Muriatic Acid's Role in the Production of Butadiene
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Butadiene Production Overview and Objectives
Butadiene, a crucial chemical compound in the production of synthetic rubber and various polymers, has been a cornerstone of the petrochemical industry for decades. The production of butadiene has evolved significantly since its initial discovery in the early 20th century, with continuous advancements in technology and processes aimed at improving efficiency and yield.
The primary objective in butadiene production is to achieve high purity and yield while minimizing energy consumption and environmental impact. Traditionally, butadiene has been produced as a by-product of ethylene production through steam cracking of naphtha or gas oil. However, the increasing demand for butadiene has led to the development of on-purpose production methods.
One of the key technologies in butadiene production is the oxidative dehydrogenation of n-butenes, where muriatic acid, also known as hydrochloric acid, plays a crucial role. This process involves the catalytic conversion of n-butenes to butadiene in the presence of oxygen and a catalyst. Muriatic acid is used in the catalyst preparation and regeneration stages, contributing to the overall efficiency of the process.
The use of muriatic acid in butadiene production aligns with the industry's goals of improving selectivity and reducing energy consumption. By optimizing the catalyst composition and reaction conditions, manufacturers can achieve higher butadiene yields while minimizing unwanted side reactions. This not only improves the economic viability of the process but also reduces the environmental footprint of butadiene production.
As the demand for butadiene continues to grow, driven by the expanding automotive and construction industries, there is a pressing need for more efficient and sustainable production methods. The role of muriatic acid in these processes is expected to evolve, with ongoing research focused on developing novel catalysts and reaction systems that can further enhance the selectivity and yield of butadiene production.
The objectives of current research and development efforts in butadiene production include:
1. Improving catalyst performance and longevity
2. Enhancing process integration to reduce energy consumption
3. Developing alternative feedstocks to reduce dependence on fossil fuels
4. Minimizing waste generation and environmental impact
5. Exploring new applications for butadiene in emerging markets
These objectives are driving innovation in the field, with a particular focus on the role of muriatic acid in catalyst systems and process optimization. As the industry moves towards more sustainable practices, the integration of green chemistry principles in butadiene production is becoming increasingly important, with muriatic acid playing a key role in enabling these advancements.
The primary objective in butadiene production is to achieve high purity and yield while minimizing energy consumption and environmental impact. Traditionally, butadiene has been produced as a by-product of ethylene production through steam cracking of naphtha or gas oil. However, the increasing demand for butadiene has led to the development of on-purpose production methods.
One of the key technologies in butadiene production is the oxidative dehydrogenation of n-butenes, where muriatic acid, also known as hydrochloric acid, plays a crucial role. This process involves the catalytic conversion of n-butenes to butadiene in the presence of oxygen and a catalyst. Muriatic acid is used in the catalyst preparation and regeneration stages, contributing to the overall efficiency of the process.
The use of muriatic acid in butadiene production aligns with the industry's goals of improving selectivity and reducing energy consumption. By optimizing the catalyst composition and reaction conditions, manufacturers can achieve higher butadiene yields while minimizing unwanted side reactions. This not only improves the economic viability of the process but also reduces the environmental footprint of butadiene production.
As the demand for butadiene continues to grow, driven by the expanding automotive and construction industries, there is a pressing need for more efficient and sustainable production methods. The role of muriatic acid in these processes is expected to evolve, with ongoing research focused on developing novel catalysts and reaction systems that can further enhance the selectivity and yield of butadiene production.
The objectives of current research and development efforts in butadiene production include:
1. Improving catalyst performance and longevity
2. Enhancing process integration to reduce energy consumption
3. Developing alternative feedstocks to reduce dependence on fossil fuels
4. Minimizing waste generation and environmental impact
5. Exploring new applications for butadiene in emerging markets
These objectives are driving innovation in the field, with a particular focus on the role of muriatic acid in catalyst systems and process optimization. As the industry moves towards more sustainable practices, the integration of green chemistry principles in butadiene production is becoming increasingly important, with muriatic acid playing a key role in enabling these advancements.
Market Analysis for Butadiene and Muriatic Acid
The global market for butadiene and muriatic acid (hydrochloric acid) is closely intertwined with various industrial sectors, particularly the petrochemical and polymer industries. Butadiene, a key component in synthetic rubber production, has witnessed fluctuating demand patterns in recent years. The automotive industry, a major consumer of butadiene-derived products, has been a significant driver of market growth. However, the shift towards electric vehicles may potentially impact long-term demand for certain rubber products.
