Neoprene's Position in Advanced Medical Equipment Seals
AUG 5, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neoprene Seal Evolution
Neoprene, a synthetic rubber developed by DuPont in 1930, has undergone significant evolution in its application for medical equipment seals. Initially used primarily in industrial settings, neoprene's unique properties quickly attracted attention from the medical field. The material's resistance to oils, chemicals, and weathering, combined with its flexibility and durability, made it an ideal candidate for sealing applications in medical devices.
In the 1950s and 1960s, as medical technology advanced rapidly, neoprene began to find its way into various medical equipment seals. Its early applications included seals for respiratory devices, such as oxygen masks and ventilators, where its ability to maintain an airtight seal was crucial. During this period, manufacturers focused on improving the material's purity and reducing potential allergens to make it more suitable for medical use.
The 1970s and 1980s saw a significant leap in neoprene's medical applications. With the advent of more sophisticated medical equipment, such as dialysis machines and infusion pumps, neoprene seals became integral components. Researchers and engineers worked on enhancing the material's properties, developing specialized formulations that could withstand repeated sterilization processes without degradation.
In the 1990s and early 2000s, the focus shifted towards improving neoprene's biocompatibility. This era saw the development of medical-grade neoprene with reduced extractables and leachables, making it safer for use in direct contact with bodily fluids. Manufacturers also began exploring neoprene composites, combining it with other materials to achieve specific performance characteristics for different medical applications.
The past two decades have witnessed further refinement in neoprene seal technology for medical equipment. Advancements in polymer science have led to the creation of ultra-pure neoprene variants with enhanced chemical resistance and longer service life. These improvements have made neoprene seals suitable for use in cutting-edge medical technologies, such as robotic surgical equipment and advanced diagnostic devices.
Today, neoprene continues to evolve to meet the stringent requirements of modern medical equipment. Recent developments include the integration of antimicrobial properties into neoprene seals, addressing concerns about hospital-acquired infections. Additionally, researchers are exploring ways to enhance neoprene's performance at extreme temperatures and pressures, expanding its potential applications in specialized medical equipment.
In the 1950s and 1960s, as medical technology advanced rapidly, neoprene began to find its way into various medical equipment seals. Its early applications included seals for respiratory devices, such as oxygen masks and ventilators, where its ability to maintain an airtight seal was crucial. During this period, manufacturers focused on improving the material's purity and reducing potential allergens to make it more suitable for medical use.
The 1970s and 1980s saw a significant leap in neoprene's medical applications. With the advent of more sophisticated medical equipment, such as dialysis machines and infusion pumps, neoprene seals became integral components. Researchers and engineers worked on enhancing the material's properties, developing specialized formulations that could withstand repeated sterilization processes without degradation.
In the 1990s and early 2000s, the focus shifted towards improving neoprene's biocompatibility. This era saw the development of medical-grade neoprene with reduced extractables and leachables, making it safer for use in direct contact with bodily fluids. Manufacturers also began exploring neoprene composites, combining it with other materials to achieve specific performance characteristics for different medical applications.
The past two decades have witnessed further refinement in neoprene seal technology for medical equipment. Advancements in polymer science have led to the creation of ultra-pure neoprene variants with enhanced chemical resistance and longer service life. These improvements have made neoprene seals suitable for use in cutting-edge medical technologies, such as robotic surgical equipment and advanced diagnostic devices.
Today, neoprene continues to evolve to meet the stringent requirements of modern medical equipment. Recent developments include the integration of antimicrobial properties into neoprene seals, addressing concerns about hospital-acquired infections. Additionally, researchers are exploring ways to enhance neoprene's performance at extreme temperatures and pressures, expanding its potential applications in specialized medical equipment.
Medical Equipment Demand
The demand for advanced medical equipment seals, particularly those utilizing neoprene, has been steadily increasing in recent years. This growth is primarily driven by the expanding healthcare industry, technological advancements in medical devices, and the rising prevalence of chronic diseases worldwide. The global medical equipment market, which heavily relies on high-quality seals, is projected to reach significant value in the coming years, with a substantial portion attributed to sealing components.
