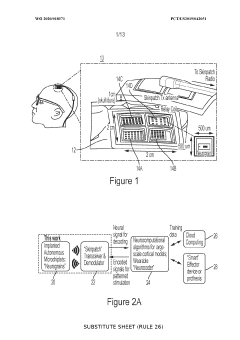
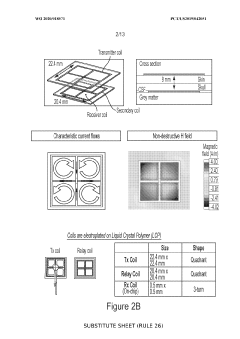
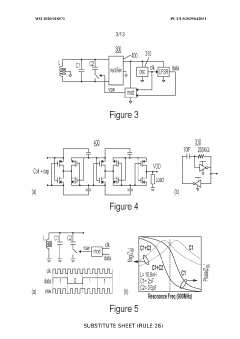
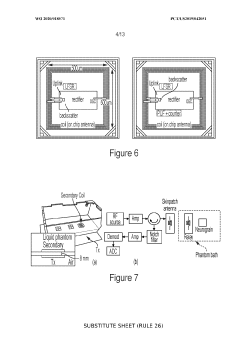
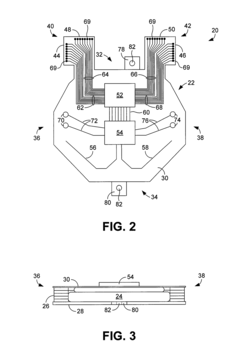
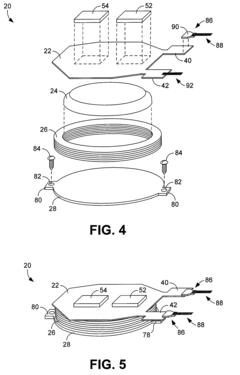
Neural interface materials for next-generation Brain-Computer Interfaces implants
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neural Interface Materials Background and Objectives
Neural interface materials have evolved significantly since the first crude electrodes were used for brain stimulation in the 1870s. The field has progressed from simple metal wires to sophisticated microelectrode arrays capable of recording from hundreds of neurons simultaneously. This evolution reflects the growing understanding of neural signaling mechanisms and the development of materials science, which together have enabled increasingly precise interactions with neural tissue.
The current landscape of neural interface materials encompasses a diverse range of approaches, including metallic electrodes (platinum, gold, iridium oxide), silicon-based microelectrode arrays, polymer-based flexible electrodes, and carbon-based nanomaterials. Each material category offers distinct advantages in terms of electrical properties, biocompatibility, mechanical flexibility, and longevity. Recent advances in nanomaterials and conducting polymers have particularly accelerated progress in this field.
The primary objective of next-generation neural interface materials research is to overcome the fundamental limitations of current implantable technologies. These limitations include poor long-term stability, mechanical mismatch with neural tissue, inflammatory responses, and signal degradation over time. Developing materials that can maintain stable neural recordings for decades rather than months or years represents a critical goal for enabling truly practical brain-computer interface (BCI) implants.
Another key objective is to enhance the spatial and temporal resolution of neural recordings. Current technologies often face a trade-off between coverage area and signal quality. Next-generation materials aim to enable high-density electrode arrays that can simultaneously record from thousands or even millions of neurons with single-cell resolution, while maintaining minimal tissue damage and inflammatory response.
Biocompatibility remains a paramount concern, with research objectives focused on developing materials that not only avoid triggering immune responses but potentially promote beneficial tissue integration. This includes exploring biomimetic approaches that incorporate elements of the extracellular matrix or anti-inflammatory compounds to improve the tissue-electrode interface.
Wireless power and data transmission capabilities represent another crucial objective, requiring materials that can efficiently transduce energy and transmit signals without generating excessive heat or requiring bulky components. This includes exploration of piezoelectric materials, wireless resonant coupling, and ultrasonic power transfer technologies.
The ultimate goal of neural interface materials research is to enable a new generation of BCI implants that can seamlessly integrate with neural tissue, providing stable, high-resolution neural recordings and stimulation capabilities over decades of use. Such technologies would revolutionize treatments for neurological disorders and potentially enable entirely new forms of human-computer interaction.
