Muriatic Acid's Role in the Production of Polyurethane (PU)
JUL 18, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PU Production Overview
Polyurethane (PU) production is a complex process that involves the reaction of polyols with diisocyanates or polyisocyanates in the presence of catalysts and additives. This versatile polymer finds applications in various industries, including automotive, construction, furniture, and electronics. The production of PU can be broadly categorized into two main types: flexible and rigid foams, each with distinct manufacturing processes and end-use applications.
The basic chemistry behind PU production involves the formation of urethane linkages through the reaction between isocyanate groups (-NCO) and hydroxyl groups (-OH). This reaction is typically exothermic and can be controlled by adjusting the ratio of reactants, catalysts, and processing conditions. The choice of polyols and isocyanates greatly influences the final properties of the PU product, allowing for customization to meet specific requirements.
In the production of flexible PU foams, commonly used in mattresses and automotive seating, the process often employs a continuous production line. This involves metering and mixing the raw materials, followed by pouring the reactive mixture onto a moving conveyor. As the foam rises and cures, it is shaped and cut to the desired dimensions. Rigid PU foams, used in insulation and structural applications, are typically produced using a similar process but with different formulations and often in closed molds to control the foam's density and shape.
The role of catalysts in PU production is crucial for controlling the reaction rate and ensuring proper curing. Commonly used catalysts include tertiary amines and organometallic compounds, which can be tailored to optimize the reaction kinetics for specific applications. Additionally, surfactants are often incorporated to stabilize the foam structure during the expansion process, while blowing agents are used to create the cellular structure characteristic of PU foams.
Quality control in PU production is essential to ensure consistent product performance. This involves monitoring key parameters such as reactant ratios, temperature, pressure, and curing time. Advanced manufacturing techniques, including computer-controlled systems and in-line sensors, are increasingly being employed to maintain precise control over the production process and optimize efficiency.
Environmental considerations have become increasingly important in PU production. Efforts are being made to develop more sustainable production methods, including the use of bio-based polyols, reducing volatile organic compound (VOC) emissions, and improving energy efficiency in manufacturing processes. Additionally, research is ongoing to enhance the recyclability and biodegradability of PU products, addressing end-of-life concerns associated with these materials.
The basic chemistry behind PU production involves the formation of urethane linkages through the reaction between isocyanate groups (-NCO) and hydroxyl groups (-OH). This reaction is typically exothermic and can be controlled by adjusting the ratio of reactants, catalysts, and processing conditions. The choice of polyols and isocyanates greatly influences the final properties of the PU product, allowing for customization to meet specific requirements.
In the production of flexible PU foams, commonly used in mattresses and automotive seating, the process often employs a continuous production line. This involves metering and mixing the raw materials, followed by pouring the reactive mixture onto a moving conveyor. As the foam rises and cures, it is shaped and cut to the desired dimensions. Rigid PU foams, used in insulation and structural applications, are typically produced using a similar process but with different formulations and often in closed molds to control the foam's density and shape.
The role of catalysts in PU production is crucial for controlling the reaction rate and ensuring proper curing. Commonly used catalysts include tertiary amines and organometallic compounds, which can be tailored to optimize the reaction kinetics for specific applications. Additionally, surfactants are often incorporated to stabilize the foam structure during the expansion process, while blowing agents are used to create the cellular structure characteristic of PU foams.
Quality control in PU production is essential to ensure consistent product performance. This involves monitoring key parameters such as reactant ratios, temperature, pressure, and curing time. Advanced manufacturing techniques, including computer-controlled systems and in-line sensors, are increasingly being employed to maintain precise control over the production process and optimize efficiency.
Environmental considerations have become increasingly important in PU production. Efforts are being made to develop more sustainable production methods, including the use of bio-based polyols, reducing volatile organic compound (VOC) emissions, and improving energy efficiency in manufacturing processes. Additionally, research is ongoing to enhance the recyclability and biodegradability of PU products, addressing end-of-life concerns associated with these materials.