The Asia-Pacific region, led by China and India, continues to dominate the butadiene market due to rapid industrialization and increasing automotive production. North America and Europe maintain stable market shares, primarily driven by the tire and polymer industries. The Middle East has emerged as a growing market, capitalizing on its petrochemical infrastructure.
Muriatic acid, while not directly used in butadiene production, plays a crucial role in various industrial processes that support the overall petrochemical industry. Its market is more diversified, with applications ranging from steel pickling to water treatment. The construction industry's growth, particularly in developing economies, has bolstered the demand for muriatic acid in recent years.
The pricing dynamics of both butadiene and muriatic acid are heavily influenced by raw material costs and energy prices. Butadiene prices have shown volatility, largely due to fluctuations in crude oil prices and naphtha cracking economics. Muriatic acid prices, while generally more stable, can be affected by changes in chlor-alkali production rates and industrial demand cycles.
Environmental regulations have become increasingly stringent, impacting both markets. For butadiene, there's a growing emphasis on developing bio-based alternatives to reduce reliance on fossil fuels. The muriatic acid market faces challenges related to handling and disposal regulations, driving innovations in safer production and application methods.
The COVID-19 pandemic temporarily disrupted supply chains and demand patterns for both chemicals. However, the markets have shown resilience, with recovery trends observed as industrial activities resumed. The long-term outlook for both butadiene and muriatic acid remains cautiously optimistic, with growth opportunities in emerging markets and potential for technological advancements in production processes and applications.
The Asia-Pacific region, led by China and India, continues to dominate the butadiene market due to rapid industrialization and increasing automotive production. North America and Europe maintain stable market shares, primarily driven by the tire and polymer industries. The Middle East has emerged as a growing market, capitalizing on its petrochemical infrastructure.
Muriatic acid, while not directly used in butadiene production, plays a crucial role in various industrial processes that support the overall petrochemical industry. Its market is more diversified, with applications ranging from steel pickling to water treatment. The construction industry's growth, particularly in developing economies, has bolstered the demand for muriatic acid in recent years.
The pricing dynamics of both butadiene and muriatic acid are heavily influenced by raw material costs and energy prices. Butadiene prices have shown volatility, largely due to fluctuations in crude oil prices and naphtha cracking economics. Muriatic acid prices, while generally more stable, can be affected by changes in chlor-alkali production rates and industrial demand cycles.
Environmental regulations have become increasingly stringent, impacting both markets. For butadiene, there's a growing emphasis on developing bio-based alternatives to reduce reliance on fossil fuels. The muriatic acid market faces challenges related to handling and disposal regulations, driving innovations in safer production and application methods.
The COVID-19 pandemic temporarily disrupted supply chains and demand patterns for both chemicals. However, the markets have shown resilience, with recovery trends observed as industrial activities resumed. The long-term outlook for both butadiene and muriatic acid remains cautiously optimistic, with growth opportunities in emerging markets and potential for technological advancements in production processes and applications.
Technical Challenges in Butadiene Synthesis
The synthesis of butadiene faces several technical challenges, primarily due to the complex nature of the production process and the involvement of muriatic acid (hydrochloric acid) as a key component. One of the main difficulties lies in controlling the reaction conditions to optimize yield and selectivity. The dehydrogenation of n-butane or butenes to butadiene requires precise temperature and pressure control, as well as the use of specific catalysts. Maintaining these conditions consistently across large-scale industrial production can be problematic.
Another significant challenge is the corrosive nature of muriatic acid, which can lead to equipment degradation and potential safety hazards. This necessitates the use of specialized materials and equipment designs that can withstand prolonged exposure to the acid. Additionally, the handling and storage of large quantities of muriatic acid pose logistical and safety concerns that must be carefully managed.
The purification of butadiene from the reaction mixture presents its own set of challenges. The separation of butadiene from other C4 hydrocarbons and byproducts requires sophisticated distillation and extraction processes. The presence of muriatic acid in the mixture complicates this separation, as it can form azeotropes with certain components, making traditional distillation methods less effective.
Environmental concerns also pose significant technical challenges in butadiene production. The use of muriatic acid and the generation of chlorinated byproducts necessitate stringent emission control and waste treatment processes. Developing efficient methods for neutralizing and disposing of acid waste, as well as capturing and treating volatile organic compounds, is crucial for meeting environmental regulations.
