Longitudinal Studies on Muscimol in Psychotropic Analysis
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Research Background and Objectives
Muscimol, a potent GABA receptor agonist, has been a subject of significant interest in psychotropic research for several decades. This naturally occurring psychoactive compound, found primarily in Amanita mushroom species, has garnered attention due to its unique pharmacological properties and potential therapeutic applications. The evolution of muscimol research can be traced back to the mid-20th century when scientists first isolated and identified the compound.
The primary objective of longitudinal studies on muscimol in psychotropic analysis is to comprehensively understand its long-term effects on the human brain and behavior. These studies aim to elucidate the compound's mechanism of action, its impact on neurotransmitter systems, and its potential for treating various neurological and psychiatric disorders. By examining muscimol's effects over extended periods, researchers seek to uncover any cumulative or adaptive changes in neural circuits and cognitive functions.
One of the key drivers behind muscimol research is its selectivity for GABA-A receptors, which play a crucial role in regulating neuronal excitability. This specificity makes muscimol an invaluable tool for investigating GABAergic neurotransmission and its implications in various brain functions, including anxiety, sleep, and memory. Longitudinal studies are particularly important in this context, as they allow researchers to observe how chronic exposure to muscimol may alter receptor sensitivity and neural plasticity over time.
The technological advancements in neuroimaging and electrophysiology have significantly contributed to the progress of muscimol research. These tools enable scientists to visualize and quantify the compound's effects on brain activity and connectivity in real-time, providing unprecedented insights into its mechanisms of action. Furthermore, the development of sophisticated animal models and improved methods for synthesizing and administering muscimol have expanded the scope and reliability of longitudinal studies.
As research in this field progresses, there is a growing interest in exploring muscimol's potential therapeutic applications. Studies are investigating its efficacy in treating conditions such as epilepsy, anxiety disorders, and sleep disturbances. The long-term nature of these investigations is crucial for assessing both the sustained benefits and potential risks associated with prolonged muscimol use.
Another important aspect of muscimol research is its role in understanding the broader implications of GABAergic modulation in the brain. By studying muscimol's effects over time, researchers hope to gain insights into the fundamental principles of neural inhibition and its role in maintaining cognitive and emotional balance. This knowledge could potentially lead to the development of novel therapeutic strategies for a wide range of neurological and psychiatric conditions.
The primary objective of longitudinal studies on muscimol in psychotropic analysis is to comprehensively understand its long-term effects on the human brain and behavior. These studies aim to elucidate the compound's mechanism of action, its impact on neurotransmitter systems, and its potential for treating various neurological and psychiatric disorders. By examining muscimol's effects over extended periods, researchers seek to uncover any cumulative or adaptive changes in neural circuits and cognitive functions.
One of the key drivers behind muscimol research is its selectivity for GABA-A receptors, which play a crucial role in regulating neuronal excitability. This specificity makes muscimol an invaluable tool for investigating GABAergic neurotransmission and its implications in various brain functions, including anxiety, sleep, and memory. Longitudinal studies are particularly important in this context, as they allow researchers to observe how chronic exposure to muscimol may alter receptor sensitivity and neural plasticity over time.
The technological advancements in neuroimaging and electrophysiology have significantly contributed to the progress of muscimol research. These tools enable scientists to visualize and quantify the compound's effects on brain activity and connectivity in real-time, providing unprecedented insights into its mechanisms of action. Furthermore, the development of sophisticated animal models and improved methods for synthesizing and administering muscimol have expanded the scope and reliability of longitudinal studies.
As research in this field progresses, there is a growing interest in exploring muscimol's potential therapeutic applications. Studies are investigating its efficacy in treating conditions such as epilepsy, anxiety disorders, and sleep disturbances. The long-term nature of these investigations is crucial for assessing both the sustained benefits and potential risks associated with prolonged muscimol use.
Another important aspect of muscimol research is its role in understanding the broader implications of GABAergic modulation in the brain. By studying muscimol's effects over time, researchers hope to gain insights into the fundamental principles of neural inhibition and its role in maintaining cognitive and emotional balance. This knowledge could potentially lead to the development of novel therapeutic strategies for a wide range of neurological and psychiatric conditions.
Psychotropic Market Analysis
The psychotropic market has experienced significant growth in recent years, driven by increasing awareness of mental health issues and the rising prevalence of neurological disorders. The global psychotropic drugs market was valued at approximately $29 billion in 2020 and is projected to reach $40 billion by 2027, growing at a CAGR of around 4.5% during the forecast period.
