Muriatic Acid's Role in the Production of Polyphenylene Ether (PPE)
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PPE Production Overview
Polyphenylene Ether (PPE) is a high-performance engineering thermoplastic known for its exceptional heat resistance, dimensional stability, and electrical insulation properties. The production of PPE involves a complex oxidative coupling polymerization process, where muriatic acid, also known as hydrochloric acid (HCl), plays a crucial role.
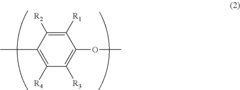
The synthesis of PPE typically begins with the oxidative coupling of 2,6-dimethylphenol monomers in the presence of a catalyst system. This reaction is carried out in an organic solvent, usually toluene or xylene, with oxygen as the oxidizing agent. The catalyst system commonly consists of a copper salt and an amine ligand, which work together to facilitate the formation of carbon-oxygen bonds between the phenol units.
Muriatic acid enters the production process at several key stages. Firstly, it is used to prepare the copper catalyst by dissolving copper metal or copper salts. The acidic environment helps maintain the copper in its active form and prevents the formation of inactive copper complexes. Additionally, muriatic acid aids in controlling the pH of the reaction mixture, which is critical for optimizing the polymerization rate and achieving the desired molecular weight distribution of the PPE polymer.
During the polymerization reaction, muriatic acid also serves as a proton source, which is essential for the termination of growing polymer chains. This controlled termination helps regulate the molecular weight of the final PPE product, directly influencing its mechanical and thermal properties. Furthermore, the acid assists in the removal of residual catalyst and unreacted monomers from the polymer solution after the reaction is complete.
In the post-polymerization stages, muriatic acid is utilized in the purification and isolation of PPE. It helps in the precipitation of the polymer from the reaction mixture and aids in washing away impurities. The acid treatment also contributes to the stabilization of the polymer by removing any remaining reactive species that could lead to degradation during processing or use.
The concentration and quantity of muriatic acid used in PPE production must be carefully controlled to achieve the desired polymer properties and to minimize environmental impact. Excess acid can lead to unwanted side reactions or degradation of the polymer, while insufficient acid may result in incomplete polymerization or inadequate purification.
As environmental concerns grow, manufacturers are continuously exploring ways to optimize the use of muriatic acid in PPE production. This includes developing more efficient catalyst systems that require less acid, implementing closed-loop recycling systems for the acid, and investigating alternative acidic compounds that may offer similar benefits with reduced environmental footprint.
The synthesis of PPE typically begins with the oxidative coupling of 2,6-dimethylphenol monomers in the presence of a catalyst system. This reaction is carried out in an organic solvent, usually toluene or xylene, with oxygen as the oxidizing agent. The catalyst system commonly consists of a copper salt and an amine ligand, which work together to facilitate the formation of carbon-oxygen bonds between the phenol units.
Muriatic acid enters the production process at several key stages. Firstly, it is used to prepare the copper catalyst by dissolving copper metal or copper salts. The acidic environment helps maintain the copper in its active form and prevents the formation of inactive copper complexes. Additionally, muriatic acid aids in controlling the pH of the reaction mixture, which is critical for optimizing the polymerization rate and achieving the desired molecular weight distribution of the PPE polymer.
During the polymerization reaction, muriatic acid also serves as a proton source, which is essential for the termination of growing polymer chains. This controlled termination helps regulate the molecular weight of the final PPE product, directly influencing its mechanical and thermal properties. Furthermore, the acid assists in the removal of residual catalyst and unreacted monomers from the polymer solution after the reaction is complete.
In the post-polymerization stages, muriatic acid is utilized in the purification and isolation of PPE. It helps in the precipitation of the polymer from the reaction mixture and aids in washing away impurities. The acid treatment also contributes to the stabilization of the polymer by removing any remaining reactive species that could lead to degradation during processing or use.
The concentration and quantity of muriatic acid used in PPE production must be carefully controlled to achieve the desired polymer properties and to minimize environmental impact. Excess acid can lead to unwanted side reactions or degradation of the polymer, while insufficient acid may result in incomplete polymerization or inadequate purification.
As environmental concerns grow, manufacturers are continuously exploring ways to optimize the use of muriatic acid in PPE production. This includes developing more efficient catalyst systems that require less acid, implementing closed-loop recycling systems for the acid, and investigating alternative acidic compounds that may offer similar benefits with reduced environmental footprint.