Energy efficiency is another area of technical difficulty. The endothermic nature of the dehydrogenation reaction requires substantial energy input, which impacts the overall economics of the process. Improving heat integration and developing more efficient catalysts are ongoing challenges in optimizing the energy consumption of butadiene production.
Catalyst development and management present additional technical hurdles. The catalysts used in butadiene synthesis must be highly selective and resistant to deactivation in the presence of muriatic acid. Developing catalysts that maintain their activity over extended periods and can be easily regenerated is a key focus of research in this field.
Lastly, process control and automation pose significant challenges in butadiene production. The complexity of the reaction system, coupled with the need for precise control of multiple parameters, requires sophisticated monitoring and control systems. Implementing advanced process control strategies, such as model predictive control, to optimize production while ensuring safety and product quality is an ongoing area of development in the industry.
Another significant challenge is the corrosive nature of muriatic acid, which can lead to equipment degradation and potential safety hazards. This necessitates the use of specialized materials and equipment designs that can withstand prolonged exposure to the acid. Additionally, the handling and storage of large quantities of muriatic acid pose logistical and safety concerns that must be carefully managed.
The purification of butadiene from the reaction mixture presents its own set of challenges. The separation of butadiene from other C4 hydrocarbons and byproducts requires sophisticated distillation and extraction processes. The presence of muriatic acid in the mixture complicates this separation, as it can form azeotropes with certain components, making traditional distillation methods less effective.
Environmental concerns also pose significant technical challenges in butadiene production. The use of muriatic acid and the generation of chlorinated byproducts necessitate stringent emission control and waste treatment processes. Developing efficient methods for neutralizing and disposing of acid waste, as well as capturing and treating volatile organic compounds, is crucial for meeting environmental regulations.
Energy efficiency is another area of technical difficulty. The endothermic nature of the dehydrogenation reaction requires substantial energy input, which impacts the overall economics of the process. Improving heat integration and developing more efficient catalysts are ongoing challenges in optimizing the energy consumption of butadiene production.
Catalyst development and management present additional technical hurdles. The catalysts used in butadiene synthesis must be highly selective and resistant to deactivation in the presence of muriatic acid. Developing catalysts that maintain their activity over extended periods and can be easily regenerated is a key focus of research in this field.
Lastly, process control and automation pose significant challenges in butadiene production. The complexity of the reaction system, coupled with the need for precise control of multiple parameters, requires sophisticated monitoring and control systems. Implementing advanced process control strategies, such as model predictive control, to optimize production while ensuring safety and product quality is an ongoing area of development in the industry.
Current Muriatic Acid-Based Butadiene Production Processes
01 Production and purification of muriatic acid
Muriatic acid, also known as hydrochloric acid, can be produced and purified through various industrial processes. These methods often involve the reaction of chlorine with hydrogen or the treatment of chloride salts with sulfuric acid. Purification techniques may include distillation or membrane separation to remove impurities and achieve desired concentrations.- Production and purification of muriatic acid: Muriatic acid, also known as hydrochloric acid, can be produced and purified through various industrial processes. These methods often involve the reaction of chlorine with hydrogen or the treatment of salt (sodium chloride) with sulfuric acid. Purification techniques may include distillation or membrane separation to remove impurities and achieve the desired concentration.
- Applications in metal treatment and surface cleaning: Muriatic acid is widely used in metal treatment processes, such as pickling, etching, and surface cleaning. It can effectively remove rust, scale, and other contaminants from metal surfaces. The acid is particularly useful in preparing metal surfaces for further treatment, such as painting or electroplating.
- Use in construction and building materials: Muriatic acid finds applications in the construction industry, particularly in the treatment and cleaning of concrete, brick, and other masonry surfaces. It can be used to remove efflorescence, stains, and mineral deposits. Additionally, the acid is employed in the production of certain building materials and in the preparation of surfaces for renovation or restoration work.
- Environmental and safety considerations: Handling and disposal of muriatic acid require careful consideration of environmental and safety factors. Proper storage, transportation, and use of the acid are essential to prevent accidents and minimize environmental impact. Neutralization techniques and specialized equipment may be employed to manage acid waste and emissions. Safety measures include the use of protective gear and proper ventilation in work areas.
- Industrial and chemical processing applications: Muriatic acid is a versatile chemical used in various industrial processes and chemical manufacturing. It serves as a reagent in the production of other chemicals, as a pH regulator in water treatment, and as a catalyst in certain reactions. The acid is also utilized in the food industry for processing and in the pharmaceutical sector for drug synthesis and purification.