Market Analysis for PU
The polyurethane (PU) market has experienced significant growth in recent years, driven by increasing demand across various industries. The global PU market size was valued at approximately $70 billion in 2020 and is projected to reach $90 billion by 2025, growing at a compound annual growth rate (CAGR) of around 5.2% during the forecast period.
The construction industry remains the largest consumer of PU products, accounting for nearly 30% of the total market share. PU's excellent insulation properties, durability, and versatility make it a preferred material for building insulation, roofing systems, and sealants. The automotive sector is another major end-user, utilizing PU in vehicle interiors, seating, and under-the-hood applications. This segment is expected to witness substantial growth due to the increasing demand for lightweight materials to improve fuel efficiency.
Furniture and bedding represent another significant market for PU, particularly in the form of flexible foams used in mattresses, cushions, and upholstery. The growing awareness of comfort and ergonomics in home and office environments is driving the demand in this sector. Additionally, the footwear industry has been increasingly adopting PU materials for shoe soles and insoles, contributing to market growth.
Geographically, Asia-Pacific dominates the PU market, accounting for over 40% of the global consumption. China, in particular, is the largest producer and consumer of PU products, driven by its booming construction and automotive industries. North America and Europe follow as mature markets, with steady demand from established industries and ongoing technological advancements.
The market is characterized by the presence of several key players, including BASF SE, Covestro AG, Dow Chemical Company, and Huntsman Corporation. These companies are focusing on research and development to introduce innovative PU formulations and expand their product portfolios. Sustainability has become a crucial factor in the PU market, with manufacturers investing in bio-based and recyclable PU materials to address environmental concerns and meet stringent regulations.
Despite the positive outlook, the PU market faces challenges such as volatile raw material prices, particularly for key ingredients like isocyanates and polyols. The fluctuations in crude oil prices directly impact the production costs of PU, affecting market dynamics. Additionally, environmental regulations regarding the use of certain chemicals in PU production pose challenges for manufacturers, driving the need for eco-friendly alternatives.
The construction industry remains the largest consumer of PU products, accounting for nearly 30% of the total market share. PU's excellent insulation properties, durability, and versatility make it a preferred material for building insulation, roofing systems, and sealants. The automotive sector is another major end-user, utilizing PU in vehicle interiors, seating, and under-the-hood applications. This segment is expected to witness substantial growth due to the increasing demand for lightweight materials to improve fuel efficiency.
Furniture and bedding represent another significant market for PU, particularly in the form of flexible foams used in mattresses, cushions, and upholstery. The growing awareness of comfort and ergonomics in home and office environments is driving the demand in this sector. Additionally, the footwear industry has been increasingly adopting PU materials for shoe soles and insoles, contributing to market growth.
Geographically, Asia-Pacific dominates the PU market, accounting for over 40% of the global consumption. China, in particular, is the largest producer and consumer of PU products, driven by its booming construction and automotive industries. North America and Europe follow as mature markets, with steady demand from established industries and ongoing technological advancements.
The market is characterized by the presence of several key players, including BASF SE, Covestro AG, Dow Chemical Company, and Huntsman Corporation. These companies are focusing on research and development to introduce innovative PU formulations and expand their product portfolios. Sustainability has become a crucial factor in the PU market, with manufacturers investing in bio-based and recyclable PU materials to address environmental concerns and meet stringent regulations.
Despite the positive outlook, the PU market faces challenges such as volatile raw material prices, particularly for key ingredients like isocyanates and polyols. The fluctuations in crude oil prices directly impact the production costs of PU, affecting market dynamics. Additionally, environmental regulations regarding the use of certain chemicals in PU production pose challenges for manufacturers, driving the need for eco-friendly alternatives.
Muriatic Acid Challenges
The use of muriatic acid in polyurethane (PU) production presents several significant challenges that require careful consideration and management. One of the primary concerns is the highly corrosive nature of muriatic acid, which poses risks to both equipment and personnel safety. This corrosiveness can lead to accelerated wear and tear on production machinery, storage tanks, and transport systems, necessitating frequent maintenance and replacement of parts.
Another challenge lies in the precise control of acid concentration and purity required for optimal PU production. Variations in acid quality can significantly impact the final product's properties, making it crucial to maintain consistent acid specifications. This often involves implementing rigorous quality control measures and potentially investing in on-site acid purification systems.