Muscimol, a potent GABA receptor agonist derived from various species of mushrooms, has garnered increasing attention in the psychotropic market. While not currently approved for clinical use, ongoing research into its potential therapeutic applications has sparked interest among pharmaceutical companies and investors.
The market for novel psychotropic compounds like muscimol is primarily driven by the growing demand for more effective treatments for anxiety disorders, depression, and other neuropsychiatric conditions. Traditional psychotropic medications often come with significant side effects and limited efficacy for certain patient populations, creating a need for alternative treatment options.
Longitudinal studies on muscimol in psychotropic analysis have revealed promising results in preclinical and early-stage clinical trials. These studies have demonstrated muscimol's potential in treating various neurological and psychiatric disorders, including anxiety, insomnia, and certain forms of epilepsy. The compound's unique mechanism of action and relatively low toxicity profile have made it an attractive candidate for further research and development.
The psychotropic market is highly competitive, with major pharmaceutical companies investing heavily in research and development of novel compounds. Several biotechnology firms have also entered the space, focusing on the development of psychedelic-inspired drugs, including muscimol-based therapies. This increased competition has led to a surge in patent filings and strategic partnerships within the industry.
Regulatory bodies, such as the FDA and EMA, have shown increased openness to psychedelic-inspired treatments, paving the way for potential future approvals of muscimol-based therapies. This shift in regulatory stance has further fueled investor interest and market growth potential for novel psychotropic compounds.
Despite the promising outlook, challenges remain in the commercialization of muscimol-based therapies. These include the need for extensive clinical trials to establish safety and efficacy, potential stigma associated with psychedelic-inspired treatments, and the complexities of drug scheduling and controlled substance regulations.
Muscimol, a potent GABA receptor agonist derived from various species of mushrooms, has garnered increasing attention in the psychotropic market. While not currently approved for clinical use, ongoing research into its potential therapeutic applications has sparked interest among pharmaceutical companies and investors.
The market for novel psychotropic compounds like muscimol is primarily driven by the growing demand for more effective treatments for anxiety disorders, depression, and other neuropsychiatric conditions. Traditional psychotropic medications often come with significant side effects and limited efficacy for certain patient populations, creating a need for alternative treatment options.
Longitudinal studies on muscimol in psychotropic analysis have revealed promising results in preclinical and early-stage clinical trials. These studies have demonstrated muscimol's potential in treating various neurological and psychiatric disorders, including anxiety, insomnia, and certain forms of epilepsy. The compound's unique mechanism of action and relatively low toxicity profile have made it an attractive candidate for further research and development.
The psychotropic market is highly competitive, with major pharmaceutical companies investing heavily in research and development of novel compounds. Several biotechnology firms have also entered the space, focusing on the development of psychedelic-inspired drugs, including muscimol-based therapies. This increased competition has led to a surge in patent filings and strategic partnerships within the industry.
Regulatory bodies, such as the FDA and EMA, have shown increased openness to psychedelic-inspired treatments, paving the way for potential future approvals of muscimol-based therapies. This shift in regulatory stance has further fueled investor interest and market growth potential for novel psychotropic compounds.
Despite the promising outlook, challenges remain in the commercialization of muscimol-based therapies. These include the need for extensive clinical trials to establish safety and efficacy, potential stigma associated with psychedelic-inspired treatments, and the complexities of drug scheduling and controlled substance regulations.
Muscimol Study Challenges
Conducting longitudinal studies on muscimol in psychotropic analysis presents several significant challenges that researchers must navigate. One of the primary difficulties lies in maintaining consistent dosing and administration protocols over extended periods. Muscimol's short half-life and rapid metabolism necessitate frequent dosing, which can be problematic for long-term studies. This issue is compounded by the potential for tolerance development, requiring careful dose adjustments to maintain therapeutic effects.
Another major challenge is the management of side effects and potential adverse reactions over time. While muscimol is generally considered to have a favorable safety profile, prolonged exposure may lead to unexpected physiological or psychological effects. Researchers must implement robust monitoring systems to detect and address any emerging issues promptly, which can be resource-intensive and complex in long-term studies.