02 Applications in metal treatment and surface cleaning
Muriatic acid is widely used in metal treatment processes, such as pickling, etching, and surface cleaning. It effectively removes rust, scale, and other contaminants from metal surfaces, preparing them for further processing or coating. The acid's strong reactivity makes it suitable for various industrial cleaning applications.Expand Specific Solutions03 Use in construction and building materials
In the construction industry, muriatic acid is utilized for cleaning masonry, concrete, and other building materials. It can remove efflorescence, mortar residues, and stains from surfaces. Additionally, it is used in the production of certain construction materials and in the treatment of swimming pool water to maintain proper pH levels.Expand Specific Solutions04 Environmental and safety considerations
Handling and disposal of muriatic acid require careful attention to environmental and safety regulations. Proper storage, transportation, and use of protective equipment are essential. Neutralization techniques and specialized waste treatment processes are employed to mitigate the environmental impact of acid disposal and to ensure worker safety.Expand Specific Solutions05 Innovations in muriatic acid formulations
Recent developments in muriatic acid formulations focus on improving its effectiveness and safety for specific applications. These innovations include the addition of corrosion inhibitors, surfactants, or other additives to enhance performance in cleaning or industrial processes. Some formulations aim to reduce fumes or improve the acid's stability for prolonged storage and use.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The production of butadiene using muriatic acid is at a mature stage in the chemical industry, with a significant global market size. The technology has been well-established, with major players like BASF, Reliance Industries, and China Petroleum & Chemical Corp. leading the field. The market is characterized by ongoing research and development efforts, as evidenced by the involvement of academic institutions such as Nanjing Tech University and Beijing University of Chemical Technology. Companies like Genomatica are exploring bio-based alternatives, indicating a shift towards more sustainable production methods. The competitive landscape is diverse, with petrochemical giants, specialized chemical companies, and research institutions all contributing to advancements in butadiene production technology.
Genomatica, Inc.
Technical Solution: Genomatica, Inc. has developed a bio-based approach to butadiene production that incorporates muriatic acid in a novel fermentation process. Their technology uses genetically engineered microorganisms to convert renewable feedstocks into 2,3-butanediol, which is then dehydrated using muriatic acid as a catalyst. This bio-catalytic route achieves a butadiene yield of up to 85% from the 2,3-butanediol intermediate [7]. Genomatica's process integrates advanced fermentation control systems with efficient downstream processing, resulting in a 40% reduction in carbon footprint compared to petroleum-based butadiene production [8]. The company has also developed a proprietary acid recovery system that enables the recycling of over 95% of the muriatic acid used in the process.
Strengths: High yield, renewable feedstock, significantly reduced carbon footprint. Weaknesses: Potential scalability challenges, sensitivity to feedstock quality and availability.
UOP LLC
Technical Solution: UOP LLC has pioneered a breakthrough in butadiene production using muriatic acid as a key component in their Oxo-D™ process. This technology utilizes a unique oxychlorination reaction where muriatic acid is combined with oxygen and C4 hydrocarbons in the presence of a multifunctional catalyst. The process achieves a single-pass butadiene yield of up to 65% and offers significant advantages in terms of feedstock flexibility [5]. UOP's approach incorporates advanced reactor designs that optimize mass transfer and reaction kinetics, resulting in a 25% reduction in energy consumption compared to conventional steam cracking methods [6]. The company has also developed proprietary separation technologies that enable efficient recovery and recycling of muriatic acid, minimizing waste and improving process economics.
Strengths: High single-pass yield, feedstock flexibility, energy-efficient process. Weaknesses: Complex catalyst system may require frequent regeneration, potential safety concerns with oxychlorination reactions.
Innovative Approaches in Muriatic Acid Utilization
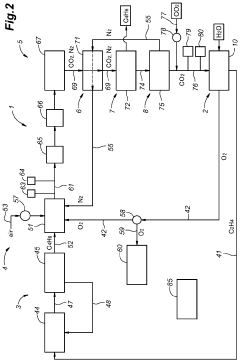
Integration of n-c4/n-c4=/bd separation system for on-purpose butadiene synthesis
PatentActiveUS20170204025A1
Innovation
- The process integrates a dehydrogenation unit, butane extraction columns, oxydehydrogenation units, and light gas separation units to efficiently generate and separate butene and butadiene streams, utilizing solvent extraction systems and recycling to maximize butadiene recovery, with specific steps including passing n-butane through dehydrogenation, butane extraction, and oxydehydrogenation units, and using cold-box condensing units for separation.
