer wellness, gaming, productivity enhancement, and specialized professional applications in high-precision fields.
Pricing models vary significantly across the market. Medical-grade implantable BCI devices typically range from $15,000 to $150,000 depending on complexity and capabilities, while non-invasive consumer-oriented devices range from $300 to $3,000.
Market barriers include stringent regulatory requirements, particularly for implantable devices requiring long-term safety evaluations. The FDA and equivalent international bodies have established rigorous testing protocols for neural implants, with approval processes often extending 3-5 years. Reimbursement challenges also persist, as many insurance providers classify advanced neural interfaces as experimental technologies.
Current Challenges in Long-term BCI Biocompatibility
The biocompatibility of implantable Brain-Computer Interface (BCI) devices presents significant challenges for long-term applications. Despite advances in materials science and neural engineering, the foreign body response remains a primary obstacle. When neural implants are introduced into brain tissue, the immune system initiates an inflammatory cascade that can lead to device encapsulation by glial scarring. This process gradually degrades signal quality and ultimately compromises device functionality, with studies showing significant signal deterioration within 6-12 months post-implantation in many cases.
Material degradation poses another critical challenge. Current electrode materials, including platinum, iridium oxide, and silicon, experience corrosion and structural breakdown when exposed to the harsh biochemical environment of the brain. This degradation not only affects recording fidelity but also potentially releases toxic byproducts into surrounding neural tissue, causing secondary damage beyond the initial implantation trauma.
Micromotion between rigid implants and soft brain tissue exacerbates biocompatibility issues. The mechanical mismatch creates continuous microtrauma as natural brain movements occur during respiration, cardiovascular pulsation, and head movements. This persistent irritation maintains a chronic inflammatory state and accelerates the foreign body response, creating a negative feedback loop that shortens device lifespan.
Infection risks represent a persistent concern for transcutaneous BCI systems. The interface between external components and implanted elements creates potential pathways for microbial invasion. Current sterilization techniques and antimicrobial coatings provide initial protection, but their efficacy diminishes over time, leaving patients vulnerable to delayed-onset infections that may necessitate device removal and extensive antibiotic treatment.
Long-term electrical stimulation effects remain incompletely understood. Continuous electrical stimulation can alter local tissue properties through electrochemical reactions at the electrode-tissue interface. These changes may include pH shifts, reactive oxygen species generation, and altered ion concentrations that potentially damage neurons and supporting cells over extended periods.
The challenge of achieving stable neural recordings is compounded by natural neural plasticity. The brain's adaptive nature means that neural networks continuously reorganize, potentially shifting away from recording sites. This biological dynamism requires BCI systems capable of adapting to changing neural landscapes—a capability current fixed-position electrodes largely lack.
Addressing these biocompatibility challenges requires interdisciplinary approaches combining materials science, immunology, neuroscience, and engineering. Recent research directions include ultra-soft biomimetic materials, anti-inflammatory coatings, wireless power transmission to eliminate transcutaneous components, and closed-loop systems capable of adjusting stimulation parameters based on tissue response monitoring.
Material degradation poses another critical challenge. Current electrode materials, including platinum, iridium oxide, and silicon, experience corrosion and structural breakdown when exposed to the harsh biochemical environment of the brain. This degradation not only affects recording fidelity but also potentially releases toxic byproducts into surrounding neural tissue, causing secondary damage beyond the initial implantation trauma.
Micromotion between rigid implants and soft brain tissue exacerbates biocompatibility issues. The mechanical mismatch creates continuous microtrauma as natural brain movements occur during respiration, cardiovascular pulsation, and head movements. This persistent irritation maintains a chronic inflammatory state and accelerates the foreign body response, creating a negative feedback loop that shortens device lifespan.
Infection risks represent a persistent concern for transcutaneous BCI systems. The interface between external components and implanted elements creates potential pathways for microbial invasion. Current sterilization techniques and antimicrobial coatings provide initial protection, but their efficacy diminishes over time, leaving patients vulnerable to delayed-onset infections that may necessitate device removal and extensive antibiotic treatment.
Long-term electrical stimulation effects remain incompletely understood. Continuous electrical stimulation can alter local tissue properties through electrochemical reactions at the electrode-tissue interface. These changes may include pH shifts, reactive oxygen species generation, and altered ion concentrations that potentially damage neurons and supporting cells over extended periods.
The challenge of achieving stable neural recordings is compounded by natural neural plasticity. The brain's adaptive nature means that neural networks continuously reorganize, potentially shifting away from recording sites. This biological dynamism requires BCI systems capable of adapting to changing neural landscapes—a capability current fixed-position electrodes largely lack.