Market Analysis for PPE
The global market for Polyphenylene Ether (PPE) has been experiencing steady growth, driven by increasing demand in various end-use industries such as automotive, electronics, and consumer goods. PPE's unique properties, including high heat resistance, dimensional stability, and excellent electrical insulation, make it a preferred material in these sectors.
In the automotive industry, PPE is widely used in under-hood components, electrical connectors, and exterior parts due to its ability to withstand high temperatures and maintain structural integrity. The growing trend towards electric vehicles has further boosted the demand for PPE, as it is essential in battery components and electrical systems.
The electronics sector represents another significant market for PPE, particularly in the production of circuit boards, connectors, and housings for electronic devices. With the rapid advancement of technology and the increasing miniaturization of electronic components, the demand for high-performance materials like PPE continues to rise.
Consumer goods, including household appliances and power tools, also contribute to the PPE market growth. The material's durability, heat resistance, and electrical insulation properties make it ideal for applications in these products, enhancing their performance and longevity.
Geographically, Asia-Pacific dominates the PPE market, with China and India being the major consumers. The region's robust manufacturing sector, particularly in electronics and automotive industries, drives this demand. North America and Europe follow, with significant consumption in advanced manufacturing and automotive sectors.
The PPE market faces challenges from environmental concerns and the push for more sustainable materials. However, ongoing research and development efforts are focused on improving the recyclability and sustainability of PPE, which could potentially open new market opportunities.
Key players in the PPE market include SABIC, Asahi Kasei Corporation, Mitsubishi Chemical Corporation, and Evonik Industries AG. These companies are investing in research and development to enhance PPE properties and expand its applications, further driving market growth.
The role of muriatic acid in PPE production is crucial, as it is used in the oxidative coupling process of 2,6-xylenol to form PPE. This process efficiency directly impacts the production costs and quality of PPE, influencing its market competitiveness. Innovations in this production process, particularly in the use and recovery of muriatic acid, could lead to significant advancements in PPE manufacturing, potentially affecting market dynamics and pricing strategies.
In the automotive industry, PPE is widely used in under-hood components, electrical connectors, and exterior parts due to its ability to withstand high temperatures and maintain structural integrity. The growing trend towards electric vehicles has further boosted the demand for PPE, as it is essential in battery components and electrical systems.
The electronics sector represents another significant market for PPE, particularly in the production of circuit boards, connectors, and housings for electronic devices. With the rapid advancement of technology and the increasing miniaturization of electronic components, the demand for high-performance materials like PPE continues to rise.
Consumer goods, including household appliances and power tools, also contribute to the PPE market growth. The material's durability, heat resistance, and electrical insulation properties make it ideal for applications in these products, enhancing their performance and longevity.
Geographically, Asia-Pacific dominates the PPE market, with China and India being the major consumers. The region's robust manufacturing sector, particularly in electronics and automotive industries, drives this demand. North America and Europe follow, with significant consumption in advanced manufacturing and automotive sectors.
The PPE market faces challenges from environmental concerns and the push for more sustainable materials. However, ongoing research and development efforts are focused on improving the recyclability and sustainability of PPE, which could potentially open new market opportunities.
Key players in the PPE market include SABIC, Asahi Kasei Corporation, Mitsubishi Chemical Corporation, and Evonik Industries AG. These companies are investing in research and development to enhance PPE properties and expand its applications, further driving market growth.
The role of muriatic acid in PPE production is crucial, as it is used in the oxidative coupling process of 2,6-xylenol to form PPE. This process efficiency directly impacts the production costs and quality of PPE, influencing its market competitiveness. Innovations in this production process, particularly in the use and recovery of muriatic acid, could lead to significant advancements in PPE manufacturing, potentially affecting market dynamics and pricing strategies.
Muriatic Acid Challenges
The use of muriatic acid in the production of Polyphenylene Ether (PPE) presents several significant challenges that need to be addressed for efficient and safe manufacturing processes. One of the primary concerns is the highly corrosive nature of muriatic acid, which can lead to equipment degradation and potential safety hazards in the production environment. This necessitates the use of specialized, acid-resistant materials for storage tanks, pipelines, and reaction vessels, significantly increasing production costs and maintenance requirements.
Another challenge lies in the precise control of acid concentration and purity. Muriatic acid used in PPE production must meet stringent quality standards to ensure consistent product quality. Variations in acid concentration can lead to inconsistencies in the polymerization process, affecting the final properties of the PPE. Maintaining the required purity levels and managing impurities in industrial-scale production can be technically demanding and resource-intensive.