Manufacturing method of butadiene
PatentPendingUS20240158321A1
Innovation
- A manufacturing method that involves an electrolytic reduction process to produce ethylene and oxygen from carbon dioxide and water, followed by dimerization to form butene, and then oxidative dehydrogenation to produce butadiene, where the produced carbon dioxide is reused as a raw material in the electrolytic reduction process, reducing greenhouse gas emissions and raw material costs.
Environmental Impact and Sustainability Considerations
The production of butadiene using muriatic acid, also known as hydrochloric acid, raises significant environmental and sustainability concerns. The process involves the use of corrosive chemicals and generates potentially harmful byproducts, necessitating careful consideration of its ecological impact.
One of the primary environmental concerns is the potential for acid emissions. Muriatic acid is highly corrosive and can contribute to air pollution if not properly contained and managed. These emissions can lead to acid rain, which has detrimental effects on ecosystems, including soil acidification and damage to aquatic environments. To mitigate this risk, advanced scrubbing systems and emission control technologies must be implemented in production facilities.
Water pollution is another critical issue associated with butadiene production using muriatic acid. The process generates acidic wastewater that requires extensive treatment before discharge. If not properly managed, this wastewater can harm aquatic life and disrupt the pH balance of water bodies. Implementing closed-loop water systems and advanced wastewater treatment technologies is essential to minimize the environmental impact on local water resources.
The production process also raises concerns about resource consumption and energy efficiency. The synthesis of butadiene from muriatic acid requires significant energy inputs, contributing to greenhouse gas emissions if fossil fuels are the primary energy source. Improving process efficiency and transitioning to renewable energy sources for production facilities can help address these sustainability challenges.
Waste management is a crucial aspect of environmental considerations in butadiene production. The process generates various chemical byproducts and spent catalysts that require proper disposal or recycling. Developing effective waste management strategies, including the recovery and reuse of byproducts, is essential for minimizing the environmental footprint of the production process.
To enhance sustainability, the industry is exploring alternative production methods and greener chemistries. Bio-based routes for butadiene production and the development of less corrosive catalysts are areas of active research. These innovations aim to reduce the reliance on muriatic acid and minimize the environmental impact of butadiene production.
Regulatory compliance and industry standards play a vital role in addressing environmental concerns. Stringent regulations on emissions, waste disposal, and safety protocols are necessary to ensure responsible production practices. Companies must invest in continuous monitoring systems and implement best practices to meet or exceed environmental standards.
In conclusion, while muriatic acid plays a crucial role in butadiene production, the environmental and sustainability challenges it poses are significant. Addressing these concerns requires a multifaceted approach, combining technological innovations, process optimizations, and regulatory compliance to minimize the ecological footprint of butadiene production and move towards more sustainable manufacturing practices.
One of the primary environmental concerns is the potential for acid emissions. Muriatic acid is highly corrosive and can contribute to air pollution if not properly contained and managed. These emissions can lead to acid rain, which has detrimental effects on ecosystems, including soil acidification and damage to aquatic environments. To mitigate this risk, advanced scrubbing systems and emission control technologies must be implemented in production facilities.
Water pollution is another critical issue associated with butadiene production using muriatic acid. The process generates acidic wastewater that requires extensive treatment before discharge. If not properly managed, this wastewater can harm aquatic life and disrupt the pH balance of water bodies. Implementing closed-loop water systems and advanced wastewater treatment technologies is essential to minimize the environmental impact on local water resources.
The production process also raises concerns about resource consumption and energy efficiency. The synthesis of butadiene from muriatic acid requires significant energy inputs, contributing to greenhouse gas emissions if fossil fuels are the primary energy source. Improving process efficiency and transitioning to renewable energy sources for production facilities can help address these sustainability challenges.
Waste management is a crucial aspect of environmental considerations in butadiene production. The process generates various chemical byproducts and spent catalysts that require proper disposal or recycling. Developing effective waste management strategies, including the recovery and reuse of byproducts, is essential for minimizing the environmental footprint of the production process.
To enhance sustainability, the industry is exploring alternative production methods and greener chemistries. Bio-based routes for butadiene production and the development of less corrosive catalysts are areas of active research. These innovations aim to reduce the reliance on muriatic acid and minimize the environmental impact of butadiene production.
