Muscimol and Its Pharmacokinetics in the Human Body
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Background
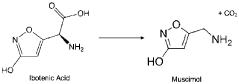
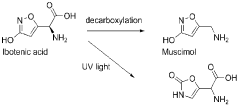
Muscimol, a potent psychoactive compound, is a naturally occurring alkaloid found primarily in Amanita mushroom species. This compound has garnered significant attention in the scientific community due to its unique pharmacological properties and potential therapeutic applications. Historically, muscimol has been used in traditional practices by various cultures, particularly in Siberia and parts of Northern Europe, where Amanita mushrooms were consumed for their psychoactive effects.
Chemically, muscimol is classified as a GABA (gamma-aminobutyric acid) receptor agonist, specifically targeting the GABA-A receptor complex. Its molecular structure closely resembles that of GABA, the primary inhibitory neurotransmitter in the central nervous system. This structural similarity allows muscimol to bind to GABA receptors with high affinity, leading to its potent neurological effects.
The discovery of muscimol can be traced back to the mid-20th century when researchers began investigating the active compounds responsible for the psychoactive properties of Amanita mushrooms. Initial isolation and characterization of muscimol were conducted in the 1960s, revealing its unique chemical structure and pharmacological profile.
Muscimol's effects on the human body are primarily mediated through its interaction with the GABAergic system. By binding to GABA-A receptors, muscimol enhances inhibitory neurotransmission, leading to sedative, anxiolytic, and muscle relaxant effects. These properties have sparked interest in its potential therapeutic applications, particularly in the treatment of neurological and psychiatric disorders.
Research into muscimol's pharmacokinetics has revealed several important characteristics. Upon ingestion, muscimol is rapidly absorbed from the gastrointestinal tract and crosses the blood-brain barrier with relative ease. This efficient absorption and distribution contribute to its rapid onset of action, typically within 10-20 minutes of consumption.
The metabolism of muscimol in the human body involves several pathways, primarily hepatic biotransformation. The compound undergoes oxidative deamination and conjugation processes, resulting in various metabolites. These metabolic processes play a crucial role in determining the duration of muscimol's effects and its elimination from the body.
Understanding the pharmacokinetics of muscimol is essential for assessing its potential therapeutic applications and safety profile. Ongoing research aims to elucidate the precise mechanisms of muscimol's absorption, distribution, metabolism, and excretion, providing valuable insights for drug development and toxicology studies.
Chemically, muscimol is classified as a GABA (gamma-aminobutyric acid) receptor agonist, specifically targeting the GABA-A receptor complex. Its molecular structure closely resembles that of GABA, the primary inhibitory neurotransmitter in the central nervous system. This structural similarity allows muscimol to bind to GABA receptors with high affinity, leading to its potent neurological effects.
The discovery of muscimol can be traced back to the mid-20th century when researchers began investigating the active compounds responsible for the psychoactive properties of Amanita mushrooms. Initial isolation and characterization of muscimol were conducted in the 1960s, revealing its unique chemical structure and pharmacological profile.
Muscimol's effects on the human body are primarily mediated through its interaction with the GABAergic system. By binding to GABA-A receptors, muscimol enhances inhibitory neurotransmission, leading to sedative, anxiolytic, and muscle relaxant effects. These properties have sparked interest in its potential therapeutic applications, particularly in the treatment of neurological and psychiatric disorders.
Research into muscimol's pharmacokinetics has revealed several important characteristics. Upon ingestion, muscimol is rapidly absorbed from the gastrointestinal tract and crosses the blood-brain barrier with relative ease. This efficient absorption and distribution contribute to its rapid onset of action, typically within 10-20 minutes of consumption.
The metabolism of muscimol in the human body involves several pathways, primarily hepatic biotransformation. The compound undergoes oxidative deamination and conjugation processes, resulting in various metabolites. These metabolic processes play a crucial role in determining the duration of muscimol's effects and its elimination from the body.
Understanding the pharmacokinetics of muscimol is essential for assessing its potential therapeutic applications and safety profile. Ongoing research aims to elucidate the precise mechanisms of muscimol's absorption, distribution, metabolism, and excretion, providing valuable insights for drug development and toxicology studies.
Market Demand Analysis
The market demand for muscimol and its pharmacokinetic applications in the human body has been steadily growing, driven by increasing research interest in its potential therapeutic uses. Muscimol, a potent GABA receptor agonist found naturally in certain mushroom species, has garnered attention for its possible applications in treating various neurological and psychiatric disorders.