The handling and transportation of muriatic acid pose additional challenges. Strict safety protocols must be implemented to prevent accidental spills or exposure, which can have severe environmental and health consequences. This includes specialized training for personnel, robust containment systems, and comprehensive emergency response plans. The regulatory compliance associated with the storage, use, and disposal of muriatic acid adds another layer of complexity to the production process.
Environmental concerns also present significant challenges in the use of muriatic acid for PPE production. The potential for acid emissions and the generation of acidic waste streams necessitate advanced treatment and neutralization processes. Implementing effective pollution control measures and adhering to increasingly stringent environmental regulations can be both technically challenging and costly for manufacturers.
Furthermore, the energy-intensive nature of processes involving muriatic acid contributes to the overall carbon footprint of PPE production. As industries face growing pressure to reduce their environmental impact, finding ways to optimize energy consumption and explore more sustainable alternatives to muriatic acid becomes a critical challenge.
Lastly, the volatility in the supply and pricing of muriatic acid can pose challenges for PPE manufacturers. Fluctuations in availability or cost can significantly impact production schedules and overall manufacturing economics. Developing strategies to mitigate supply chain risks and exploring alternative acid sources or production methods become important considerations for ensuring the stability and sustainability of PPE production processes.
Another challenge lies in the precise control of acid concentration and purity. Muriatic acid used in PPE production must meet stringent quality standards to ensure consistent product quality. Variations in acid concentration can lead to inconsistencies in the polymerization process, affecting the final properties of the PPE. Maintaining the required purity levels and managing impurities in industrial-scale production can be technically demanding and resource-intensive.
The handling and transportation of muriatic acid pose additional challenges. Strict safety protocols must be implemented to prevent accidental spills or exposure, which can have severe environmental and health consequences. This includes specialized training for personnel, robust containment systems, and comprehensive emergency response plans. The regulatory compliance associated with the storage, use, and disposal of muriatic acid adds another layer of complexity to the production process.
Environmental concerns also present significant challenges in the use of muriatic acid for PPE production. The potential for acid emissions and the generation of acidic waste streams necessitate advanced treatment and neutralization processes. Implementing effective pollution control measures and adhering to increasingly stringent environmental regulations can be both technically challenging and costly for manufacturers.
Furthermore, the energy-intensive nature of processes involving muriatic acid contributes to the overall carbon footprint of PPE production. As industries face growing pressure to reduce their environmental impact, finding ways to optimize energy consumption and explore more sustainable alternatives to muriatic acid becomes a critical challenge.
Lastly, the volatility in the supply and pricing of muriatic acid can pose challenges for PPE manufacturers. Fluctuations in availability or cost can significantly impact production schedules and overall manufacturing economics. Developing strategies to mitigate supply chain risks and exploring alternative acid sources or production methods become important considerations for ensuring the stability and sustainability of PPE production processes.
Current Muriatic Acid Use
01 Industrial applications of muriatic acid
Muriatic acid, also known as hydrochloric acid, has various industrial applications. It is used in metal cleaning and pickling processes, particularly in the steel industry. The acid is also employed in the production of chemicals, water treatment, and as a pH regulator in various industrial processes.- Industrial applications of muriatic acid: Muriatic acid, also known as hydrochloric acid, has various industrial applications. It is used in metal cleaning and pickling processes, particularly in the steel industry. The acid is also employed in the production of chemicals, water treatment, and as a pH regulator in various industrial processes.
- Cleaning and etching applications: Muriatic acid is widely used in cleaning and etching applications. It is effective in removing rust, scale, and other mineral deposits from surfaces. The acid is commonly used in household cleaning products, pool maintenance, and masonry cleaning. Its strong acidic properties make it suitable for etching concrete and other materials.
- Waste treatment and environmental applications: Muriatic acid plays a role in waste treatment and environmental applications. It is used in the treatment of industrial wastewater, neutralization of alkaline effluents, and in flue gas desulfurization processes. The acid also finds applications in soil remediation and as a pH adjuster in environmental monitoring.
- Chemical synthesis and production: Muriatic acid is an important reagent in chemical synthesis and production processes. It is used in the manufacture of various chemicals, including chlorides, dyes, and pharmaceuticals. The acid serves as a catalyst in organic synthesis reactions and is employed in the production of plastics and rubber compounds.