Neoprene, a synthetic rubber known for its excellent chemical resistance, flexibility, and durability, has found extensive applications in medical equipment seals. Its unique properties make it particularly suitable for use in various medical devices, including diagnostic equipment, surgical instruments, and patient monitoring systems. The demand for neoprene seals in medical applications is closely tied to the growth of these sectors.
One of the key factors driving the demand for neoprene seals in medical equipment is the increasing focus on infection control and prevention in healthcare settings. Neoprene's resistance to various chemicals and its ability to maintain a tight seal under diverse conditions make it an ideal material for preventing contamination and ensuring the sterility of medical devices. This has become especially crucial in light of recent global health crises and the heightened awareness of healthcare-associated infections.
The aging population in many developed countries is another significant driver of demand for medical equipment seals. As the elderly population grows, there is an increased need for advanced medical devices for diagnosis, treatment, and monitoring of age-related conditions. This demographic shift has led to a surge in demand for medical equipment that requires high-performance seals, including those made from neoprene.
Furthermore, the trend towards miniaturization and portability in medical devices has created new opportunities for neoprene seals. As medical equipment becomes smaller and more compact, the need for precise, reliable sealing solutions that can perform in confined spaces has grown. Neoprene's versatility and ability to be molded into complex shapes make it well-suited for these applications.
The COVID-19 pandemic has also had a significant impact on the demand for medical equipment seals. The sudden surge in demand for ventilators, respiratory equipment, and other critical care devices has highlighted the importance of reliable sealing solutions in medical applications. This has led to increased interest in materials like neoprene that can meet the stringent requirements of medical-grade seals.
Neoprene, a synthetic rubber known for its excellent chemical resistance, flexibility, and durability, has found extensive applications in medical equipment seals. Its unique properties make it particularly suitable for use in various medical devices, including diagnostic equipment, surgical instruments, and patient monitoring systems. The demand for neoprene seals in medical applications is closely tied to the growth of these sectors.
One of the key factors driving the demand for neoprene seals in medical equipment is the increasing focus on infection control and prevention in healthcare settings. Neoprene's resistance to various chemicals and its ability to maintain a tight seal under diverse conditions make it an ideal material for preventing contamination and ensuring the sterility of medical devices. This has become especially crucial in light of recent global health crises and the heightened awareness of healthcare-associated infections.
The aging population in many developed countries is another significant driver of demand for medical equipment seals. As the elderly population grows, there is an increased need for advanced medical devices for diagnosis, treatment, and monitoring of age-related conditions. This demographic shift has led to a surge in demand for medical equipment that requires high-performance seals, including those made from neoprene.
Furthermore, the trend towards miniaturization and portability in medical devices has created new opportunities for neoprene seals. As medical equipment becomes smaller and more compact, the need for precise, reliable sealing solutions that can perform in confined spaces has grown. Neoprene's versatility and ability to be molded into complex shapes make it well-suited for these applications.
The COVID-19 pandemic has also had a significant impact on the demand for medical equipment seals. The sudden surge in demand for ventilators, respiratory equipment, and other critical care devices has highlighted the importance of reliable sealing solutions in medical applications. This has led to increased interest in materials like neoprene that can meet the stringent requirements of medical-grade seals.
Neoprene Seal Challenges
Neoprene, a synthetic rubber known for its versatility and durability, faces several challenges in its application as a sealing material for advanced medical equipment. One of the primary issues is its limited chemical resistance, particularly when exposed to certain oils, solvents, and aggressive chemicals commonly used in medical environments. This vulnerability can lead to degradation of the seal over time, potentially compromising the integrity of the medical equipment.
Another significant challenge is Neoprene's temperature limitations. While it performs well in moderate temperature ranges, it may not maintain its sealing properties effectively in extreme heat or cold conditions that some medical devices may encounter during sterilization processes or in specialized storage environments. This temperature sensitivity can restrict its use in certain high-performance medical applications.
Neoprene's compression set characteristics also present a challenge in medical sealing applications. Over time and under constant pressure, Neoprene seals may experience permanent deformation, reducing their ability to maintain an effective seal. This is particularly problematic in medical equipment that requires long-term, consistent sealing performance to ensure patient safety and equipment reliability.