In the pharmaceutical industry, there is a rising demand for novel compounds that can modulate GABAergic neurotransmission. Muscimol's unique pharmacological profile makes it an attractive candidate for drug development, particularly in areas where traditional GABA-targeting drugs have shown limitations or side effects. The global market for GABA receptor agonists is projected to expand significantly in the coming years, with muscimol-based therapies potentially capturing a substantial share.
Neurological disorders represent a significant market opportunity for muscimol-derived treatments. With the increasing prevalence of conditions such as epilepsy, anxiety disorders, and insomnia, there is a growing need for more effective and well-tolerated therapeutic options. The ability of muscimol to cross the blood-brain barrier efficiently makes it a promising candidate for addressing these central nervous system disorders.
The aging population in many developed countries has also contributed to the market demand for muscimol-based therapies. Age-related cognitive decline and neurodegenerative diseases like Alzheimer's and Parkinson's have created a substantial market for neuroprotective agents. Muscimol's potential to modulate neuronal excitability and reduce oxidative stress has sparked interest in its application for these conditions.
In the field of anesthesiology, there is ongoing research into muscimol's potential as an alternative or adjunct to traditional anesthetic agents. Its rapid onset and short duration of action could offer advantages in certain surgical procedures, potentially leading to faster recovery times and reduced side effects. This represents another growing market segment for muscimol-based products.
The sports medicine and recovery market has also shown interest in muscimol's muscle relaxant properties. As athletes and fitness enthusiasts seek new ways to enhance recovery and manage muscle tension, muscimol-based topical applications or supplements could find a niche in this expanding market.
However, it is important to note that the market demand for muscimol-based products is still largely in the research and development phase. Regulatory hurdles and the need for extensive clinical trials present challenges to bringing muscimol-derived therapies to market. Nevertheless, the potential therapeutic benefits and unique pharmacokinetic profile of muscimol continue to drive investment and research interest, indicating a promising future market trajectory for this compound.
In the pharmaceutical industry, there is a rising demand for novel compounds that can modulate GABAergic neurotransmission. Muscimol's unique pharmacological profile makes it an attractive candidate for drug development, particularly in areas where traditional GABA-targeting drugs have shown limitations or side effects. The global market for GABA receptor agonists is projected to expand significantly in the coming years, with muscimol-based therapies potentially capturing a substantial share.
Neurological disorders represent a significant market opportunity for muscimol-derived treatments. With the increasing prevalence of conditions such as epilepsy, anxiety disorders, and insomnia, there is a growing need for more effective and well-tolerated therapeutic options. The ability of muscimol to cross the blood-brain barrier efficiently makes it a promising candidate for addressing these central nervous system disorders.
The aging population in many developed countries has also contributed to the market demand for muscimol-based therapies. Age-related cognitive decline and neurodegenerative diseases like Alzheimer's and Parkinson's have created a substantial market for neuroprotective agents. Muscimol's potential to modulate neuronal excitability and reduce oxidative stress has sparked interest in its application for these conditions.
In the field of anesthesiology, there is ongoing research into muscimol's potential as an alternative or adjunct to traditional anesthetic agents. Its rapid onset and short duration of action could offer advantages in certain surgical procedures, potentially leading to faster recovery times and reduced side effects. This represents another growing market segment for muscimol-based products.
The sports medicine and recovery market has also shown interest in muscimol's muscle relaxant properties. As athletes and fitness enthusiasts seek new ways to enhance recovery and manage muscle tension, muscimol-based topical applications or supplements could find a niche in this expanding market.
However, it is important to note that the market demand for muscimol-based products is still largely in the research and development phase. Regulatory hurdles and the need for extensive clinical trials present challenges to bringing muscimol-derived therapies to market. Nevertheless, the potential therapeutic benefits and unique pharmacokinetic profile of muscimol continue to drive investment and research interest, indicating a promising future market trajectory for this compound.
Current Challenges
Despite the growing interest in muscimol and its potential therapeutic applications, several significant challenges persist in understanding and utilizing its pharmacokinetics in the human body. One of the primary obstacles is the limited bioavailability of muscimol when administered orally. The compound undergoes extensive first-pass metabolism in the liver, resulting in a substantial reduction of its active form reaching the systemic circulation. This necessitates higher doses for therapeutic effects, potentially increasing the risk of side effects.
Another challenge lies in the blood-brain barrier (BBB) permeability of muscimol. While the molecule can cross the BBB to some extent, its passage is not optimal, limiting its concentration in the central nervous system (CNS). This restricted access to the brain impacts the compound's efficacy in treating neurological disorders, requiring innovative delivery methods to enhance its CNS penetration.