Addressing these biocompatibility challenges requires interdisciplinary approaches combining materials science, immunology, neuroscience, and engineering. Recent research directions include ultra-soft biomimetic materials, anti-inflammatory coatings, wireless power transmission to eliminate transcutaneous components, and closed-loop systems capable of adjusting stimulation parameters based on tissue response monitoring.
Existing Safety Evaluation Methodologies
01 Biocompatibility and material safety for implantable BCI devices
Implantable brain-computer interfaces require biocompatible materials that minimize tissue reaction and inflammation. Advanced materials such as flexible polymers, biocompatible metals, and coatings are used to reduce foreign body responses and ensure long-term stability within neural tissue. These materials must undergo rigorous testing to prevent adverse reactions, toxicity, or degradation that could compromise patient safety or device functionality.- Biocompatibility and tissue integration of implantable BCI devices: Implantable brain-computer interface devices require biocompatible materials and designs to minimize tissue damage and inflammatory responses. Advanced coatings and materials can improve long-term integration with neural tissue, reducing scarring and maintaining signal quality. These innovations focus on reducing foreign body reactions and ensuring stable performance of the implant over extended periods, which is crucial for patient safety.
- Infection prevention and sterilization techniques: Safety protocols for implantable BCI devices include specialized sterilization methods and antimicrobial coatings to prevent infections. These techniques address the significant risk of microbial contamination during and after implantation procedures. Innovations in this area include self-sterilizing surfaces, controlled-release antimicrobial agents, and hermetic sealing technologies that maintain sterility throughout the device's lifetime.
- Electrical safety and electromagnetic compatibility: Implantable BCI devices must incorporate robust electrical safety features to prevent tissue damage from current leakage or electrical malfunctions. This includes isolation circuits, surge protection, and electromagnetic shielding to protect both the device and the patient. These safety mechanisms ensure compatibility with external electromagnetic fields from everyday environments and medical equipment such as MRI machines.
- Long-term monitoring and failure detection systems: Advanced monitoring systems are integrated into implantable BCI devices to continuously assess performance and detect potential failures before they compromise patient safety. These systems include self-diagnostic capabilities, wireless monitoring of critical parameters, and redundant safety mechanisms. Early warning systems can alert medical professionals to degradation in device performance, allowing for intervention before safety is compromised.
- Minimally invasive implantation and removal procedures: Innovative surgical techniques and device designs focus on minimizing the invasiveness of BCI implantation and removal procedures. These approaches reduce surgical trauma, lower infection risks, and improve recovery outcomes. Developments include flexible electrodes that can be delivered through smaller cranial openings, dissolvable components that eliminate the need for removal surgeries, and modular designs that allow for partial device replacement without disturbing integrated components.
02 Infection prevention and sterilization protocols
Safety measures for implantable BCI devices include specialized sterilization techniques and antimicrobial coatings to prevent infections. Surgical protocols are designed to minimize contamination risks during implantation procedures. Some devices incorporate infection monitoring systems and drug-eluting components that can release antimicrobial agents to maintain a sterile environment around the implant, reducing the risk of post-surgical complications.Expand Specific Solutions03 Electrical safety and electromagnetic compatibility
Implantable BCI devices must maintain electrical safety through proper insulation, current limiting mechanisms, and protection against electromagnetic interference. Safety features include surge protection circuits, fault detection systems, and electromagnetic shielding to prevent device malfunction. These measures protect neural tissue from electrical damage while ensuring the device operates reliably in the presence of external electromagnetic fields from common electronic devices.Expand Specific Solutions04 Long-term monitoring and failure prevention systems
Advanced monitoring systems are integrated into implantable BCI devices to continuously assess performance and detect potential failures before they cause harm. These systems monitor parameters such as electrode impedance, signal quality, power consumption, and tissue response. Some devices incorporate redundant components, self-diagnostic capabilities, and remote monitoring features that allow healthcare providers to identify issues requiring intervention without invasive procedures.Expand Specific Solutions05 Minimally invasive implantation techniques
Safety innovations in BCI technology include minimally invasive implantation methods that reduce surgical trauma and recovery time. These techniques utilize specialized delivery systems, microelectrode arrays, and flexible substrates that can be inserted through smaller cranial openings. Some approaches employ endovascular techniques or utilize natural anatomical pathways to access target brain regions, significantly reducing the risks associated with traditional open brain surgery.Expand Specific Solutions
Leading Organizations in BCI Implant Research
The Brain-Computer Interface (BCI) implantable devices safety evaluation landscape is currently in an early growth phase, with market size projected to expand significantly as clinical applications advance. The technology maturity varies considerably among key players, with established medical device companies like NeuroPace and emerging BCI specialists such as Neuralink and Precision Neuroscience leading commercial development. Academic institutions including California Institute of Technology, Tsinghua University, and Johns Hopkins University contribute fundamental research on long-term biocompatibility and neural tissue interactions. The competitive environment features a blend of private companies focused on clinical applications and research institutions developing safety protocols. Regulatory frameworks for long-term safety assessment are still evolving, creating both challenges and opportunities for organizations investing in comprehensive safety evaluation methodologies.