The current landscape of neural interface materials encompasses a diverse range of approaches, including metallic electrodes (platinum, gold, iridium oxide), silicon-based microelectrode arrays, polymer-based flexible electrodes, and carbon-based nanomaterials. Each material category offers distinct advantages in terms of electrical properties, biocompatibility, mechanical flexibility, and longevity. Recent advances in nanomaterials and conducting polymers have particularly accelerated progress in this field.
The primary objective of next-generation neural interface materials research is to overcome the fundamental limitations of current implantable technologies. These limitations include poor long-term stability, mechanical mismatch with neural tissue, inflammatory responses, and signal degradation over time. Developing materials that can maintain stable neural recordings for decades rather than months or years represents a critical goal for enabling truly practical brain-computer interface (BCI) implants.
Another key objective is to enhance the spatial and temporal resolution of neural recordings. Current technologies often face a trade-off between coverage area and signal quality. Next-generation materials aim to enable high-density electrode arrays that can simultaneously record from thousands or even millions of neurons with single-cell resolution, while maintaining minimal tissue damage and inflammatory response.
Biocompatibility remains a paramount concern, with research objectives focused on developing materials that not only avoid triggering immune responses but potentially promote beneficial tissue integration. This includes exploring biomimetic approaches that incorporate elements of the extracellular matrix or anti-inflammatory compounds to improve the tissue-electrode interface.
Wireless power and data transmission capabilities represent another crucial objective, requiring materials that can efficiently transduce energy and transmit signals without generating excessive heat or requiring bulky components. This includes exploration of piezoelectric materials, wireless resonant coupling, and ultrasonic power transfer technologies.
The ultimate goal of neural interface materials research is to enable a new generation of BCI implants that can seamlessly integrate with neural tissue, providing stable, high-resolution neural recordings and stimulation capabilities over decades of use. Such technologies would revolutionize treatments for neurological disorders and potentially enable entirely new forms of human-computer interaction.
Market Analysis for BCI Implant Technologies
The global Brain-Computer Interface (BCI) implant market is experiencing significant growth, driven by advancements in neural interface materials and increasing applications across medical and non-medical sectors. Current market valuations place the BCI implant segment at approximately $1.2 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 15.3% through 2030, potentially reaching $3.8 billion by the end of the decade.
Medical applications currently dominate the market landscape, accounting for nearly 70% of total market share. Within this segment, treatments for neurological disorders such as epilepsy, Parkinson's disease, and paralysis represent the primary revenue generators. The therapeutic potential of next-generation neural interface materials is particularly promising for conditions previously considered untreatable, creating substantial market opportunities.
Consumer applications, while currently smaller at about 20% of the market, are showing the fastest growth trajectory with annual expansion rates exceeding 25%. This surge is primarily fueled by gaming, virtual reality, and emerging cognitive enhancement applications. Military and defense applications constitute the remaining 10%, focusing on enhanced soldier capabilities and remote operation systems.
Geographically, North America leads with approximately 45% of the global market share, followed by Europe (25%) and Asia-Pacific (20%). China and South Korea are demonstrating the most aggressive growth rates in the Asia-Pacific region, supported by substantial government investments in neurotechnology research and development.
Key market drivers include aging global populations, increasing prevalence of neurological disorders, and growing acceptance of implantable medical technologies. The convergence of miniaturization technologies, biocompatible materials science, and artificial intelligence is accelerating market expansion beyond traditional medical applications.
Significant market barriers persist, including high development and implementation costs, stringent regulatory approval processes, and concerns regarding data privacy and security. The average development timeline for new BCI implant technologies remains lengthy at 5-7 years, with regulatory approval adding an additional 2-3 years in major markets.
Reimbursement landscapes vary significantly across regions, with public healthcare systems in Europe providing broader coverage for BCI implants than private insurance-dominated markets like the United States. This disparity creates uneven market penetration and adoption rates globally.
Medical applications currently dominate the market landscape, accounting for nearly 70% of total market share. Within this segment, treatments for neurological disorders such as epilepsy, Parkinson's disease, and paralysis represent the primary revenue generators. The therapeutic potential of next-generation neural interface materials is particularly promising for conditions previously considered untreatable, creating substantial market opportunities.
Consumer applications, while currently smaller at about 20% of the market, are showing the fastest growth trajectory with annual expansion rates exceeding 25%. This surge is primarily fueled by gaming, virtual reality, and emerging cognitive enhancement applications. Military and defense applications constitute the remaining 10%, focusing on enhanced soldier capabilities and remote operation systems.