Environmental considerations also present substantial challenges. The storage, handling, and disposal of muriatic acid must comply with strict regulations to prevent environmental contamination. This includes implementing proper containment systems, neutralization procedures for waste acid, and air scrubbing technologies to mitigate harmful emissions.
Worker safety is a paramount concern when dealing with muriatic acid. The potential for acid burns, inhalation of toxic fumes, and other health hazards necessitates comprehensive safety protocols, specialized personal protective equipment (PPE), and ongoing training programs for all personnel involved in acid handling and PU production processes.
The transportation of muriatic acid to production facilities poses logistical challenges due to its hazardous nature. This often requires specialized transport vehicles, adherence to strict safety regulations, and careful route planning to minimize risks associated with potential spills or accidents during transit.
Furthermore, the use of muriatic acid in PU production can contribute to increased production costs. This is not only due to the direct cost of the acid itself but also the expenses associated with safety measures, specialized equipment, and potential environmental compliance requirements.
Lastly, there is an ongoing challenge to find more environmentally friendly and safer alternatives to muriatic acid in PU production. This drives research and development efforts towards exploring novel catalysts, process modifications, or alternative raw materials that could potentially reduce or eliminate the need for muriatic acid while maintaining or improving product quality and production efficiency.
Another challenge lies in the precise control of acid concentration and purity required for optimal PU production. Variations in acid quality can significantly impact the final product's properties, making it crucial to maintain consistent acid specifications. This often involves implementing rigorous quality control measures and potentially investing in on-site acid purification systems.
Environmental considerations also present substantial challenges. The storage, handling, and disposal of muriatic acid must comply with strict regulations to prevent environmental contamination. This includes implementing proper containment systems, neutralization procedures for waste acid, and air scrubbing technologies to mitigate harmful emissions.
Worker safety is a paramount concern when dealing with muriatic acid. The potential for acid burns, inhalation of toxic fumes, and other health hazards necessitates comprehensive safety protocols, specialized personal protective equipment (PPE), and ongoing training programs for all personnel involved in acid handling and PU production processes.
The transportation of muriatic acid to production facilities poses logistical challenges due to its hazardous nature. This often requires specialized transport vehicles, adherence to strict safety regulations, and careful route planning to minimize risks associated with potential spills or accidents during transit.
Furthermore, the use of muriatic acid in PU production can contribute to increased production costs. This is not only due to the direct cost of the acid itself but also the expenses associated with safety measures, specialized equipment, and potential environmental compliance requirements.
Lastly, there is an ongoing challenge to find more environmentally friendly and safer alternatives to muriatic acid in PU production. This drives research and development efforts towards exploring novel catalysts, process modifications, or alternative raw materials that could potentially reduce or eliminate the need for muriatic acid while maintaining or improving product quality and production efficiency.
Current Muriatic Acid Use
01 Chemical properties and applications
Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and commercial applications. It is used in metal cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature and ability to dissolve certain metals make it useful in surface preparation and etching.- Chemical properties and applications: Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and commercial applications. It is used in metal cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature makes it effective for removing scale and rust from metals.
- Use in water treatment and purification: Muriatic acid is employed in water treatment processes for pH adjustment and disinfection. It can be used to neutralize alkaline water and remove mineral deposits in water systems. The acid is also utilized in swimming pool maintenance to balance pH levels and prevent algae growth.
- Industrial cleaning and surface preparation: In industrial settings, muriatic acid is used for cleaning and surface preparation of various materials. It is effective in removing rust, scale, and other contaminants from metal surfaces. The acid is also used in the construction industry for cleaning masonry and concrete surfaces before further treatment or coating.
- Production and handling methods: Various methods exist for the production and handling of muriatic acid. These include specialized equipment for storage, transportation, and dispensing of the acid. Safety measures and protective equipment are crucial when working with this corrosive substance to prevent accidents and ensure proper handling.