NeuroPace, Inc.
Technical Solution: NeuroPace has established one of the most robust long-term safety evaluation protocols for implantable brain-computer interfaces, leveraging their extensive experience with their RNS® System for epilepsy treatment. Their methodology includes a structured 10-year follow-up program for implanted patients, with comprehensive data collection at regular intervals to track device performance, tissue response, and neurological outcomes. The company employs a multi-modal safety assessment approach combining clinical evaluations, neuroimaging, and electrophysiological recordings to detect subtle changes in neural tissue or device function. Their safety evaluation framework incorporates automated seizure detection algorithms that continuously adapt to patient-specific patterns, allowing for personalized safety monitoring. NeuroPace has developed specialized MRI-conditional protocols enabling safe imaging of patients with implants, facilitating long-term monitoring of tissue-device interactions. The company maintains a centralized adverse event database with sophisticated analytics to identify potential safety signals across their patient population, enabling rapid response to emerging safety concerns[3][4].
Strengths: Extensive clinical experience with FDA-approved implantable neural devices; established long-term patient monitoring infrastructure; proven track record of device longevity and safety. Weaknesses: Primary focus on epilepsy applications may limit generalizability to other BCI applications; relatively conservative approach to innovation compared to newer competitors; higher cost structure for safety monitoring systems.
Precision Neuroscience Corp.
Technical Solution: Precision Neuroscience has developed an innovative approach to long-term safety evaluation for their ultra-thin, minimally invasive brain-computer interface technology. Their Layer 7 Cortical Interface utilizes a film-like array of electrodes thinner than a human hair, designed specifically to minimize long-term tissue damage and inflammatory response. The company's safety evaluation protocol centers on their proprietary "reversible implantation" methodology, allowing for device removal with minimal tissue disruption if safety concerns arise. Their evaluation framework includes specialized imaging techniques to monitor the brain-device interface at microscopic resolution over extended periods, tracking cellular responses and potential microstructural changes. Precision employs machine learning algorithms to analyze neural signal quality degradation patterns as early indicators of safety issues before clinical symptoms manifest. The company has established a tiered biocompatibility testing program that evaluates materials stability under accelerated aging conditions simulating years of implantation within weeks. Their safety monitoring includes regular assessment of blood-brain barrier integrity and regional cerebral blood flow around implantation sites to detect subtle inflammatory processes[5][6].
Strengths: Ultra-thin electrode technology minimizing tissue damage; reversible implantation approach allowing device removal if needed; specialized high-resolution imaging protocols for interface monitoring. Weaknesses: Relatively new company with limited long-term clinical data; smaller scale operations may limit comprehensive safety testing resources; technology optimized for cortical surface recording rather than deep brain structures.
Critical Patents and Research on Neural Tissue Integration
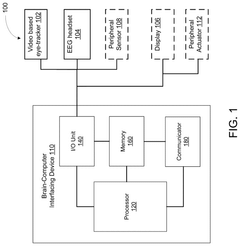
Brain-computer interface device, operation method therefor, and brain-computer interface system
PatentWO2025156995A1
Innovation
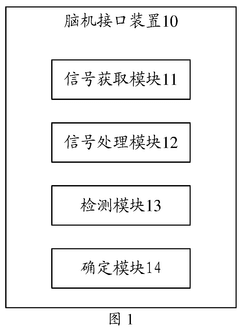
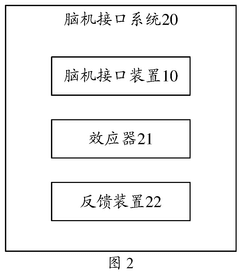
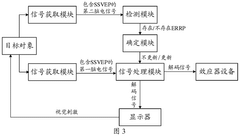
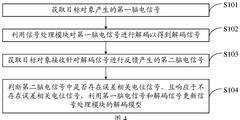
- By introducing signal acquisition modules, signal processing modules, detection modules and determination modules into the brain-computer interface device, the decoding model is realized using the memristor array, and error-related potential signals are detected during the interaction process, the decoding model parameters are updated, and the electroencephalopathy and memristor conductance drift phenomenon are overcome.