Geographically, North America leads with approximately 45% of the global market share, followed by Europe (25%) and Asia-Pacific (20%). China and South Korea are demonstrating the most aggressive growth rates in the Asia-Pacific region, supported by substantial government investments in neurotechnology research and development.
Key market drivers include aging global populations, increasing prevalence of neurological disorders, and growing acceptance of implantable medical technologies. The convergence of miniaturization technologies, biocompatible materials science, and artificial intelligence is accelerating market expansion beyond traditional medical applications.
Significant market barriers persist, including high development and implementation costs, stringent regulatory approval processes, and concerns regarding data privacy and security. The average development timeline for new BCI implant technologies remains lengthy at 5-7 years, with regulatory approval adding an additional 2-3 years in major markets.
Reimbursement landscapes vary significantly across regions, with public healthcare systems in Europe providing broader coverage for BCI implants than private insurance-dominated markets like the United States. This disparity creates uneven market penetration and adoption rates globally.
Current Challenges in Neural Interface Materials
Despite significant advancements in neural interface technologies, several critical challenges persist in the development of materials for next-generation Brain-Computer Interface (BCI) implants. The foremost challenge remains biocompatibility, as materials must coexist with neural tissue without triggering inflammatory responses or scar tissue formation that degrades signal quality over time. Current materials often face a "foreign body response" where the immune system isolates the implant, significantly reducing long-term efficacy.
Mechanical mismatch presents another substantial hurdle. Brain tissue is extremely soft (approximately 1-10 kPa), while traditional electronic materials are orders of magnitude stiffer. This disparity causes micromotion between the implant and surrounding tissue during normal brain movements, leading to chronic inflammation and signal deterioration. Developing materials with elastic properties that match neural tissue remains technically challenging.
Durability in the harsh physiological environment poses significant difficulties. Neural interfaces must withstand constant exposure to corrosive bodily fluids, enzymatic activity, and mechanical stress while maintaining functionality for years or even decades. Current materials often degrade within months to a few years, necessitating replacement surgeries that increase patient risk.
Signal-to-noise ratio optimization continues to challenge engineers. Background neural activity and electromagnetic interference can overwhelm the minute electrical signals being recorded. Materials must simultaneously provide excellent electrical conductivity for signal recording while offering appropriate insulation properties to prevent cross-talk between channels.
Power requirements represent another critical limitation. Implantable neural interfaces typically require external power sources or batteries that need replacement, complicating long-term implantation. Materials that enable wireless power transmission or energy harvesting from the body itself remain underdeveloped.
Fabrication complexity also impedes progress. Manufacturing techniques must produce microscale or nanoscale features while maintaining material integrity and functionality. Current processes often involve complex, multi-step procedures that limit scalability and increase production costs.
Regulatory hurdles compound these technical challenges. Novel materials must undergo extensive safety testing and clinical trials before approval, a process that can take years and cost millions. This regulatory landscape significantly slows innovation and commercial implementation of promising new neural interface materials.
Mechanical mismatch presents another substantial hurdle. Brain tissue is extremely soft (approximately 1-10 kPa), while traditional electronic materials are orders of magnitude stiffer. This disparity causes micromotion between the implant and surrounding tissue during normal brain movements, leading to chronic inflammation and signal deterioration. Developing materials with elastic properties that match neural tissue remains technically challenging.
Durability in the harsh physiological environment poses significant difficulties. Neural interfaces must withstand constant exposure to corrosive bodily fluids, enzymatic activity, and mechanical stress while maintaining functionality for years or even decades. Current materials often degrade within months to a few years, necessitating replacement surgeries that increase patient risk.
Signal-to-noise ratio optimization continues to challenge engineers. Background neural activity and electromagnetic interference can overwhelm the minute electrical signals being recorded. Materials must simultaneously provide excellent electrical conductivity for signal recording while offering appropriate insulation properties to prevent cross-talk between channels.
Power requirements represent another critical limitation. Implantable neural interfaces typically require external power sources or batteries that need replacement, complicating long-term implantation. Materials that enable wireless power transmission or energy harvesting from the body itself remain underdeveloped.