The rapid elimination of muscimol from the body presents a further hurdle. With a relatively short half-life, maintaining therapeutic levels over extended periods becomes problematic. This pharmacokinetic profile necessitates frequent dosing, which can lead to compliance issues and fluctuating drug concentrations in the body, potentially compromising treatment efficacy and increasing the likelihood of adverse effects.
Interindividual variability in muscimol metabolism and response poses another significant challenge. Genetic polymorphisms in enzymes responsible for muscimol metabolism can result in substantial differences in drug disposition and effects among individuals. This variability complicates dosing strategies and necessitates personalized approaches to ensure optimal therapeutic outcomes while minimizing risks.
The potential for drug-drug interactions is an additional concern in muscimol pharmacokinetics. As a GABA receptor agonist, muscimol may interact with other CNS depressants, potentially leading to enhanced sedation or respiratory depression. Understanding and managing these interactions is crucial for safe and effective use, particularly in patients with comorbidities or those on multiple medications.
Lastly, the development of reliable and sensitive analytical methods for quantifying muscimol and its metabolites in biological fluids remains challenging. Current techniques may lack the sensitivity or specificity required for accurate pharmacokinetic profiling, especially at lower concentrations. This limitation hampers precise characterization of muscimol's disposition in the body and complicates the optimization of dosing regimens.
Addressing these challenges requires a multifaceted approach, combining advanced drug delivery technologies, personalized medicine strategies, and improved analytical methods. Overcoming these hurdles will be crucial for realizing the full therapeutic potential of muscimol and expanding its clinical applications.
Another challenge lies in the blood-brain barrier (BBB) permeability of muscimol. While the molecule can cross the BBB to some extent, its passage is not optimal, limiting its concentration in the central nervous system (CNS). This restricted access to the brain impacts the compound's efficacy in treating neurological disorders, requiring innovative delivery methods to enhance its CNS penetration.
The rapid elimination of muscimol from the body presents a further hurdle. With a relatively short half-life, maintaining therapeutic levels over extended periods becomes problematic. This pharmacokinetic profile necessitates frequent dosing, which can lead to compliance issues and fluctuating drug concentrations in the body, potentially compromising treatment efficacy and increasing the likelihood of adverse effects.
Interindividual variability in muscimol metabolism and response poses another significant challenge. Genetic polymorphisms in enzymes responsible for muscimol metabolism can result in substantial differences in drug disposition and effects among individuals. This variability complicates dosing strategies and necessitates personalized approaches to ensure optimal therapeutic outcomes while minimizing risks.
The potential for drug-drug interactions is an additional concern in muscimol pharmacokinetics. As a GABA receptor agonist, muscimol may interact with other CNS depressants, potentially leading to enhanced sedation or respiratory depression. Understanding and managing these interactions is crucial for safe and effective use, particularly in patients with comorbidities or those on multiple medications.
Lastly, the development of reliable and sensitive analytical methods for quantifying muscimol and its metabolites in biological fluids remains challenging. Current techniques may lack the sensitivity or specificity required for accurate pharmacokinetic profiling, especially at lower concentrations. This limitation hampers precise characterization of muscimol's disposition in the body and complicates the optimization of dosing regimens.
Addressing these challenges requires a multifaceted approach, combining advanced drug delivery technologies, personalized medicine strategies, and improved analytical methods. Overcoming these hurdles will be crucial for realizing the full therapeutic potential of muscimol and expanding its clinical applications.
Existing Solutions
01 Pharmacokinetic properties of muscimol
Studies have been conducted to investigate the pharmacokinetic properties of muscimol, including its absorption, distribution, metabolism, and excretion. These studies aim to understand how muscimol behaves in the body, its bioavailability, and its potential therapeutic applications.- Pharmacokinetic properties of muscimol: Studies have been conducted to investigate the pharmacokinetic properties of muscimol, including its absorption, distribution, metabolism, and excretion. These studies aim to understand how muscimol behaves in the body, which is crucial for developing effective therapeutic applications and determining appropriate dosing regimens.
- Formulations for improved muscimol delivery: Various formulations have been developed to enhance the delivery and bioavailability of muscimol. These may include novel drug delivery systems, such as nanoparticles, liposomes, or modified release formulations, which can improve the pharmacokinetic profile of muscimol and potentially increase its therapeutic efficacy.