The material's permeability to certain gases and liquids is another concern in medical applications. While Neoprene offers good resistance to many substances, it may allow the passage of some gases or fluids, which could be critical in maintaining sterile environments or containing hazardous materials in medical settings.
Furthermore, Neoprene's biocompatibility and potential for leaching compounds pose challenges in medical applications. Although generally considered safe, there are concerns about potential long-term effects of Neoprene in direct or indirect contact with biological tissues or fluids. This necessitates careful consideration and testing for each specific medical application.
The manufacturing process of Neoprene seals for medical equipment also presents challenges. Achieving consistent quality, precise dimensions, and surface finishes required for high-performance medical seals can be difficult, especially for complex geometries or miniaturized components increasingly common in advanced medical devices.
Lastly, the growing demand for more environmentally friendly materials in the medical industry poses a challenge for Neoprene. As a synthetic rubber, it is not biodegradable and its production process has environmental implications. This has led to increased pressure to find more sustainable alternatives, potentially limiting Neoprene's future in certain medical sealing applications.
Another significant challenge is Neoprene's temperature limitations. While it performs well in moderate temperature ranges, it may not maintain its sealing properties effectively in extreme heat or cold conditions that some medical devices may encounter during sterilization processes or in specialized storage environments. This temperature sensitivity can restrict its use in certain high-performance medical applications.
Neoprene's compression set characteristics also present a challenge in medical sealing applications. Over time and under constant pressure, Neoprene seals may experience permanent deformation, reducing their ability to maintain an effective seal. This is particularly problematic in medical equipment that requires long-term, consistent sealing performance to ensure patient safety and equipment reliability.
The material's permeability to certain gases and liquids is another concern in medical applications. While Neoprene offers good resistance to many substances, it may allow the passage of some gases or fluids, which could be critical in maintaining sterile environments or containing hazardous materials in medical settings.
Furthermore, Neoprene's biocompatibility and potential for leaching compounds pose challenges in medical applications. Although generally considered safe, there are concerns about potential long-term effects of Neoprene in direct or indirect contact with biological tissues or fluids. This necessitates careful consideration and testing for each specific medical application.
The manufacturing process of Neoprene seals for medical equipment also presents challenges. Achieving consistent quality, precise dimensions, and surface finishes required for high-performance medical seals can be difficult, especially for complex geometries or miniaturized components increasingly common in advanced medical devices.
Lastly, the growing demand for more environmentally friendly materials in the medical industry poses a challenge for Neoprene. As a synthetic rubber, it is not biodegradable and its production process has environmental implications. This has led to increased pressure to find more sustainable alternatives, potentially limiting Neoprene's future in certain medical sealing applications.
Current Neoprene Solutions
01 Composition and synthesis of neoprene
Neoprene is a synthetic rubber produced by polymerization of chloroprene. It has various compositions and methods of synthesis, including emulsion polymerization and solution polymerization techniques. The process often involves the use of specific catalysts and additives to control the properties of the final product.- Composition and synthesis of neoprene: Neoprene is a synthetic rubber produced by polymerization of chloroprene. It is known for its resistance to oil, heat, and weathering. The manufacturing process involves careful control of reaction conditions and may include various additives to enhance specific properties.
- Applications of neoprene in protective gear: Neoprene is widely used in the production of protective gear such as wetsuits, diving suits, and other water-resistant clothing. Its flexibility, insulation properties, and durability make it ideal for these applications. Neoprene can be combined with other materials to enhance its performance in specific use cases.
- Neoprene in industrial applications: Neoprene finds extensive use in various industrial applications due to its resistance to chemicals, oils, and extreme temperatures. It is used in the production of gaskets, hoses, conveyor belts, and other mechanical components that require durability and flexibility in harsh environments.
- Modifications and improvements to neoprene: Ongoing research focuses on improving neoprene's properties through various modifications. This includes the development of new formulations, incorporation of additives, and creation of neoprene composites to enhance specific characteristics such as flame resistance, electrical insulation, or environmental sustainability.
- Neoprene foam and cellular structures: Neoprene can be processed into foam or cellular structures, which are useful in applications requiring cushioning, insulation, or buoyancy. These forms of neoprene are used in products such as laptop sleeves, mouse pads, and flotation devices. The manufacturing process involves careful control of cell size and distribution.