The ethical considerations surrounding extended psychotropic substance administration pose additional hurdles. Institutional review boards and ethics committees may have concerns about the long-term impact on participants' mental health and cognitive function. This necessitates the development of comprehensive safety protocols and exit strategies for study participants, adding layers of complexity to study design and execution.
Data collection and analysis in longitudinal muscimol studies present their own set of challenges. The need for consistent, standardized assessment methods across multiple time points is crucial but can be difficult to maintain, especially when dealing with subjective measures of psychological states. Furthermore, the potential for participant attrition over extended study periods can compromise data integrity and statistical power, requiring careful planning and oversampling strategies.
Controlling for confounding variables becomes increasingly complex in longitudinal designs. External factors such as lifestyle changes, concurrent medications, or environmental stressors can significantly impact study outcomes. Researchers must develop sophisticated methods to account for these variables without compromising the study's focus on muscimol's effects.
Lastly, the financial and logistical demands of conducting long-term studies on muscimol are substantial. Securing funding for extended research periods, maintaining dedicated research staff, and ensuring consistent access to high-quality muscimol supplies over years can be daunting tasks. These practical considerations often limit the scope and duration of longitudinal studies, potentially constraining the depth of knowledge that can be gained about muscimol's long-term psychotropic effects.
Another major challenge is the management of side effects and potential adverse reactions over time. While muscimol is generally considered to have a favorable safety profile, prolonged exposure may lead to unexpected physiological or psychological effects. Researchers must implement robust monitoring systems to detect and address any emerging issues promptly, which can be resource-intensive and complex in long-term studies.
The ethical considerations surrounding extended psychotropic substance administration pose additional hurdles. Institutional review boards and ethics committees may have concerns about the long-term impact on participants' mental health and cognitive function. This necessitates the development of comprehensive safety protocols and exit strategies for study participants, adding layers of complexity to study design and execution.
Data collection and analysis in longitudinal muscimol studies present their own set of challenges. The need for consistent, standardized assessment methods across multiple time points is crucial but can be difficult to maintain, especially when dealing with subjective measures of psychological states. Furthermore, the potential for participant attrition over extended study periods can compromise data integrity and statistical power, requiring careful planning and oversampling strategies.
Controlling for confounding variables becomes increasingly complex in longitudinal designs. External factors such as lifestyle changes, concurrent medications, or environmental stressors can significantly impact study outcomes. Researchers must develop sophisticated methods to account for these variables without compromising the study's focus on muscimol's effects.
Lastly, the financial and logistical demands of conducting long-term studies on muscimol are substantial. Securing funding for extended research periods, maintaining dedicated research staff, and ensuring consistent access to high-quality muscimol supplies over years can be daunting tasks. These practical considerations often limit the scope and duration of longitudinal studies, potentially constraining the depth of knowledge that can be gained about muscimol's long-term psychotropic effects.
Current Muscimol Analysis Techniques
01 Pharmaceutical compositions containing muscimol
Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for treating neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.- Pharmaceutical compositions containing muscimol: Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include muscimol as an active ingredient, often in combination with other compounds or excipients. The formulations are designed to treat neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.
- Methods of administering muscimol: Various methods for administering muscimol have been developed, including oral, topical, and parenteral routes. Some approaches focus on targeted delivery to specific areas of the body, such as the brain or central nervous system. Novel delivery systems may be used to enhance bioavailability or control release of the compound.
- Muscimol derivatives and analogs: Research has been conducted on developing muscimol derivatives and analogs with improved pharmacological properties. These modified compounds may have enhanced potency, selectivity, or reduced side effects compared to muscimol itself. Some derivatives are designed to cross the blood-brain barrier more effectively or to have longer-lasting effects.
- Use of muscimol in combination therapies: Muscimol is often used in combination with other therapeutic agents to enhance treatment efficacy. These combination therapies may target multiple aspects of a disorder or condition, potentially leading to synergistic effects. The combinations are explored for various indications, including neurological and psychiatric disorders.
- Muscimol in neurostimulation and neuromodulation: Muscimol is investigated for its potential in neurostimulation and neuromodulation techniques. It may be used in conjunction with electrical or optical stimulation methods to influence neural activity. These approaches are explored for treating neurological disorders, pain management, and other conditions affecting the nervous system.