Fabrication complexity also impedes progress. Manufacturing techniques must produce microscale or nanoscale features while maintaining material integrity and functionality. Current processes often involve complex, multi-step procedures that limit scalability and increase production costs.
Regulatory hurdles compound these technical challenges. Novel materials must undergo extensive safety testing and clinical trials before approval, a process that can take years and cost millions. This regulatory landscape significantly slows innovation and commercial implementation of promising new neural interface materials.
State-of-the-Art Neural Interface Material Solutions
01 Biocompatible materials for neural interfaces
Biocompatible materials are essential for neural interfaces to minimize tissue rejection and inflammation. These materials include polymers, hydrogels, and other substrates that can safely interface with neural tissue. The biocompatibility ensures long-term stability of the neural interface and reduces scar tissue formation, which can impede signal transmission. These materials often incorporate coatings or surface modifications to enhance integration with neural tissue.- Biocompatible materials for neural interfaces: Biocompatible materials are essential for neural interfaces to minimize tissue rejection and inflammation when implanted in the body. These materials include flexible polymers, hydrogels, and certain metals that can safely interface with neural tissue. The biocompatibility ensures long-term stability of the neural interface and reduces scar tissue formation, which can degrade signal quality over time. Advanced coatings and surface modifications can further enhance integration with neural tissue.
- Flexible and stretchable electrode materials: Flexible and stretchable materials are used to create neural interfaces that can conform to the natural curvature and movement of neural tissues. These materials include conductive polymers, carbon-based nanomaterials, and thin metal films on elastic substrates. The mechanical compliance reduces tissue damage and improves long-term recording stability by minimizing micro-motion between the electrode and tissue. These materials enable the development of neural interfaces that can maintain functionality during body movement.
- Nanomaterials for enhanced neural signal detection: Nanomaterials such as carbon nanotubes, graphene, and gold nanoparticles are incorporated into neural interfaces to enhance signal detection capabilities. These materials provide high surface area-to-volume ratios, excellent electrical conductivity, and unique surface properties that improve the electrode-tissue interface. Nanomaterial-based electrodes can detect weaker neural signals and provide higher spatial resolution compared to conventional materials, enabling more precise neural recording and stimulation.
- Smart materials with stimulus-responsive properties: Smart materials that can change their properties in response to external stimuli are being developed for advanced neural interfaces. These include shape-memory polymers, temperature-responsive hydrogels, and electrically switchable materials. Such materials can facilitate minimally invasive implantation, controlled drug release at the interface site, or adaptive mechanical properties to match tissue characteristics. These stimulus-responsive properties enable neural interfaces that can dynamically adapt to changing physiological conditions.
- Integration of neural interfaces with machine learning systems: Advanced neural interface materials are being designed to work in conjunction with machine learning algorithms to improve signal processing and interpretation. These systems incorporate specialized materials that can generate consistent, high-quality neural signals that are optimized for machine learning analysis. The combination of advanced materials science and artificial intelligence enables more accurate decoding of neural signals, adaptive calibration of neural interfaces, and development of more intuitive brain-computer interfaces for various applications.
02 Flexible and stretchable neural interface materials
Flexible and stretchable materials are developed for neural interfaces to better match the mechanical properties of brain tissue. These materials can conform to the curved surfaces of the brain and accommodate natural movements, reducing mechanical stress and tissue damage. Examples include thin-film polymers, elastomers, and mesh-like structures that maintain electrical functionality while providing mechanical compliance. This flexibility is crucial for long-term implantation and stable neural recording.Expand Specific Solutions03 Conductive materials for signal transmission
Specialized conductive materials are used in neural interfaces to effectively transmit electrical signals between neural tissue and electronic devices. These materials include metallic nanostructures, conductive polymers, carbon-based materials like graphene, and composite materials that combine conductivity with biocompatibility. The design of these materials focuses on optimizing signal-to-noise ratio while minimizing impedance to ensure accurate neural recording and stimulation.Expand Specific Solutions04 Neural interface materials with drug delivery capabilities
Advanced neural interface materials incorporate drug delivery capabilities to reduce inflammation, prevent infection, or deliver therapeutic agents directly to neural tissue. These materials often feature porous structures, degradable components, or reservoirs that can store and release drugs in a controlled manner. This dual functionality helps maintain the long-term performance of neural interfaces by actively managing the tissue response at the implant site.Expand Specific Solutions05 AI-integrated neural interface materials
Emerging neural interface materials integrate artificial intelligence capabilities directly into the material structure. These smart materials can adapt to changing neural signals, self-calibrate, or process information at the interface level. They often incorporate neuromorphic computing elements or machine learning algorithms that can operate with minimal power requirements. This integration of AI with neural interface materials enables more sophisticated brain-machine interactions and improved signal processing capabilities.Expand Specific Solutions
Leading Organizations in BCI Implant Development
The neural interface materials market for next-generation Brain-Computer Interface implants is currently in an early growth phase, characterized by rapid technological advancement and increasing commercial interest. The global BCI market is projected to expand significantly, driven by medical applications and emerging consumer use cases. Technologically, the field shows varying maturity levels across different approaches. Leading research institutions like MIT, EPFL, and University of Michigan are advancing fundamental materials science, while companies including Neuralink, Precision Neuroscience, and Cognixion are commercializing innovative implantable solutions. Chinese institutions such as Peking University and Shanghai Jiao Tong University are making significant contributions to flexible electronics and biocompatible materials, indicating a globally competitive landscape with both established players and emerging startups actively developing proprietary technologies.