02 Applications of neoprene in protective gear
Neoprene is widely used in the production of protective gear due to its excellent insulation and water-resistant properties. It is commonly used in wetsuits, diving suits, and other water sports equipment. The material's flexibility and durability make it suitable for various protective applications in different industries.Expand Specific Solutions03 Neoprene foam production and properties
Neoprene foam is a versatile material with unique properties such as excellent insulation, buoyancy, and shock absorption. The production process involves the introduction of gas bubbles into the neoprene compound during polymerization or through post-curing techniques. Various methods are employed to control the foam's density and cell structure.Expand Specific Solutions04 Neoprene blends and composites
Neoprene can be blended with other materials or used in composites to enhance its properties or create specialized products. These blends and composites often combine the beneficial properties of neoprene with those of other materials, resulting in improved performance characteristics such as increased strength, chemical resistance, or specific electrical properties.Expand Specific Solutions05 Neoprene in adhesive and sealant applications
Neoprene-based adhesives and sealants are widely used in various industries due to their excellent bonding strength, flexibility, and resistance to weathering and chemicals. These products are formulated to provide strong adhesion to a wide range of substrates and can be used in both indoor and outdoor applications.Expand Specific Solutions
Key Neoprene Manufacturers
The market for advanced medical equipment seals, particularly those utilizing neoprene, is in a growth phase, driven by increasing demand for sophisticated medical devices. The global market size for medical seals is expanding, with a projected CAGR of 5-7% over the next five years. Technologically, neoprene seals are mature but continue to evolve, with companies like Sumitomo Rubber Industries and Consort Medical leading innovations. Emerging players such as Mercator MedSystems and Aesculap AG are focusing on niche applications, while established firms like Boston Scientific and Terumo Corp. are integrating advanced sealing solutions into their broader medical device portfolios. The competitive landscape is characterized by a mix of specialized seal manufacturers and diversified medical technology companies, indicating a dynamic and evolving market.
Sumitomo Rubber Industries, Ltd.
Technical Solution: Sumitomo Rubber Industries has developed advanced neoprene compounds specifically tailored for medical equipment seals. Their proprietary formulation enhances the material's resistance to chemicals, oils, and extreme temperatures, making it ideal for use in critical medical applications. The company employs a unique vulcanization process that improves the neoprene's tensile strength and elongation properties, resulting in seals that maintain their integrity under high pressure and repeated sterilization cycles[1]. Sumitomo's neoprene seals also incorporate antimicrobial additives, reducing the risk of bacterial growth and enhancing overall hygiene in medical environments[3].
Strengths: Superior chemical resistance, improved durability, and antimicrobial properties. Weaknesses: Potentially higher cost compared to standard neoprene formulations, and limited customization options for specific medical devices.
Philips (China) Investment Co. Ltd.
Technical Solution: Philips has developed innovative neoprene-based sealing solutions for their advanced medical imaging equipment, particularly in MRI and CT scanners. Their proprietary neoprene compounds are engineered to provide exceptional electromagnetic shielding properties, crucial for maintaining image quality and reducing interference in sensitive diagnostic equipment[8]. Philips' neoprene seals also incorporate advanced vibration dampening characteristics, minimizing mechanical noise and improving overall system performance. The company has implemented a novel manufacturing process that allows for the creation of complex, precision-molded neoprene components with tight tolerances, ensuring optimal sealing in intricate medical device assemblies[9]. Additionally, Philips has developed neoprene formulations with enhanced thermal management properties, addressing the heat dissipation challenges in high-powered medical imaging systems.
Strengths: Excellent electromagnetic shielding, vibration dampening, and precision molding capabilities. Weaknesses: High specialization may limit broader application, and potential for increased production costs due to complex manufacturing processes.