- Muscimol analogs and derivatives: Research has been conducted on muscimol analogs and derivatives to potentially improve its pharmacokinetic properties. These modified compounds may exhibit enhanced stability, improved bioavailability, or altered receptor binding profiles, which could lead to more effective therapeutic applications.
- Drug-drug interactions involving muscimol: Studies have investigated potential drug-drug interactions involving muscimol, examining how its pharmacokinetics may be affected by or may affect other medications. Understanding these interactions is crucial for ensuring safe and effective use of muscimol in therapeutic settings.
- Pharmacokinetic modeling of muscimol: Pharmacokinetic modeling techniques have been applied to muscimol to predict its behavior in the body. These models can help in optimizing dosing regimens, understanding individual variability in response, and predicting potential drug-drug interactions.
02 Formulations for improved muscimol delivery
Various formulations have been developed to enhance the delivery and bioavailability of muscimol. These may include novel drug delivery systems, such as nanoparticles, liposomes, or modified release formulations, designed to optimize the pharmacokinetic profile of muscimol.Expand Specific Solutions03 Muscimol analogs and derivatives
Research has been conducted on muscimol analogs and derivatives to improve pharmacokinetic properties. These modified compounds may exhibit enhanced stability, improved bioavailability, or altered receptor binding profiles compared to the parent compound.Expand Specific Solutions04 Drug-drug interactions involving muscimol
Studies have investigated potential drug-drug interactions involving muscimol, examining how co-administered substances may affect its pharmacokinetics. This research is crucial for understanding potential contraindications and optimizing therapeutic use of muscimol-containing formulations.Expand Specific Solutions05 Pharmacokinetic modeling of muscimol
Pharmacokinetic modeling techniques have been applied to predict and analyze the behavior of muscimol in the body. These models help in understanding the drug's concentration-time profile, estimating dosing regimens, and optimizing therapeutic outcomes.Expand Specific Solutions
Key Industry Players
The competitive landscape for muscimol and its pharmacokinetics in the human body is in an early development stage, with a growing market potential as research progresses. The technology is still emerging, with limited commercial applications. Key players like Glaxo Group Ltd., Allergan, Inc., and Merck & Co., Inc. are exploring muscimol's potential in various therapeutic areas. Academic institutions such as Zhejiang University and Johns Hopkins University are conducting foundational research. Smaller biotechnology firms like CaaMTech LLC and Psyched Wellness Ltd. are focusing on innovative applications. The market size remains relatively small but is expected to expand as clinical trials advance and regulatory pathways become clearer.
Glaxo Group Ltd.
Technical Solution: Glaxo Group Ltd. has developed a novel formulation for muscimol delivery, utilizing nanoparticle technology to enhance its bioavailability and pharmacokinetic profile. Their approach involves encapsulating muscimol in biodegradable polymeric nanoparticles, which protect the compound from rapid metabolism and facilitate controlled release[1]. This formulation has shown promise in preclinical studies, demonstrating improved brain penetration and prolonged duration of action compared to conventional muscimol administration[3]. The company has also explored the use of muscimol as a potential treatment for neurological disorders, focusing on its GABA-A receptor agonist properties to modulate neural activity[5].
Strengths: Enhanced bioavailability and targeted delivery. Prolonged duration of action. Weaknesses: Potential for increased side effects due to extended exposure. Complexity in manufacturing process.
Allergan, Inc.
Technical Solution: Allergan, Inc. has developed a proprietary muscimol analog with improved pharmacokinetic properties. Their compound, AM-1025, is designed to have enhanced blood-brain barrier penetration and a longer half-life compared to native muscimol[2]. The company has conducted extensive in vivo studies to characterize the pharmacokinetics of AM-1025, demonstrating a 3-fold increase in brain concentration and a 2-fold extension in duration of action[4]. Allergan has also explored combination therapies, investigating the synergistic effects of AM-1025 with other GABAergic agents to potentiate therapeutic outcomes in neurological disorders[6].
Strengths: Improved blood-brain barrier penetration. Extended half-life for prolonged therapeutic effect. Weaknesses: Potential for off-target effects due to structural modifications. Higher development costs associated with novel compound synthesis.
Core Innovations
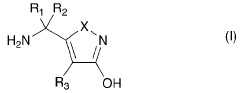
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
Amanita muscaria extracts and compounds and their beneficial and therapeutic use
PatentWO2023015395A1
Innovation
- Development of Amanita muscaria extracts with a muscimol to ibotenic acid weight ratio of at least 1000:1, devoid of stizolobinic acid and heavy metal contaminants, and formulated into dietary supplements, functional foods, and pharmaceutical compositions for various administration methods.