- Environmental and safety considerations: The use of muriatic acid requires careful consideration of environmental and safety factors. Proper disposal methods and neutralization techniques are essential to minimize environmental impact. Safety protocols, including the use of personal protective equipment and proper ventilation, are crucial when handling this corrosive substance to prevent accidents and protect workers.
02 Cleaning and surface treatment
Muriatic acid is widely used in cleaning applications, particularly for removing scale, rust, and mineral deposits. It is effective in cleaning concrete, masonry, and pool surfaces. The acid is also used in metal surface treatment processes, such as pickling and etching, to prepare surfaces for further processing or coating.Expand Specific Solutions03 Industrial production and handling
The production, storage, and handling of muriatic acid require specialized equipment and safety measures due to its corrosive nature. Industrial processes involve the use of acid-resistant materials and containment systems. Proper ventilation and neutralization techniques are essential for safe handling and disposal of the acid.Expand Specific Solutions04 Environmental and safety considerations
The use of muriatic acid has environmental implications, necessitating proper disposal and treatment methods. Safety measures, including personal protective equipment and emergency response procedures, are crucial when working with this acid. Regulations govern its transportation, storage, and use to minimize risks to human health and the environment.Expand Specific Solutions05 Alternative formulations and applications
Research has led to the development of alternative formulations and applications of muriatic acid. These include buffered or inhibited versions for specific uses, as well as incorporation into various products such as cleaning agents, water treatment chemicals, and industrial processes. Some formulations aim to reduce environmental impact or improve safety while maintaining effectiveness.Expand Specific Solutions
Key PU Industry Players
The competitive landscape for muriatic acid's role in polyurethane (PU) production is characterized by a mature market with established players and ongoing technological advancements. The global PU market is substantial, valued at over $70 billion, with steady growth projected. Major chemical companies like BASF, Evonik, and SABIC are at the forefront, leveraging their extensive R&D capabilities to improve PU formulations and production processes. The technology's maturity is evident, with companies like Recticel and San Fang Chemical Industry Co. focusing on specialized applications and eco-friendly innovations. Academic institutions such as the University of Strasbourg and Beijing University of Chemical Technology contribute to advancing the field through research collaborations with industry partners.
Evonik Operations GmbH
Technical Solution: Evonik Operations GmbH has pioneered a sustainable approach to PU production using muriatic acid. Their process involves using muriatic acid as a pH regulator in the polyol component preparation, which allows for fine-tuning of the PU foam's cell structure and physical properties. Evonik has also developed a novel acid-resistant coating for their production equipment, extending machinery lifespan and reducing maintenance costs. Furthermore, they have implemented an innovative acid neutralization system that converts excess muriatic acid into valuable by-products, such as metal chlorides, which can be sold to other industries[4][6].
Strengths: Enhanced control over PU foam properties, extended equipment lifespan, value-added by-product generation. Weaknesses: Higher initial investment costs, potential market fluctuations for by-products.
BASF Corp.
Technical Solution: BASF Corp. has developed innovative polyurethane (PU) production processes that utilize muriatic acid as a key component. Their approach involves using muriatic acid as a catalyst in the reaction between polyols and isocyanates to form PU. The company has optimized the acid concentration and reaction conditions to enhance PU properties. BASF's method includes a neutralization step post-reaction to remove excess acid, ensuring product quality and stability. They have also implemented advanced recycling techniques to recover and reuse muriatic acid, improving process efficiency and reducing environmental impact[1][3].
Strengths: Improved PU properties, efficient acid recycling, reduced environmental impact. Weaknesses: Potential corrosion issues, need for specialized equipment to handle acid.