Regulatory compliance and industry standards play a vital role in addressing environmental concerns. Stringent regulations on emissions, waste disposal, and safety protocols are necessary to ensure responsible production practices. Companies must invest in continuous monitoring systems and implement best practices to meet or exceed environmental standards.
In conclusion, while muriatic acid plays a crucial role in butadiene production, the environmental and sustainability challenges it poses are significant. Addressing these concerns requires a multifaceted approach, combining technological innovations, process optimizations, and regulatory compliance to minimize the ecological footprint of butadiene production and move towards more sustainable manufacturing practices.
Regulatory Framework for Chemical Manufacturing
The regulatory framework for chemical manufacturing plays a crucial role in ensuring the safe and responsible production of butadiene, particularly concerning the use of muriatic acid in the process. Governments and international organizations have established comprehensive regulations to address the potential risks associated with handling hazardous chemicals and to promote environmental protection.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of chemical manufacturing through the Toxic Substances Control Act (TSCA). This act requires manufacturers to report information on the production, use, and potential environmental and health effects of chemicals. The Occupational Safety and Health Administration (OSHA) sets standards for workplace safety, including specific guidelines for handling muriatic acid and other hazardous substances used in butadiene production.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which aims to improve the protection of human health and the environment from the risks posed by chemicals. Under REACH, manufacturers must register chemicals and provide safety data, including information on the use of muriatic acid in butadiene production.
Many countries have adopted the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), which provides a standardized approach to communicating chemical hazards. This system ensures that workers and consumers have access to clear information about the dangers associated with chemicals used in manufacturing processes.
Specific regulations addressing the storage, transportation, and disposal of muriatic acid are also in place. These include requirements for proper containment, labeling, and handling procedures to prevent spills and accidents. Additionally, regulations often mandate the use of personal protective equipment (PPE) for workers involved in the production process.
Environmental regulations play a significant role in the chemical manufacturing industry. Emission standards and waste management requirements are designed to minimize the environmental impact of butadiene production. Many jurisdictions require manufacturers to obtain permits and conduct regular environmental impact assessments to ensure compliance with air and water quality standards.
The regulatory landscape is continually evolving, with increasing focus on sustainability and circular economy principles. This has led to the development of regulations promoting the use of greener chemistry and more environmentally friendly production methods. Manufacturers are encouraged to explore alternative processes that reduce the reliance on hazardous substances like muriatic acid or implement more efficient recycling and recovery systems.
Compliance with these regulations is essential for chemical manufacturers, not only to avoid legal penalties but also to maintain public trust and ensure the long-term viability of their operations. As such, companies involved in butadiene production must stay informed about regulatory changes and invest in technologies and practices that meet or exceed current standards.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of chemical manufacturing through the Toxic Substances Control Act (TSCA). This act requires manufacturers to report information on the production, use, and potential environmental and health effects of chemicals. The Occupational Safety and Health Administration (OSHA) sets standards for workplace safety, including specific guidelines for handling muriatic acid and other hazardous substances used in butadiene production.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which aims to improve the protection of human health and the environment from the risks posed by chemicals. Under REACH, manufacturers must register chemicals and provide safety data, including information on the use of muriatic acid in butadiene production.
Many countries have adopted the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), which provides a standardized approach to communicating chemical hazards. This system ensures that workers and consumers have access to clear information about the dangers associated with chemicals used in manufacturing processes.
Specific regulations addressing the storage, transportation, and disposal of muriatic acid are also in place. These include requirements for proper containment, labeling, and handling procedures to prevent spills and accidents. Additionally, regulations often mandate the use of personal protective equipment (PPE) for workers involved in the production process.
Environmental regulations play a significant role in the chemical manufacturing industry. Emission standards and waste management requirements are designed to minimize the environmental impact of butadiene production. Many jurisdictions require manufacturers to obtain permits and conduct regular environmental impact assessments to ensure compliance with air and water quality standards.
The regulatory landscape is continually evolving, with increasing focus on sustainability and circular economy principles. This has led to the development of regulations promoting the use of greener chemistry and more environmentally friendly production methods. Manufacturers are encouraged to explore alternative processes that reduce the reliance on hazardous substances like muriatic acid or implement more efficient recycling and recovery systems.
Compliance with these regulations is essential for chemical manufacturers, not only to avoid legal penalties but also to maintain public trust and ensure the long-term viability of their operations. As such, companies involved in butadiene production must stay informed about regulatory changes and invest in technologies and practices that meet or exceed current standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!