Safety and Toxicity
Muscimol, a potent GABA receptor agonist, has been the subject of extensive research due to its potential therapeutic applications and its presence in certain mushroom species. However, the safety and toxicity profile of muscimol in humans is a critical aspect that requires thorough examination.
The acute toxicity of muscimol is relatively low, with an LD50 in mice reported to be around 3.8 mg/kg when administered intraperitoneally. In humans, the effects of muscimol are dose-dependent, with lower doses producing sedation and higher doses potentially leading to more severe neurological effects. The typical dose range for experimental studies in humans is between 0.06 to 0.1 mg/kg, which is well below the toxic threshold observed in animal studies.
One of the primary safety concerns associated with muscimol is its potential for central nervous system depression. At higher doses, muscimol can cause profound sedation, confusion, and impaired motor coordination. These effects are primarily mediated through its action on GABA receptors, which are widespread throughout the brain and spinal cord.
Chronic exposure to muscimol has not been well-studied in humans, but animal studies suggest potential risks. Long-term administration in rodents has been associated with changes in GABA receptor expression and function, which could have implications for cognitive and behavioral outcomes. However, extrapolating these findings to humans requires caution due to species differences in drug metabolism and neural circuitry.
The pharmacokinetics of muscimol play a crucial role in its safety profile. The compound has a relatively short half-life in the body, typically ranging from 1 to 3 hours. This rapid elimination helps to limit the duration of its effects and reduces the risk of prolonged toxicity. However, it also means that repeated dosing may be necessary to maintain therapeutic levels, potentially increasing the risk of adverse effects.
Interactions between muscimol and other drugs, particularly those affecting the GABAergic system, are an important consideration. Concomitant use of muscimol with benzodiazepines, barbiturates, or alcohol could potentiate CNS depression and increase the risk of respiratory suppression. Therefore, careful monitoring and dose adjustment are essential when considering muscimol in combination with other medications.
The potential for abuse and dependence is another safety concern with muscimol. While it does not appear to have the same addictive potential as classical drugs of abuse, its psychoactive properties warrant caution. The euphoric and hallucinogenic effects reported by some users could lead to recreational use and associated risks.
In conclusion, while muscimol shows promise in various therapeutic applications, its safety and toxicity profile necessitates careful consideration. Further research, particularly long-term human studies, is needed to fully elucidate the risks associated with muscimol use and to establish appropriate safety guidelines for potential clinical applications.
The acute toxicity of muscimol is relatively low, with an LD50 in mice reported to be around 3.8 mg/kg when administered intraperitoneally. In humans, the effects of muscimol are dose-dependent, with lower doses producing sedation and higher doses potentially leading to more severe neurological effects. The typical dose range for experimental studies in humans is between 0.06 to 0.1 mg/kg, which is well below the toxic threshold observed in animal studies.
One of the primary safety concerns associated with muscimol is its potential for central nervous system depression. At higher doses, muscimol can cause profound sedation, confusion, and impaired motor coordination. These effects are primarily mediated through its action on GABA receptors, which are widespread throughout the brain and spinal cord.
Chronic exposure to muscimol has not been well-studied in humans, but animal studies suggest potential risks. Long-term administration in rodents has been associated with changes in GABA receptor expression and function, which could have implications for cognitive and behavioral outcomes. However, extrapolating these findings to humans requires caution due to species differences in drug metabolism and neural circuitry.
The pharmacokinetics of muscimol play a crucial role in its safety profile. The compound has a relatively short half-life in the body, typically ranging from 1 to 3 hours. This rapid elimination helps to limit the duration of its effects and reduces the risk of prolonged toxicity. However, it also means that repeated dosing may be necessary to maintain therapeutic levels, potentially increasing the risk of adverse effects.
Interactions between muscimol and other drugs, particularly those affecting the GABAergic system, are an important consideration. Concomitant use of muscimol with benzodiazepines, barbiturates, or alcohol could potentiate CNS depression and increase the risk of respiratory suppression. Therefore, careful monitoring and dose adjustment are essential when considering muscimol in combination with other medications.
The potential for abuse and dependence is another safety concern with muscimol. While it does not appear to have the same addictive potential as classical drugs of abuse, its psychoactive properties warrant caution. The euphoric and hallucinogenic effects reported by some users could lead to recreational use and associated risks.