Precision Neuroscience Corp.
Technical Solution: Precision Neuroscience has developed the Layer 7 Cortical Interface, an ultra-thin, flexible neural interface that resembles a piece of cellophane and can be inserted through a minimally invasive cranial slit without traditional open-brain surgery. The device utilizes a film-based array of micron-scale electrodes arranged on a substrate thinner than a human hair (approximately 20 μm thick), allowing it to conform to the brain's surface without penetrating tissue. Their proprietary materials combine polymer substrates with metal electrode arrays that maintain conductivity while allowing flexibility. The interface targets the brain's outer layer (cortex) and uses a specialized insertion system that unfolds the film across the brain surface. Their electrode materials incorporate novel coatings that enhance signal quality and reduce impedance at the electrode-tissue interface, improving long-term recording stability and biocompatibility.
Strengths: Minimally invasive insertion reduces surgical risks and brain trauma; flexible materials better match brain mechanical properties, potentially reducing foreign body response; reversible implantation allows for device removal if needed. Weaknesses: Surface positioning limits access to deeper brain structures; may have lower signal resolution compared to penetrating electrodes; relatively new technology with limited long-term clinical data on material durability and performance.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered several breakthrough neural interface materials for BCI implants. Their researchers developed fiber-based neural probes that integrate multiple functions including optical stimulation, electrical recording, and drug delivery within a single flexible fiber. These fibers are created using thermal drawing processes that maintain the integrity of conductive, optical, and fluidic components while achieving diameters as small as 85 μm. MIT's materials innovation extends to mesh electronics, ultra-flexible neural nets with feature sizes matching neurons (approximately 10 μm) and bending stiffness similar to brain tissue (~10^-15 N·m²), allowing for minimally invasive delivery through syringes as small as 100 μm in inner diameter. Their recent work includes the development of hydrogel-based electrodes that better match brain tissue mechanical properties, reducing foreign body responses. MIT researchers have also created "neuron-like electronics" using conductive polymers (PEDOT:PSS) and elastomeric substrates that can stretch and flex with brain tissue, maintaining stable neural recordings over extended periods while minimizing inflammation.
Strengths: Multifunctional capabilities combining recording, stimulation and drug delivery; tissue-like mechanical properties minimize immune response; innovative fabrication techniques enable complex architectures at neural-appropriate scales. Weaknesses: Some advanced materials face challenges in scaling to high-channel-count arrays; certain conductive polymers may have limited long-term stability in physiological environments; complex fabrication processes may present manufacturing challenges.
Critical Patents and Research in Neural Interface Materials
High density neural implants for brain-machine interfaces
PatentWO2020018571A1
Innovation
- A distributed sensor system utilizing CMOS untethered microelectronic chiplets, or neurograins, that form a network of individual neural interfacing nodes in the cortex for active recording and electrical stimulation, coupled with an epidermal skinpatch and external devices for wireless communication and power transfer, enabling high-density neural interfaces.
Neural interface systems and methods
PatentActiveUS8428732B2
Innovation
- The development of an ultra-low-power wireless implantable neural probe with a flexible substrate integrating electrodes and electronics, using asynchronous sampling methods like integrate-and-fire, and offloading processing to a backend computing device to reduce power requirements and minimize tissue disruption.