- Safety and handling of muriatic acid: Proper safety measures and handling procedures are crucial when working with muriatic acid. This includes the use of appropriate personal protective equipment, proper storage containers, and ventilation systems. Neutralization techniques and spill response protocols are important aspects of handling this corrosive substance safely in industrial and laboratory settings.
02 Cleaning and etching applications
Muriatic acid is widely used for cleaning and etching purposes. It is effective in removing rust, scale, and other deposits from metal surfaces. In the construction industry, it is used for cleaning masonry and concrete surfaces. The acid is also utilized in pool maintenance to adjust pH levels and remove stains.Expand Specific Solutions03 Production and handling of muriatic acid
The production of muriatic acid involves various processes, including the reaction of hydrogen chloride with water. Specialized equipment and safety measures are required for its production, storage, and handling due to its corrosive nature. Innovations in production methods focus on improving efficiency and reducing environmental impact.Expand Specific Solutions04 Environmental and safety considerations
Due to its corrosive nature, the use of muriatic acid requires strict safety protocols. Proper handling, storage, and disposal methods are essential to prevent environmental contamination and ensure worker safety. Innovations in this area include the development of safer formulations and improved containment systems.Expand Specific Solutions05 Alternative applications and formulations
Research is ongoing to develop alternative applications and formulations of muriatic acid. This includes its use in specialized chemical processes, such as the production of chlorine dioxide for water treatment. Modified formulations aim to reduce the acid's corrosiveness while maintaining its effectiveness for specific applications.Expand Specific Solutions
Key PPE Manufacturers
The competitive landscape for muriatic acid's role in Polyphenylene Ether (PPE) production is in a mature stage, with established players dominating the market. The global PPE market size is projected to reach significant growth, driven by increasing demand in various industries. Technologically, the process is well-established, with companies like Asahi Kasei Corp., SABIC, and Kingfa Sci. & Tech. Co., Ltd. leading in PPE production. These firms have advanced their manufacturing processes, focusing on efficiency and quality improvements. Other players like China Petroleum & Chemical Corp. and Bluestar Adisseo Co. are also contributing to the market's technological advancements, indicating a high level of competition and innovation in this sector.
SABIC Global Technologies BV
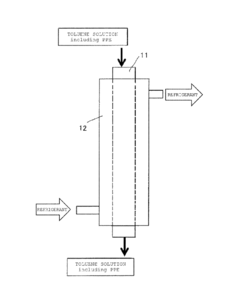
Technical Solution: SABIC has developed an innovative process for producing Polyphenylene Ether (PPE) using muriatic acid as a key component. Their method involves the oxidative coupling of 2,6-xylenol in the presence of a copper-amine catalyst system. Muriatic acid (hydrochloric acid) is used in the post-reaction workup to neutralize the alkaline reaction mixture and precipitate the PPE polymer. This process allows for precise control of molecular weight and improved product purity[1]. SABIC's technology also incorporates a solvent recovery system that recycles the muriatic acid, reducing waste and improving overall process efficiency[3]. The company has further optimized the process to minimize the formation of unwanted by-products, resulting in higher yields and better quality PPE[5].
Strengths: High-quality PPE production with precise molecular weight control, efficient use of muriatic acid through recycling, and improved product purity. Weaknesses: Potential corrosion issues due to the use of muriatic acid, requiring specialized equipment and safety measures.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a modified PPE production process that utilizes muriatic acid in conjunction with their proprietary catalyst system. Their approach involves a two-stage oxidation process, where muriatic acid is introduced in the second stage to control the reaction pH and facilitate polymer precipitation[2]. Sinopec's method incorporates a novel acid recovery system that allows for the regeneration and reuse of muriatic acid, significantly reducing raw material costs and environmental impact[4]. The company has also implemented advanced process control systems to optimize the use of muriatic acid, ensuring consistent product quality while minimizing acid consumption[6].
Strengths: Cost-effective production through acid recovery and reuse, advanced process control for consistent quality. Weaknesses: Complex two-stage process may require more sophisticated equipment and control systems.
Innovative Acid Catalysts
Method for producing polyphenylene ether
PatentActiveUS9777114B1
Innovation
- A method involving oxidative polymerization of phenol compounds with a polymerization catalyst, followed by catalyst extraction, solvent concentration, and a gel removal step using a heat exchanger to precipitate visually observable chloroform-insoluble substances, ensuring minimal gel incorporation in the final product.