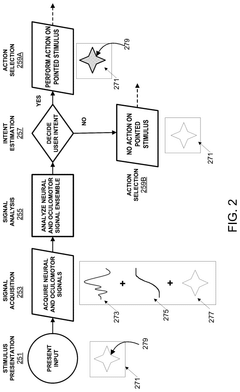
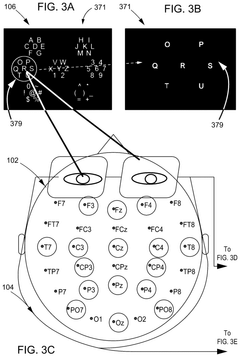
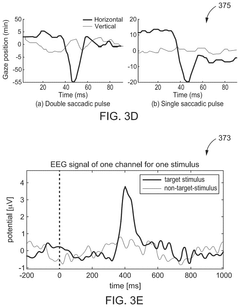
Brain-computer interface with adaptations for high-speed, accurate, and intuitive user interactions
PatentPendingUS20250093951A1
Innovation
- A hardware-agnostic integrated oculomotor-neural hybrid BCI platform that combines real-time eye-movement tracking with brain activity tracking to mediate user interactions, using a hybrid BCI system with pointing and action control features to enhance user interface design for high-speed and accurate interactions.
Regulatory Framework for Implantable Neural Devices
The regulatory landscape for implantable neural devices represents a complex framework that continues to evolve alongside technological advancements in Brain-Computer Interfaces (BCIs). Currently, the FDA's Center for Devices and Radiological Health (CDRH) serves as the primary regulatory body in the United States, classifying most implantable neural devices as Class III medical devices requiring premarket approval (PMA) due to their high-risk profile and direct interface with brain tissue.
International regulatory frameworks exhibit significant variation, with the European Union implementing the Medical Device Regulation (MDR) in 2021, which introduced more stringent requirements for clinical evidence and post-market surveillance specifically addressing long-term safety concerns. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specialized pathways for innovative medical technologies, while China's National Medical Products Administration (NMPA) has recently developed specific guidelines for neurotechnology devices.
Safety standards for implantable neural devices encompass biocompatibility testing (ISO 10993), sterility assurance (ISO 11737), electromagnetic compatibility (IEC 60601-1-2), and specific standards for active implantable medical devices (ISO 14708). These standards are continuously updated to address emerging concerns related to long-term implantation, such as material degradation, chronic tissue response, and device longevity.
A significant regulatory challenge lies in the establishment of appropriate endpoints for safety evaluation, particularly for devices intended to remain implanted for decades. Current frameworks typically require clinical trials with follow-up periods of 1-5 years, which may be insufficient to detect delayed adverse effects. This has prompted regulatory bodies to implement mandatory post-market surveillance programs and patient registries to monitor long-term outcomes.
The FDA's recent "Breakthrough Devices Program" and the EU's "Innovation Pathway" represent regulatory adaptations designed to accelerate the development of novel neural technologies while maintaining safety standards. These programs incorporate early and frequent communication between developers and regulators, flexible clinical study designs, and expedited review processes while emphasizing robust post-market data collection.
Emerging regulatory considerations include the development of standards for cybersecurity of connected neural devices, protocols for managing explanted devices, and frameworks for evaluating the psychological and social impacts of long-term neural implants. Additionally, regulatory bodies are increasingly focusing on patient-reported outcomes and quality of life measures as important safety endpoints for BCI technologies intended for chronic use.
International regulatory frameworks exhibit significant variation, with the European Union implementing the Medical Device Regulation (MDR) in 2021, which introduced more stringent requirements for clinical evidence and post-market surveillance specifically addressing long-term safety concerns. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specialized pathways for innovative medical technologies, while China's National Medical Products Administration (NMPA) has recently developed specific guidelines for neurotechnology devices.
Safety standards for implantable neural devices encompass biocompatibility testing (ISO 10993), sterility assurance (ISO 11737), electromagnetic compatibility (IEC 60601-1-2), and specific standards for active implantable medical devices (ISO 14708). These standards are continuously updated to address emerging concerns related to long-term implantation, such as material degradation, chronic tissue response, and device longevity.