Biocompatibility and Longevity Considerations
Biocompatibility and longevity represent critical challenges for neural interface materials in next-generation Brain-Computer Interface (BCI) implants. The foreign body response remains a significant barrier, as the brain's immune system recognizes implanted materials as invasive entities, triggering inflammatory responses that lead to glial scarring. This encapsulation process isolates the electrode from target neurons, progressively degrading signal quality and ultimately causing device failure.
Current materials science research focuses on developing ultra-flexible substrates that minimize mechanical mismatch between rigid electronics and soft neural tissue. Polymers such as polyimide, parylene-C, and PDMS demonstrate promising biocompatibility profiles, with elastic moduli closer to brain tissue than traditional metal or silicon-based interfaces. These materials reduce micromotion-induced inflammation and extend functional implant lifespans from months to several years in preclinical models.
Surface modification strategies have emerged as essential approaches for enhancing biocompatibility. Anti-inflammatory coatings incorporating dexamethasone or other immunosuppressive agents provide localized inflammation control without systemic effects. Biomimetic coatings using neural adhesion molecules, laminin fragments, or RGD peptide sequences actively promote neuronal integration while discouraging glial cell attachment, creating more favorable interface environments.
Degradation mechanisms in the harsh physiological environment present ongoing challenges. Material breakdown through hydrolysis, oxidation, and enzymatic activity compromises both device functionality and patient safety. Next-generation materials incorporate self-healing capabilities through dynamic chemical bonds that can reform after damage, potentially extending functional lifespans significantly. Hermetic encapsulation technologies using atomic layer deposition of ceramics or diamond-like carbon provide exceptional barrier properties against moisture ingress.
Long-term stability testing protocols have evolved to better predict in vivo performance. Accelerated aging studies simulating years of implantation within weeks help identify potential failure modes before clinical implementation. Advanced in vitro models incorporating microfluidic systems with human neural cells better replicate the dynamic biological environment than traditional static testing methods.
Regulatory considerations for long-term neural implants remain stringent, requiring extensive biocompatibility testing according to ISO 10993 standards. The FDA has recently established specialized guidance for implantable BCI devices, emphasizing the need for comprehensive chronic toxicity and carcinogenicity assessments beyond standard protocols for shorter-term medical implants.
Current materials science research focuses on developing ultra-flexible substrates that minimize mechanical mismatch between rigid electronics and soft neural tissue. Polymers such as polyimide, parylene-C, and PDMS demonstrate promising biocompatibility profiles, with elastic moduli closer to brain tissue than traditional metal or silicon-based interfaces. These materials reduce micromotion-induced inflammation and extend functional implant lifespans from months to several years in preclinical models.
Surface modification strategies have emerged as essential approaches for enhancing biocompatibility. Anti-inflammatory coatings incorporating dexamethasone or other immunosuppressive agents provide localized inflammation control without systemic effects. Biomimetic coatings using neural adhesion molecules, laminin fragments, or RGD peptide sequences actively promote neuronal integration while discouraging glial cell attachment, creating more favorable interface environments.
Degradation mechanisms in the harsh physiological environment present ongoing challenges. Material breakdown through hydrolysis, oxidation, and enzymatic activity compromises both device functionality and patient safety. Next-generation materials incorporate self-healing capabilities through dynamic chemical bonds that can reform after damage, potentially extending functional lifespans significantly. Hermetic encapsulation technologies using atomic layer deposition of ceramics or diamond-like carbon provide exceptional barrier properties against moisture ingress.
Long-term stability testing protocols have evolved to better predict in vivo performance. Accelerated aging studies simulating years of implantation within weeks help identify potential failure modes before clinical implementation. Advanced in vitro models incorporating microfluidic systems with human neural cells better replicate the dynamic biological environment than traditional static testing methods.
Regulatory considerations for long-term neural implants remain stringent, requiring extensive biocompatibility testing according to ISO 10993 standards. The FDA has recently established specialized guidance for implantable BCI devices, emphasizing the need for comprehensive chronic toxicity and carcinogenicity assessments beyond standard protocols for shorter-term medical implants.