02 Muscimol analogs and derivatives
Research focuses on developing and synthesizing muscimol analogs and derivatives. These modified compounds aim to improve upon the properties of muscimol, such as enhanced potency, selectivity, or reduced side effects. The analogs may be designed to target specific GABA receptor subtypes or to have improved pharmacokinetic profiles.Expand Specific Solutions03 Use of muscimol in neurostimulation therapies
Muscimol is explored in combination with neurostimulation techniques for treating neurological and psychiatric disorders. This approach may involve the use of muscimol to enhance or modulate the effects of electrical or magnetic stimulation of the brain, potentially improving outcomes in conditions such as depression or epilepsy.Expand Specific Solutions04 Muscimol in combination therapies
Muscimol is investigated as part of combination therapies with other active compounds. These combinations may target multiple pathways or receptors simultaneously, potentially leading to synergistic effects in treating various disorders. The combinations could include other GABA modulators, antidepressants, or anxiolytics.Expand Specific Solutions05 Novel delivery systems for muscimol
Innovative delivery systems are being developed to improve the administration and efficacy of muscimol. These may include nanoparticle formulations, transdermal patches, or controlled-release mechanisms. The goal is to enhance the bioavailability of muscimol, reduce dosing frequency, or target specific areas of the body or brain.Expand Specific Solutions
Key Players in Psychotropic Analysis
The field of longitudinal studies on muscimol in psychotropic analysis is in an early developmental stage, characterized by growing interest but limited market size. The technology's maturity is still evolving, with key players like ACADIA Pharmaceuticals, Cybin IRL, and Mindset Pharma leading research efforts. Established pharmaceutical giants such as Novartis AG and Eli Lilly & Co. are also exploring this area, indicating its potential significance. Academic institutions like Vanderbilt University and The Scripps Research Institute contribute valuable research, fostering collaboration between industry and academia. As the field progresses, it is likely to attract more investment and expand its market reach, particularly in neurological and psychiatric applications.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has developed a novel approach to studying muscimol's effects in longitudinal psychotropic analysis. Their research focuses on the long-term impact of muscimol, a GABA-A receptor agonist, on neurotransmitter systems and behavior. The company utilizes advanced neuroimaging techniques, including PET and fMRI, to track changes in brain activity over extended periods[1]. They have also implemented a proprietary algorithm to analyze longitudinal data, allowing for the detection of subtle changes in neurotransmitter levels and behavioral patterns[3]. ACADIA's studies incorporate regular assessments of cognitive function, mood, and anxiety levels to provide a comprehensive understanding of muscimol's psychotropic effects over time[5].
Strengths: Comprehensive approach combining neuroimaging and behavioral assessments. Advanced data analysis techniques for detecting subtle changes. Weaknesses: Potential for long-term side effects, limited generalizability to diverse populations.
Cybin IRL Ltd.
Technical Solution: Cybin IRL Ltd. has pioneered a unique longitudinal study design for muscimol in psychotropic analysis, focusing on its potential as a novel therapeutic for mental health disorders. Their approach involves a controlled, gradual dosing regimen over an extended period, typically 6-12 months[2]. The company utilizes cutting-edge biomarker analysis, including measurements of neuroplasticity markers and inflammatory cytokines, to track the biological effects of muscimol over time[4]. Cybin's research also incorporates regular psychometric assessments and qualitative interviews to capture the subjective experiences of participants throughout the study duration[6]. This multi-faceted approach allows for a comprehensive understanding of muscimol's long-term effects on both brain physiology and psychological well-being.
Strengths: Comprehensive biomarker and psychological assessment approach. Extended study duration for capturing long-term effects. Weaknesses: Potential for participant attrition in long-term studies, challenges in controlling for external factors over extended periods.
Innovative Muscimol Research Methods
Psychotropic drug screening device based on long-term photoconductive stimulation of neurons
PatentInactiveUS20060292549A1
Innovation
- A method using photoconductive stimulation of neuronal cultures grown on silicon dies, allowing for non-invasive, long-term stimulation and testing of psychotropic compounds, which includes a silicon die with a surface for neuronal growth, a light source for selective stimulation, and control circuitry to apply voltage, enabling the assessment of compound effects on synaptic transmission over extended periods.