Innovative Seal Patents
Neoprene medical device
PatentInactiveEP3159016A1
Innovation
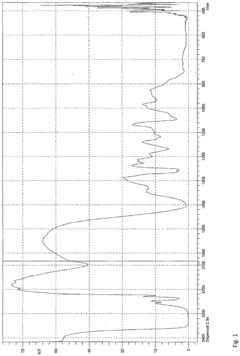
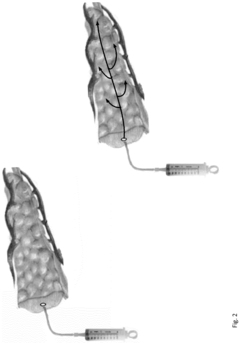
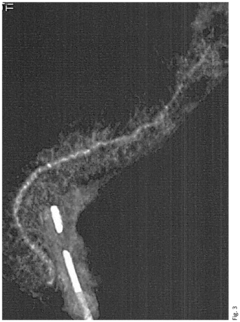
- A neoprene-based occlusive material is developed, comprising a sterile aqueous dispersion of poly(2-chloro-1,3-butadiene) stabilized at pH 13-13.5, which is injected into the pancreatic duct after surgical removal of the pancreas head, polymerizing to block pancreatic juice discharge, thereby inducing chemical pancreatectomy and preventing fistula formation.
Regulatory Compliance
Regulatory compliance is a critical aspect of Neoprene's application in advanced medical equipment seals. The medical device industry is heavily regulated to ensure patient safety and product efficacy. For Neoprene seals used in medical equipment, compliance with various regulatory standards is essential for market acceptance and legal operation.
In the United States, the Food and Drug Administration (FDA) oversees medical device regulations. Neoprene seals must comply with FDA requirements, including biocompatibility testing as per ISO 10993 standards. This ensures that the material does not cause adverse reactions when in contact with human tissues or bodily fluids. Additionally, Neoprene seals must meet FDA's Good Manufacturing Practice (GMP) guidelines to ensure consistent quality and safety.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) set stringent requirements for medical devices sold in the EU market. Neoprene seals used in medical equipment must conform to these regulations, which include comprehensive documentation, risk management, and post-market surveillance.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices. Neoprene seals must meet PMDA's standards for material safety and performance. Similarly, China's National Medical Products Administration (NMPA) has its own set of regulations that Neoprene seals must adhere to for use in medical equipment in the Chinese market.
International standards such as ISO 13485 for quality management systems in medical devices are crucial for Neoprene seal manufacturers. Compliance with this standard demonstrates a commitment to quality and safety across the entire production process. Furthermore, specific standards like ASTM D2000 for rubber products in automotive applications may be relevant for certain medical equipment seals.
Environmental regulations also play a role in Neoprene's regulatory compliance. The material must meet requirements set by agencies like the Environmental Protection Agency (EPA) in the US or the European Chemicals Agency (ECHA) regarding chemical composition and potential environmental impact.
Manufacturers of Neoprene seals for medical equipment must navigate this complex regulatory landscape, ensuring their products meet or exceed all applicable standards. This involves rigorous testing, documentation, and quality control processes. Continuous monitoring of regulatory changes and proactive adaptation to new requirements are essential for maintaining compliance and market access.
In the United States, the Food and Drug Administration (FDA) oversees medical device regulations. Neoprene seals must comply with FDA requirements, including biocompatibility testing as per ISO 10993 standards. This ensures that the material does not cause adverse reactions when in contact with human tissues or bodily fluids. Additionally, Neoprene seals must meet FDA's Good Manufacturing Practice (GMP) guidelines to ensure consistent quality and safety.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) set stringent requirements for medical devices sold in the EU market. Neoprene seals used in medical equipment must conform to these regulations, which include comprehensive documentation, risk management, and post-market surveillance.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices. Neoprene seals must meet PMDA's standards for material safety and performance. Similarly, China's National Medical Products Administration (NMPA) has its own set of regulations that Neoprene seals must adhere to for use in medical equipment in the Chinese market.
International standards such as ISO 13485 for quality management systems in medical devices are crucial for Neoprene seal manufacturers. Compliance with this standard demonstrates a commitment to quality and safety across the entire production process. Furthermore, specific standards like ASTM D2000 for rubber products in automotive applications may be relevant for certain medical equipment seals.
Environmental regulations also play a role in Neoprene's regulatory compliance. The material must meet requirements set by agencies like the Environmental Protection Agency (EPA) in the US or the European Chemicals Agency (ECHA) regarding chemical composition and potential environmental impact.