Process of producing acid-modified polyphenylene ether and polystyrenic resin composition
PatentWO1996036658A1
Innovation
- The use of fumaric acid or its derivatives as modifiers, combined with a radical generator, at room temperature and elevated temperatures (300-350°C) to produce acid-modified PPO with minimal solvent use, maintaining molecular weight and improving working environment safety.
Environmental Impact
The production of Polyphenylene Ether (PPE) using muriatic acid, also known as hydrochloric acid, has significant environmental implications that warrant careful consideration. The process involves the oxidative coupling of 2,6-xylenol, which requires the use of muriatic acid as a catalyst. This chemical reaction, while efficient in producing PPE, raises several environmental concerns.
One of the primary environmental impacts is the potential for acid emissions. Muriatic acid is a strong, corrosive substance that can release harmful fumes during the production process. These emissions, if not properly controlled, can contribute to air pollution and pose risks to both human health and the surrounding ecosystem. Stringent air quality control measures and advanced scrubbing systems are essential to mitigate these risks and ensure compliance with environmental regulations.
Water pollution is another critical concern associated with the use of muriatic acid in PPE production. The acid, if not properly handled or disposed of, can contaminate water sources, leading to acidification of aquatic environments. This can have devastating effects on aquatic life and disrupt entire ecosystems. Implementing robust wastewater treatment systems and adhering to strict disposal protocols are crucial steps in minimizing the impact on water resources.
The production process also generates hazardous waste materials that require special handling and disposal. These wastes may include spent acid solutions, contaminated catalysts, and byproducts of the reaction. Proper management of these materials is essential to prevent soil contamination and protect groundwater resources. Recycling and regeneration of the acid, where possible, can help reduce the overall environmental footprint of the production process.
Energy consumption is another factor to consider when assessing the environmental impact of PPE production using muriatic acid. The process typically requires high temperatures and pressures, resulting in significant energy demands. This energy consumption contributes to greenhouse gas emissions if fossil fuels are the primary energy source. Implementing energy-efficient technologies and exploring renewable energy options can help mitigate this aspect of the environmental impact.
The transportation and storage of muriatic acid also present potential environmental risks. Accidental spills during transport or storage can have immediate and severe consequences for the environment, including soil and water contamination. Robust safety protocols, proper containment systems, and emergency response plans are essential to minimize these risks and protect the surrounding environment.
In conclusion, while the use of muriatic acid in PPE production is an effective manufacturing process, it comes with substantial environmental challenges. Addressing these impacts requires a comprehensive approach that includes advanced emission control technologies, efficient waste management systems, and a commitment to sustainable practices throughout the production chain. As environmental regulations become increasingly stringent, the industry must continue to innovate and implement cleaner production methods to ensure the long-term sustainability of PPE manufacturing.
One of the primary environmental impacts is the potential for acid emissions. Muriatic acid is a strong, corrosive substance that can release harmful fumes during the production process. These emissions, if not properly controlled, can contribute to air pollution and pose risks to both human health and the surrounding ecosystem. Stringent air quality control measures and advanced scrubbing systems are essential to mitigate these risks and ensure compliance with environmental regulations.
Water pollution is another critical concern associated with the use of muriatic acid in PPE production. The acid, if not properly handled or disposed of, can contaminate water sources, leading to acidification of aquatic environments. This can have devastating effects on aquatic life and disrupt entire ecosystems. Implementing robust wastewater treatment systems and adhering to strict disposal protocols are crucial steps in minimizing the impact on water resources.
The production process also generates hazardous waste materials that require special handling and disposal. These wastes may include spent acid solutions, contaminated catalysts, and byproducts of the reaction. Proper management of these materials is essential to prevent soil contamination and protect groundwater resources. Recycling and regeneration of the acid, where possible, can help reduce the overall environmental footprint of the production process.
Energy consumption is another factor to consider when assessing the environmental impact of PPE production using muriatic acid. The process typically requires high temperatures and pressures, resulting in significant energy demands. This energy consumption contributes to greenhouse gas emissions if fossil fuels are the primary energy source. Implementing energy-efficient technologies and exploring renewable energy options can help mitigate this aspect of the environmental impact.
The transportation and storage of muriatic acid also present potential environmental risks. Accidental spills during transport or storage can have immediate and severe consequences for the environment, including soil and water contamination. Robust safety protocols, proper containment systems, and emergency response plans are essential to minimize these risks and protect the surrounding environment.