A significant regulatory challenge lies in the establishment of appropriate endpoints for safety evaluation, particularly for devices intended to remain implanted for decades. Current frameworks typically require clinical trials with follow-up periods of 1-5 years, which may be insufficient to detect delayed adverse effects. This has prompted regulatory bodies to implement mandatory post-market surveillance programs and patient registries to monitor long-term outcomes.
The FDA's recent "Breakthrough Devices Program" and the EU's "Innovation Pathway" represent regulatory adaptations designed to accelerate the development of novel neural technologies while maintaining safety standards. These programs incorporate early and frequent communication between developers and regulators, flexible clinical study designs, and expedited review processes while emphasizing robust post-market data collection.
Emerging regulatory considerations include the development of standards for cybersecurity of connected neural devices, protocols for managing explanted devices, and frameworks for evaluating the psychological and social impacts of long-term neural implants. Additionally, regulatory bodies are increasingly focusing on patient-reported outcomes and quality of life measures as important safety endpoints for BCI technologies intended for chronic use.
Ethical Implications of Long-term Neural Implants
The ethical landscape surrounding long-term neural implants presents complex challenges that extend beyond technical considerations. As Brain-Computer Interface (BCI) technology advances toward chronic implantation, fundamental questions arise regarding patient autonomy and informed consent. The longitudinal nature of these devices creates unprecedented scenarios where consent given pre-implantation may not adequately address future device capabilities or data usage, particularly as neural technologies evolve through software updates.
Identity and agency concerns represent another critical ethical dimension. Neural implants that influence cognitive processes or motor control may fundamentally alter a person's sense of self, raising philosophical questions about authenticity and autonomy. Research indicates that some patients with deep brain stimulation implants have reported feeling "not themselves" when devices are active, highlighting the profound relationship between neural technology and personal identity.
Privacy vulnerabilities present unique challenges with neural implants. Unlike conventional medical data, neural information may reveal thoughts, emotions, and intentions—potentially the most intimate form of personal data. The risk of unauthorized access to neural data through security breaches or commercial exploitation demands robust safeguards beyond traditional medical privacy frameworks.
Socioeconomic justice considerations cannot be overlooked. The high cost of advanced neural implants may create a "neural divide," where only privileged populations gain access to cognitive or physical enhancements. This scenario risks exacerbating existing social inequalities and creating new forms of discrimination based on neural capabilities or modifications.
Long-term psychological impacts remain largely unexplored. Patients may experience significant psychological adjustment challenges when living with neural implants, including dependency concerns, altered self-perception, and social stigma. The current research gap in understanding these long-term psychological effects represents a significant ethical blind spot in the field.
Regulatory frameworks currently struggle to address these multifaceted ethical challenges. Most medical device regulations were designed for conventional implants rather than devices that interface directly with neural tissue and cognitive processes. This regulatory gap necessitates new approaches that balance innovation with appropriate ethical safeguards, potentially including ongoing consent mechanisms, neural data protection standards, and equitable access provisions.
Identity and agency concerns represent another critical ethical dimension. Neural implants that influence cognitive processes or motor control may fundamentally alter a person's sense of self, raising philosophical questions about authenticity and autonomy. Research indicates that some patients with deep brain stimulation implants have reported feeling "not themselves" when devices are active, highlighting the profound relationship between neural technology and personal identity.
Privacy vulnerabilities present unique challenges with neural implants. Unlike conventional medical data, neural information may reveal thoughts, emotions, and intentions—potentially the most intimate form of personal data. The risk of unauthorized access to neural data through security breaches or commercial exploitation demands robust safeguards beyond traditional medical privacy frameworks.
Socioeconomic justice considerations cannot be overlooked. The high cost of advanced neural implants may create a "neural divide," where only privileged populations gain access to cognitive or physical enhancements. This scenario risks exacerbating existing social inequalities and creating new forms of discrimination based on neural capabilities or modifications.
Long-term psychological impacts remain largely unexplored. Patients may experience significant psychological adjustment challenges when living with neural implants, including dependency concerns, altered self-perception, and social stigma. The current research gap in understanding these long-term psychological effects represents a significant ethical blind spot in the field.
Regulatory frameworks currently struggle to address these multifaceted ethical challenges. Most medical device regulations were designed for conventional implants rather than devices that interface directly with neural tissue and cognitive processes. This regulatory gap necessitates new approaches that balance innovation with appropriate ethical safeguards, potentially including ongoing consent mechanisms, neural data protection standards, and equitable access provisions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!