Regulatory Framework for Implantable Neural Devices
The regulatory landscape for implantable neural devices represents a complex and evolving framework that significantly impacts the development and deployment of next-generation Brain-Computer Interface (BCI) implants. Currently, the FDA's Center for Devices and Radiological Health (CDRH) classifies most neural interface devices as Class III medical devices, requiring the most stringent regulatory oversight including premarket approval (PMA) applications with extensive clinical trial data.
In the United States, neural implants must navigate the FDA's regulatory pathway which includes demonstrating both safety and efficacy through rigorous preclinical and clinical studies. The FDA has recently established specific guidance for BCI devices, addressing unique considerations such as long-term biocompatibility, electrical safety, and cybersecurity requirements. These guidelines specifically address novel neural interface materials, requiring comprehensive testing for tissue reaction, degradation characteristics, and potential neurotoxicity.
The European regulatory framework under the Medical Device Regulation (MDR) imposes similarly stringent requirements, with neural implants falling under Class III classification. The MDR places particular emphasis on post-market surveillance and risk management throughout the device lifecycle, with specific provisions for implantable electronic devices that interface with the central nervous system.
International standards organizations have developed several relevant standards that manufacturers must adhere to, including ISO 14708 for implantable neurostimulators, IEC 60601 for medical electrical equipment safety, and ISO 10993 for biological evaluation of medical devices. These standards are continuously evolving to address emerging materials and technologies in neural interfaces.
Regulatory bodies worldwide are increasingly focusing on the ethical implications of BCI technology. This has led to the development of specific requirements regarding informed consent, data privacy, and cybersecurity for devices that can potentially access or influence neural activity. The FDA's Digital Health Innovation Action Plan and the EU's GDPR both contain provisions that impact how neural interface data must be handled and protected.
Emerging regulatory considerations specifically addressing novel neural interface materials include requirements for nanomaterial safety assessment, degradation product characterization, and long-term stability evaluation. Regulatory agencies are developing new testing protocols for advanced materials such as conducting polymers, carbon-based materials, and hydrogels that are increasingly being incorporated into next-generation neural interfaces.
The pathway to regulatory approval for novel neural interface materials typically requires a phased approach, beginning with extensive in vitro characterization, followed by preclinical animal studies, and culminating in carefully designed clinical trials with robust safety monitoring protocols. This process can take 5-7 years for truly innovative materials, representing a significant consideration in development timelines for next-generation BCI implants.
In the United States, neural implants must navigate the FDA's regulatory pathway which includes demonstrating both safety and efficacy through rigorous preclinical and clinical studies. The FDA has recently established specific guidance for BCI devices, addressing unique considerations such as long-term biocompatibility, electrical safety, and cybersecurity requirements. These guidelines specifically address novel neural interface materials, requiring comprehensive testing for tissue reaction, degradation characteristics, and potential neurotoxicity.
The European regulatory framework under the Medical Device Regulation (MDR) imposes similarly stringent requirements, with neural implants falling under Class III classification. The MDR places particular emphasis on post-market surveillance and risk management throughout the device lifecycle, with specific provisions for implantable electronic devices that interface with the central nervous system.
International standards organizations have developed several relevant standards that manufacturers must adhere to, including ISO 14708 for implantable neurostimulators, IEC 60601 for medical electrical equipment safety, and ISO 10993 for biological evaluation of medical devices. These standards are continuously evolving to address emerging materials and technologies in neural interfaces.
Regulatory bodies worldwide are increasingly focusing on the ethical implications of BCI technology. This has led to the development of specific requirements regarding informed consent, data privacy, and cybersecurity for devices that can potentially access or influence neural activity. The FDA's Digital Health Innovation Action Plan and the EU's GDPR both contain provisions that impact how neural interface data must be handled and protected.
Emerging regulatory considerations specifically addressing novel neural interface materials include requirements for nanomaterial safety assessment, degradation product characterization, and long-term stability evaluation. Regulatory agencies are developing new testing protocols for advanced materials such as conducting polymers, carbon-based materials, and hydrogels that are increasingly being incorporated into next-generation neural interfaces.
The pathway to regulatory approval for novel neural interface materials typically requires a phased approach, beginning with extensive in vitro characterization, followed by preclinical animal studies, and culminating in carefully designed clinical trials with robust safety monitoring protocols. This process can take 5-7 years for truly innovative materials, representing a significant consideration in development timelines for next-generation BCI implants.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!