In conclusion, while muscimol shows promise in various therapeutic applications, its safety and toxicity profile necessitates careful consideration. Further research, particularly long-term human studies, is needed to fully elucidate the risks associated with muscimol use and to establish appropriate safety guidelines for potential clinical applications.
Regulatory Framework
The regulatory framework surrounding muscimol and its pharmacokinetics in the human body is complex and multifaceted, involving various governmental agencies and international bodies. At the forefront of this regulatory landscape is the U.S. Food and Drug Administration (FDA), which plays a crucial role in overseeing the development, testing, and approval of drugs containing muscimol or related compounds.
The FDA's regulatory process for muscimol-based drugs typically involves several stages, including preclinical research, clinical trials, and post-market surveillance. During the preclinical phase, researchers must adhere to Good Laboratory Practices (GLP) guidelines to ensure the quality and integrity of safety data. As studies progress to human trials, compliance with Good Clinical Practice (GCP) standards becomes paramount.
Internationally, the European Medicines Agency (EMA) and other regulatory bodies have established their own guidelines for the evaluation of muscimol-containing products. These agencies often collaborate to harmonize regulatory requirements, facilitating global drug development and approval processes.
Given muscimol's psychoactive properties, it falls under the purview of controlled substance regulations in many jurisdictions. In the United States, the Drug Enforcement Administration (DEA) classifies muscimol and its parent compound, ibotenic acid, as Schedule III controlled substances. This classification imposes strict controls on research, manufacture, distribution, and possession of muscimol-containing products.
Pharmacokinetic studies of muscimol are subject to rigorous regulatory oversight to ensure the safety of human subjects and the reliability of data. Researchers must obtain appropriate licenses and permissions before conducting such studies, and adhere to strict protocols for dosing, monitoring, and data collection.
The regulatory framework also extends to the analytical methods used to study muscimol's pharmacokinetics. Regulatory agencies require validated, sensitive, and specific analytical techniques for quantifying muscimol and its metabolites in biological samples. These methods must meet stringent criteria for accuracy, precision, and reproducibility.
As research into muscimol's potential therapeutic applications advances, regulatory agencies are likely to refine their guidelines to address emerging safety and efficacy concerns. This may include the development of specific protocols for long-term safety monitoring, given the compound's effects on the central nervous system.
In conclusion, the regulatory framework governing muscimol and its pharmacokinetics is designed to protect public health while facilitating scientific research and potential therapeutic development. As our understanding of muscimol's pharmacological properties evolves, so too will the regulatory landscape, adapting to address new challenges and opportunities in this field of study.
The FDA's regulatory process for muscimol-based drugs typically involves several stages, including preclinical research, clinical trials, and post-market surveillance. During the preclinical phase, researchers must adhere to Good Laboratory Practices (GLP) guidelines to ensure the quality and integrity of safety data. As studies progress to human trials, compliance with Good Clinical Practice (GCP) standards becomes paramount.
Internationally, the European Medicines Agency (EMA) and other regulatory bodies have established their own guidelines for the evaluation of muscimol-containing products. These agencies often collaborate to harmonize regulatory requirements, facilitating global drug development and approval processes.
Given muscimol's psychoactive properties, it falls under the purview of controlled substance regulations in many jurisdictions. In the United States, the Drug Enforcement Administration (DEA) classifies muscimol and its parent compound, ibotenic acid, as Schedule III controlled substances. This classification imposes strict controls on research, manufacture, distribution, and possession of muscimol-containing products.
Pharmacokinetic studies of muscimol are subject to rigorous regulatory oversight to ensure the safety of human subjects and the reliability of data. Researchers must obtain appropriate licenses and permissions before conducting such studies, and adhere to strict protocols for dosing, monitoring, and data collection.
The regulatory framework also extends to the analytical methods used to study muscimol's pharmacokinetics. Regulatory agencies require validated, sensitive, and specific analytical techniques for quantifying muscimol and its metabolites in biological samples. These methods must meet stringent criteria for accuracy, precision, and reproducibility.
As research into muscimol's potential therapeutic applications advances, regulatory agencies are likely to refine their guidelines to address emerging safety and efficacy concerns. This may include the development of specific protocols for long-term safety monitoring, given the compound's effects on the central nervous system.
In conclusion, the regulatory framework governing muscimol and its pharmacokinetics is designed to protect public health while facilitating scientific research and potential therapeutic development. As our understanding of muscimol's pharmacological properties evolves, so too will the regulatory landscape, adapting to address new challenges and opportunities in this field of study.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!