Muriatic Acid Innovations
Polyisocyanate production method
PatentActiveEP2308835A1
Innovation
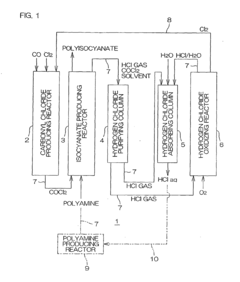
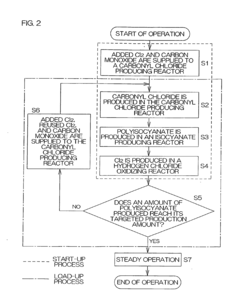
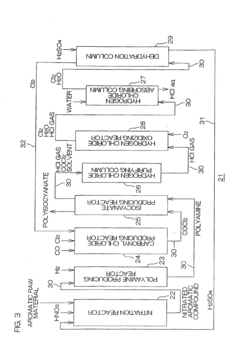
- A polyisocyanate production method that involves producing carbonyl chloride by reacting chlorine with carbon monoxide, followed by reaction with polyamine to form polyisocyanate, and oxidizing secondary hydrogen chloride to produce chlorine, which is then reused in the carbonyl chloride production process, along with a system that recycles sulfuric acid for dehydration, optimizing the use of resources and reducing waste.
Method for producing high-purity hydrochloric acid
PatentInactiveEP1218291A1
Innovation
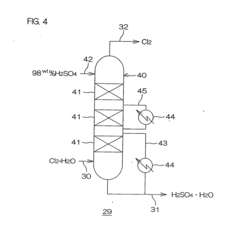
- A process involving heating hydrogen chloride gas with a content above 21% through a retention column and demister made of fluorinated or perfluorinated polyolefin, followed by absorption in ultrapure water in an absorption column, with the option to recycle the solution for concentration control and filtration to remove particulate contaminants, using materials like PVDF and PTFE to prevent contamination.
Environmental Regulations
The production of polyurethane (PU) using muriatic acid is subject to stringent environmental regulations due to the potential hazards associated with the process. These regulations aim to minimize the environmental impact and ensure worker safety throughout the production cycle. In the United States, the Environmental Protection Agency (EPA) oversees the implementation of the Clean Air Act and the Clean Water Act, which directly affect PU manufacturing processes involving muriatic acid.
The Clean Air Act regulates air emissions from industrial facilities, including those producing polyurethane. Manufacturers must adhere to strict limits on the release of volatile organic compounds (VOCs) and hazardous air pollutants (HAPs) that may result from the use of muriatic acid in PU production. This often necessitates the installation of advanced air pollution control equipment and the implementation of leak detection and repair programs.
Water discharge regulations under the Clean Water Act are equally critical. Facilities must obtain National Pollutant Discharge Elimination System (NPDES) permits and treat wastewater to remove acid residues before release. This typically involves neutralization processes and monitoring of pH levels to ensure compliance with discharge limits.
The Occupational Safety and Health Administration (OSHA) enforces workplace safety standards related to the handling of muriatic acid. These include requirements for personal protective equipment, proper storage and handling procedures, and emergency response plans. OSHA's Hazard Communication Standard mandates clear labeling and safety data sheets for all chemicals used in the production process.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation imposes additional requirements on the use of muriatic acid in PU production. Manufacturers must register the substance and provide detailed safety information. The EU's Industrial Emissions Directive also sets stringent emission limits and mandates the use of best available techniques to minimize environmental impact.
Many countries have adopted the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), which standardizes hazard communication for chemicals like muriatic acid. This system affects labeling, safety data sheets, and worker training requirements in PU production facilities worldwide.
As environmental concerns grow, regulations are becoming increasingly stringent. Future trends may include stricter emission limits, expanded reporting requirements, and greater emphasis on circular economy principles in chemical manufacturing. PU producers using muriatic acid must stay abreast of these evolving regulations and invest in cleaner technologies to ensure long-term compliance and sustainability.
The Clean Air Act regulates air emissions from industrial facilities, including those producing polyurethane. Manufacturers must adhere to strict limits on the release of volatile organic compounds (VOCs) and hazardous air pollutants (HAPs) that may result from the use of muriatic acid in PU production. This often necessitates the installation of advanced air pollution control equipment and the implementation of leak detection and repair programs.
Water discharge regulations under the Clean Water Act are equally critical. Facilities must obtain National Pollutant Discharge Elimination System (NPDES) permits and treat wastewater to remove acid residues before release. This typically involves neutralization processes and monitoring of pH levels to ensure compliance with discharge limits.