In conclusion, while the use of muriatic acid in PPE production is an effective manufacturing process, it comes with substantial environmental challenges. Addressing these impacts requires a comprehensive approach that includes advanced emission control technologies, efficient waste management systems, and a commitment to sustainable practices throughout the production chain. As environmental regulations become increasingly stringent, the industry must continue to innovate and implement cleaner production methods to ensure the long-term sustainability of PPE manufacturing.
Safety Regulations
The production of Polyphenylene Ether (PPE) using muriatic acid requires strict adherence to safety regulations due to the hazardous nature of the chemicals involved. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) in the United States, as well as their counterparts in other countries, have established comprehensive guidelines for handling and storing muriatic acid in industrial settings.
One of the primary safety concerns is the corrosive nature of muriatic acid. Workers must be provided with appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, face shields, and protective clothing. Proper training on the safe handling and use of muriatic acid is mandatory for all personnel involved in the production process. This includes instruction on emergency procedures and the use of safety equipment such as eyewash stations and safety showers.
Ventilation is another critical aspect of safety regulations in PPE production facilities. Adequate ventilation systems must be in place to prevent the accumulation of harmful fumes and vapors. Local exhaust ventilation should be installed at points where muriatic acid is used or transferred to minimize worker exposure. Regular air quality monitoring is required to ensure that exposure levels remain within permissible limits.
Storage and transportation of muriatic acid are subject to strict regulations. The acid must be stored in properly labeled, corrosion-resistant containers in well-ventilated areas away from incompatible materials. Secondary containment systems are often required to prevent spills from spreading. Transportation of muriatic acid must comply with hazardous materials regulations, including proper packaging, labeling, and documentation.
Waste management is another crucial aspect of safety regulations in PPE production. Proper disposal methods for muriatic acid and its byproducts must be implemented to prevent environmental contamination. This may include neutralization processes or specialized waste treatment facilities. Companies are required to maintain detailed records of waste generation, storage, and disposal.
Emergency response plans are a mandatory component of safety regulations. These plans must outline procedures for handling spills, leaks, and other potential accidents involving muriatic acid. Regular drills and training sessions should be conducted to ensure that all employees are familiar with emergency protocols. Facilities must also have appropriate fire suppression systems and emergency communication equipment in place.
Compliance with these safety regulations is typically monitored through regular inspections and audits by regulatory agencies. Companies are required to maintain detailed documentation of their safety practices, including training records, incident reports, and equipment maintenance logs. Failure to comply with these regulations can result in significant fines, legal liabilities, and potential shutdown of production facilities.
One of the primary safety concerns is the corrosive nature of muriatic acid. Workers must be provided with appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, face shields, and protective clothing. Proper training on the safe handling and use of muriatic acid is mandatory for all personnel involved in the production process. This includes instruction on emergency procedures and the use of safety equipment such as eyewash stations and safety showers.
Ventilation is another critical aspect of safety regulations in PPE production facilities. Adequate ventilation systems must be in place to prevent the accumulation of harmful fumes and vapors. Local exhaust ventilation should be installed at points where muriatic acid is used or transferred to minimize worker exposure. Regular air quality monitoring is required to ensure that exposure levels remain within permissible limits.
Storage and transportation of muriatic acid are subject to strict regulations. The acid must be stored in properly labeled, corrosion-resistant containers in well-ventilated areas away from incompatible materials. Secondary containment systems are often required to prevent spills from spreading. Transportation of muriatic acid must comply with hazardous materials regulations, including proper packaging, labeling, and documentation.
Waste management is another crucial aspect of safety regulations in PPE production. Proper disposal methods for muriatic acid and its byproducts must be implemented to prevent environmental contamination. This may include neutralization processes or specialized waste treatment facilities. Companies are required to maintain detailed records of waste generation, storage, and disposal.
Emergency response plans are a mandatory component of safety regulations. These plans must outline procedures for handling spills, leaks, and other potential accidents involving muriatic acid. Regular drills and training sessions should be conducted to ensure that all employees are familiar with emergency protocols. Facilities must also have appropriate fire suppression systems and emergency communication equipment in place.
Compliance with these safety regulations is typically monitored through regular inspections and audits by regulatory agencies. Companies are required to maintain detailed documentation of their safety practices, including training records, incident reports, and equipment maintenance logs. Failure to comply with these regulations can result in significant fines, legal liabilities, and potential shutdown of production facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!