Manufacturers of Neoprene seals for medical equipment must navigate this complex regulatory landscape, ensuring their products meet or exceed all applicable standards. This involves rigorous testing, documentation, and quality control processes. Continuous monitoring of regulatory changes and proactive adaptation to new requirements are essential for maintaining compliance and market access.
Biocompatibility Assessment
Biocompatibility assessment is a critical aspect of evaluating Neoprene's suitability for advanced medical equipment seals. This process involves rigorous testing to ensure that the material does not cause adverse reactions when in contact with biological systems, particularly in medical applications.
Neoprene, a synthetic rubber, has demonstrated promising characteristics for use in medical seals. Its resistance to oils, chemicals, and weathering makes it an attractive option for various medical devices. However, the biocompatibility of Neoprene must be thoroughly examined to meet stringent regulatory requirements and ensure patient safety.
The assessment typically begins with in vitro cytotoxicity tests, which evaluate the material's potential to cause cell death or inhibit cell growth. These tests involve exposing cell cultures to Neoprene extracts and observing any adverse effects. Following this, sensitization tests are conducted to determine if Neoprene can trigger allergic reactions in living organisms.
Irritation tests are another crucial component of the biocompatibility assessment. These tests evaluate whether Neoprene causes inflammation or irritation when in contact with skin, eyes, or mucous membranes. Additionally, systemic toxicity tests are performed to assess any potential harmful effects on the body's organs and systems resulting from long-term exposure to the material.
For medical equipment seals that may come into contact with blood, hemocompatibility tests are essential. These tests evaluate Neoprene's interaction with blood components, including its potential to cause hemolysis or thrombosis. Furthermore, genotoxicity and carcinogenicity studies are conducted to assess any potential mutagenic or cancer-causing properties of the material.
The biocompatibility assessment also considers the specific application of Neoprene in medical equipment seals. For instance, if the seal is intended for use in implantable devices, additional long-term implantation studies may be required to evaluate the material's performance and safety over extended periods.
It is important to note that biocompatibility assessments must adhere to international standards, such as ISO 10993, which provides guidelines for biological evaluation of medical devices. Compliance with these standards ensures that the assessment is comprehensive and meets global regulatory requirements.
The results of these biocompatibility assessments play a crucial role in determining Neoprene's suitability for advanced medical equipment seals. Positive outcomes from these tests can pave the way for Neoprene's adoption in various medical applications, while any identified issues may necessitate further material modifications or alternative solutions.
Neoprene, a synthetic rubber, has demonstrated promising characteristics for use in medical seals. Its resistance to oils, chemicals, and weathering makes it an attractive option for various medical devices. However, the biocompatibility of Neoprene must be thoroughly examined to meet stringent regulatory requirements and ensure patient safety.
The assessment typically begins with in vitro cytotoxicity tests, which evaluate the material's potential to cause cell death or inhibit cell growth. These tests involve exposing cell cultures to Neoprene extracts and observing any adverse effects. Following this, sensitization tests are conducted to determine if Neoprene can trigger allergic reactions in living organisms.
Irritation tests are another crucial component of the biocompatibility assessment. These tests evaluate whether Neoprene causes inflammation or irritation when in contact with skin, eyes, or mucous membranes. Additionally, systemic toxicity tests are performed to assess any potential harmful effects on the body's organs and systems resulting from long-term exposure to the material.
For medical equipment seals that may come into contact with blood, hemocompatibility tests are essential. These tests evaluate Neoprene's interaction with blood components, including its potential to cause hemolysis or thrombosis. Furthermore, genotoxicity and carcinogenicity studies are conducted to assess any potential mutagenic or cancer-causing properties of the material.
The biocompatibility assessment also considers the specific application of Neoprene in medical equipment seals. For instance, if the seal is intended for use in implantable devices, additional long-term implantation studies may be required to evaluate the material's performance and safety over extended periods.
It is important to note that biocompatibility assessments must adhere to international standards, such as ISO 10993, which provides guidelines for biological evaluation of medical devices. Compliance with these standards ensures that the assessment is comprehensive and meets global regulatory requirements.
The results of these biocompatibility assessments play a crucial role in determining Neoprene's suitability for advanced medical equipment seals. Positive outcomes from these tests can pave the way for Neoprene's adoption in various medical applications, while any identified issues may necessitate further material modifications or alternative solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!