Antidepressant-psilocybin co-treatment to assist psychotherapy
PatentWO2022259046A1
Innovation
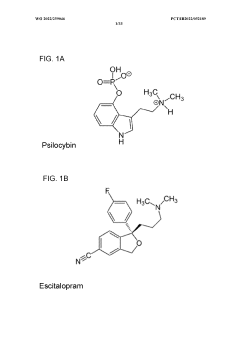
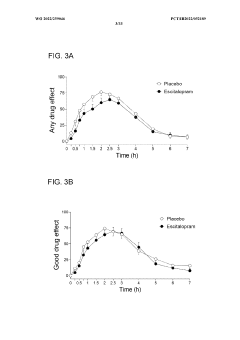
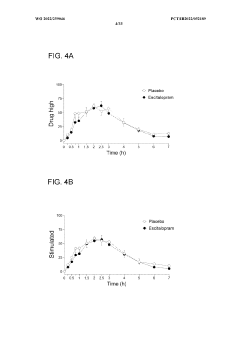
- Administering an antidepressant, such as escitalopram, before psilocybin to enhance positive psychological effects and reduce negative acute effects, by pretreating with the antidepressant and then administering psilocybin, which stimulates serotonin receptors and inhibits serotonin transport.
Ethical Considerations in Longitudinal Studies
Longitudinal studies on muscimol in psychotropic analysis present unique ethical challenges that require careful consideration throughout the research process. The extended duration of these studies, combined with the potent psychoactive effects of muscimol, necessitates a robust ethical framework to protect participants and ensure scientific integrity.
One primary ethical concern is the potential for long-term psychological effects on study participants. Muscimol, as a GABA receptor agonist, can induce altered states of consciousness and may have lasting impacts on cognitive function and mental health. Researchers must implement comprehensive screening procedures to exclude individuals with pre-existing mental health conditions or those at high risk for adverse reactions.
Informed consent is a critical ethical component in these studies. Participants must be fully aware of the potential risks, including both short-term and long-term effects of muscimol exposure. The consent process should be ongoing, allowing participants to withdraw at any point without consequence. Additionally, researchers should provide clear information about the study's duration and the commitment required from participants.
Privacy and confidentiality are paramount in longitudinal psychotropic research. As data is collected over extended periods, robust safeguards must be in place to protect participants' personal information and research data. This includes secure data storage systems, anonymization protocols, and strict access controls to prevent unauthorized use or disclosure of sensitive information.
The potential for dependency or addiction is another significant ethical consideration. While muscimol is not typically associated with high addiction potential, repeated exposure over time may lead to psychological dependence or altered reward pathways. Researchers must closely monitor participants for signs of dependence and have protocols in place for intervention and support if needed.
Ethical guidelines must also address the management of adverse events and long-term follow-up care. This includes establishing clear protocols for handling acute reactions during study sessions and providing ongoing support and monitoring after the study concludes. Long-term follow-up is essential to identify and address any delayed effects that may emerge months or years after the study's completion.
Lastly, the ethical use of research findings must be considered. Results from longitudinal studies on muscimol could have significant implications for drug development and mental health treatment. Researchers have a responsibility to ensure that their findings are disseminated accurately and used ethically, avoiding misrepresentation or exploitation for commercial gain at the expense of participant well-being.
One primary ethical concern is the potential for long-term psychological effects on study participants. Muscimol, as a GABA receptor agonist, can induce altered states of consciousness and may have lasting impacts on cognitive function and mental health. Researchers must implement comprehensive screening procedures to exclude individuals with pre-existing mental health conditions or those at high risk for adverse reactions.
Informed consent is a critical ethical component in these studies. Participants must be fully aware of the potential risks, including both short-term and long-term effects of muscimol exposure. The consent process should be ongoing, allowing participants to withdraw at any point without consequence. Additionally, researchers should provide clear information about the study's duration and the commitment required from participants.
Privacy and confidentiality are paramount in longitudinal psychotropic research. As data is collected over extended periods, robust safeguards must be in place to protect participants' personal information and research data. This includes secure data storage systems, anonymization protocols, and strict access controls to prevent unauthorized use or disclosure of sensitive information.
The potential for dependency or addiction is another significant ethical consideration. While muscimol is not typically associated with high addiction potential, repeated exposure over time may lead to psychological dependence or altered reward pathways. Researchers must closely monitor participants for signs of dependence and have protocols in place for intervention and support if needed.
Ethical guidelines must also address the management of adverse events and long-term follow-up care. This includes establishing clear protocols for handling acute reactions during study sessions and providing ongoing support and monitoring after the study concludes. Long-term follow-up is essential to identify and address any delayed effects that may emerge months or years after the study's completion.