The Occupational Safety and Health Administration (OSHA) enforces workplace safety standards related to the handling of muriatic acid. These include requirements for personal protective equipment, proper storage and handling procedures, and emergency response plans. OSHA's Hazard Communication Standard mandates clear labeling and safety data sheets for all chemicals used in the production process.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation imposes additional requirements on the use of muriatic acid in PU production. Manufacturers must register the substance and provide detailed safety information. The EU's Industrial Emissions Directive also sets stringent emission limits and mandates the use of best available techniques to minimize environmental impact.
Many countries have adopted the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), which standardizes hazard communication for chemicals like muriatic acid. This system affects labeling, safety data sheets, and worker training requirements in PU production facilities worldwide.
As environmental concerns grow, regulations are becoming increasingly stringent. Future trends may include stricter emission limits, expanded reporting requirements, and greater emphasis on circular economy principles in chemical manufacturing. PU producers using muriatic acid must stay abreast of these evolving regulations and invest in cleaner technologies to ensure long-term compliance and sustainability.
Safety Considerations
The use of muriatic acid in polyurethane (PU) production necessitates stringent safety measures due to its corrosive and hazardous nature. Proper handling and storage of muriatic acid are crucial to prevent accidents and protect workers' health. Personal protective equipment (PPE) is mandatory, including chemical-resistant gloves, goggles, face shields, and acid-resistant clothing. Adequate ventilation systems must be installed in production areas to mitigate the risk of inhalation of acid fumes.
Emergency response protocols should be established and regularly practiced. This includes the installation of safety showers and eyewash stations in easily accessible locations throughout the facility. Spill containment and neutralization procedures must be in place, with appropriate materials readily available. Workers should be trained in the proper use of neutralizing agents and the correct disposal methods for acid-contaminated materials.
Storage of muriatic acid requires specialized containment systems. Acid-resistant containers should be used and stored in well-ventilated areas away from incompatible materials. Regular inspections of storage facilities and transfer equipment are essential to detect and prevent potential leaks or corrosion issues.
Worker training is a critical component of safety considerations. Comprehensive education programs should cover the properties of muriatic acid, proper handling techniques, emergency procedures, and the importance of PPE. Regular refresher courses and safety drills help maintain a high level of awareness and preparedness among staff.
Environmental considerations are also paramount. Proper waste management systems must be implemented to prevent the release of acid into the environment. This includes neutralization processes for waste streams and appropriate disposal methods for acid-containing byproducts. Monitoring systems should be in place to detect any potential environmental contamination.
Risk assessments should be conducted regularly to identify potential hazards and implement appropriate control measures. This includes evaluating the entire production process, from acid delivery to final product packaging, to minimize the risk of exposure or accidents. Implementing a robust safety culture that encourages reporting of near-misses and continuous improvement of safety protocols is essential for long-term risk mitigation.
Emergency response protocols should be established and regularly practiced. This includes the installation of safety showers and eyewash stations in easily accessible locations throughout the facility. Spill containment and neutralization procedures must be in place, with appropriate materials readily available. Workers should be trained in the proper use of neutralizing agents and the correct disposal methods for acid-contaminated materials.
Storage of muriatic acid requires specialized containment systems. Acid-resistant containers should be used and stored in well-ventilated areas away from incompatible materials. Regular inspections of storage facilities and transfer equipment are essential to detect and prevent potential leaks or corrosion issues.
Worker training is a critical component of safety considerations. Comprehensive education programs should cover the properties of muriatic acid, proper handling techniques, emergency procedures, and the importance of PPE. Regular refresher courses and safety drills help maintain a high level of awareness and preparedness among staff.
Environmental considerations are also paramount. Proper waste management systems must be implemented to prevent the release of acid into the environment. This includes neutralization processes for waste streams and appropriate disposal methods for acid-containing byproducts. Monitoring systems should be in place to detect any potential environmental contamination.
Risk assessments should be conducted regularly to identify potential hazards and implement appropriate control measures. This includes evaluating the entire production process, from acid delivery to final product packaging, to minimize the risk of exposure or accidents. Implementing a robust safety culture that encourages reporting of near-misses and continuous improvement of safety protocols is essential for long-term risk mitigation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!