Lastly, the ethical use of research findings must be considered. Results from longitudinal studies on muscimol could have significant implications for drug development and mental health treatment. Researchers have a responsibility to ensure that their findings are disseminated accurately and used ethically, avoiding misrepresentation or exploitation for commercial gain at the expense of participant well-being.
Regulatory Framework for Psychotropic Research
The regulatory framework for psychotropic research involving longitudinal studies on muscimol is complex and multifaceted, requiring careful navigation to ensure compliance and ethical conduct. At the international level, the United Nations Convention on Psychotropic Substances of 1971 provides the overarching guidelines for the control of psychotropic substances, including muscimol. This treaty establishes a classification system for psychotropic substances and outlines the requirements for their research, manufacture, and distribution.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing psychotropic research. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for reviewing and approving clinical trials involving psychotropic substances. Researchers must obtain an Investigational New Drug (IND) application before conducting human trials with muscimol. Additionally, the Drug Enforcement Administration (DEA) regulates the use of controlled substances in research, requiring researchers to obtain appropriate licenses and registrations.
The European Medicines Agency (EMA) provides guidance for conducting clinical trials in the European Union, including those involving psychotropic substances. The EMA's Committee for Medicinal Products for Human Use (CHMP) offers scientific advice on the design and conduct of clinical trials, ensuring that research protocols meet rigorous standards of safety and efficacy.
Ethical considerations are paramount in psychotropic research, particularly in longitudinal studies. Institutional Review Boards (IRBs) or Ethics Committees play a critical role in reviewing and approving research protocols. These bodies ensure that studies adhere to principles outlined in the Declaration of Helsinki and the Belmont Report, focusing on respect for persons, beneficence, and justice.
Data protection regulations, such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US, impose strict requirements on the handling of sensitive personal data collected during longitudinal studies. Researchers must implement robust data management systems to ensure participant privacy and data security throughout the study duration.
Specific to longitudinal studies, regulatory bodies often require detailed plans for long-term follow-up and monitoring of participants. This includes protocols for adverse event reporting, interim analyses, and study termination criteria. The International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) guidelines provide a unified standard for designing, conducting, recording, and reporting clinical trials involving human subjects.
In conclusion, navigating the regulatory landscape for longitudinal studies on muscimol in psychotropic analysis requires a comprehensive understanding of international treaties, national regulations, ethical guidelines, and data protection laws. Researchers must engage with multiple regulatory bodies and adhere to stringent protocols to ensure the scientific validity, ethical integrity, and legal compliance of their studies.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing psychotropic research. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for reviewing and approving clinical trials involving psychotropic substances. Researchers must obtain an Investigational New Drug (IND) application before conducting human trials with muscimol. Additionally, the Drug Enforcement Administration (DEA) regulates the use of controlled substances in research, requiring researchers to obtain appropriate licenses and registrations.
The European Medicines Agency (EMA) provides guidance for conducting clinical trials in the European Union, including those involving psychotropic substances. The EMA's Committee for Medicinal Products for Human Use (CHMP) offers scientific advice on the design and conduct of clinical trials, ensuring that research protocols meet rigorous standards of safety and efficacy.
Ethical considerations are paramount in psychotropic research, particularly in longitudinal studies. Institutional Review Boards (IRBs) or Ethics Committees play a critical role in reviewing and approving research protocols. These bodies ensure that studies adhere to principles outlined in the Declaration of Helsinki and the Belmont Report, focusing on respect for persons, beneficence, and justice.
Data protection regulations, such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US, impose strict requirements on the handling of sensitive personal data collected during longitudinal studies. Researchers must implement robust data management systems to ensure participant privacy and data security throughout the study duration.
Specific to longitudinal studies, regulatory bodies often require detailed plans for long-term follow-up and monitoring of participants. This includes protocols for adverse event reporting, interim analyses, and study termination criteria. The International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) guidelines provide a unified standard for designing, conducting, recording, and reporting clinical trials involving human subjects.
In conclusion, navigating the regulatory landscape for longitudinal studies on muscimol in psychotropic analysis requires a comprehensive understanding of international treaties, national regulations, ethical guidelines, and data protection laws. Researchers must engage with multiple regulatory bodies and adhere to stringent protocols to ensure the scientific validity, ethical integrity, and legal compliance of their studies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!