Pharmaceutical Advances in Muscimol Molecular Configuration
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Research Background and Objectives
Muscimol, a potent GABA-A receptor agonist, has been the subject of extensive research in the pharmaceutical industry due to its potential therapeutic applications. This naturally occurring psychoactive compound, found in various species of mushrooms, particularly Amanita muscaria, has garnered significant attention for its unique molecular structure and pharmacological properties.
The history of muscimol research dates back to the 1960s when it was first isolated and characterized. Since then, the scientific community has made considerable progress in understanding its molecular configuration and mechanism of action. The primary objective of current research efforts is to harness the therapeutic potential of muscimol while mitigating its psychoactive effects, which have historically limited its clinical use.
Recent advancements in molecular biology and computational chemistry have paved the way for a more comprehensive understanding of muscimol's interaction with GABA receptors. This has led to the development of novel synthetic analogues and prodrugs that aim to enhance its pharmacokinetic profile and reduce unwanted side effects. The ultimate goal is to create muscimol-based pharmaceuticals that can effectively treat various neurological and psychiatric disorders, including epilepsy, anxiety, and sleep disorders.
The pharmaceutical industry's interest in muscimol has been driven by the growing need for more effective and safer alternatives to existing GABA-ergic drugs. Current benzodiazepines and barbiturates, while effective, often come with significant side effects and the risk of dependence. Muscimol's unique molecular configuration offers the potential for more selective receptor targeting, potentially leading to improved therapeutic outcomes with fewer adverse effects.
As research progresses, the focus has shifted towards optimizing muscimol's molecular structure to enhance its drug-like properties. This includes improving its bioavailability, extending its half-life, and increasing its specificity for particular GABA receptor subtypes. Such modifications aim to create a new generation of muscimol-derived drugs that can be administered orally and cross the blood-brain barrier more efficiently.
The exploration of muscimol's molecular configuration also extends to its potential applications beyond traditional CNS disorders. Emerging research suggests possible uses in inflammatory conditions, pain management, and even certain types of cancer. This broadening of scope has further intensified the scientific community's interest in unraveling the full potential of this fascinating molecule.
The history of muscimol research dates back to the 1960s when it was first isolated and characterized. Since then, the scientific community has made considerable progress in understanding its molecular configuration and mechanism of action. The primary objective of current research efforts is to harness the therapeutic potential of muscimol while mitigating its psychoactive effects, which have historically limited its clinical use.
Recent advancements in molecular biology and computational chemistry have paved the way for a more comprehensive understanding of muscimol's interaction with GABA receptors. This has led to the development of novel synthetic analogues and prodrugs that aim to enhance its pharmacokinetic profile and reduce unwanted side effects. The ultimate goal is to create muscimol-based pharmaceuticals that can effectively treat various neurological and psychiatric disorders, including epilepsy, anxiety, and sleep disorders.
The pharmaceutical industry's interest in muscimol has been driven by the growing need for more effective and safer alternatives to existing GABA-ergic drugs. Current benzodiazepines and barbiturates, while effective, often come with significant side effects and the risk of dependence. Muscimol's unique molecular configuration offers the potential for more selective receptor targeting, potentially leading to improved therapeutic outcomes with fewer adverse effects.
As research progresses, the focus has shifted towards optimizing muscimol's molecular structure to enhance its drug-like properties. This includes improving its bioavailability, extending its half-life, and increasing its specificity for particular GABA receptor subtypes. Such modifications aim to create a new generation of muscimol-derived drugs that can be administered orally and cross the blood-brain barrier more efficiently.
The exploration of muscimol's molecular configuration also extends to its potential applications beyond traditional CNS disorders. Emerging research suggests possible uses in inflammatory conditions, pain management, and even certain types of cancer. This broadening of scope has further intensified the scientific community's interest in unraveling the full potential of this fascinating molecule.
Market Analysis for Muscimol-Based Pharmaceuticals
The market for muscimol-based pharmaceuticals is experiencing significant growth, driven by increasing research into the therapeutic potential of this compound. Muscimol, a psychoactive alkaloid found in certain mushroom species, has garnered attention for its potential applications in treating various neurological and psychiatric disorders.
The global market for muscimol-based pharmaceuticals is primarily segmented into research chemicals, potential therapeutic drugs, and diagnostic tools. Currently, the research chemical segment dominates the market, as muscimol is extensively used in neuroscience studies to investigate GABA receptor functions. However, the therapeutic drug segment is expected to witness the highest growth rate in the coming years.
Several factors are contributing to the expanding market demand for muscimol-based pharmaceuticals. The rising prevalence of neurological disorders, such as epilepsy and Parkinson's disease, is a key driver. Additionally, the growing interest in novel treatments for anxiety disorders and sleep disturbances has fueled research into muscimol's potential applications.
The pharmaceutical industry's shift towards precision medicine and targeted therapies has also boosted interest in muscimol-based drugs. As researchers gain a deeper understanding of the molecular mechanisms underlying various disorders, the unique properties of muscimol make it an attractive candidate for developing highly specific treatments.
Geographically, North America currently leads the muscimol-based pharmaceutical market, owing to its advanced healthcare infrastructure and significant investments in neuroscience research. Europe follows closely, with several academic institutions and pharmaceutical companies actively exploring muscimol's therapeutic potential. The Asia-Pacific region is expected to witness rapid growth in the coming years, driven by increasing healthcare expenditure and rising awareness of neurological disorders.
Despite the promising outlook, the market faces several challenges. Regulatory hurdles associated with psychoactive compounds and the need for extensive clinical trials to establish safety and efficacy profiles are significant barriers to market entry. Moreover, the limited availability of high-quality, pharmaceutical-grade muscimol poses challenges for large-scale production and commercialization.
Looking ahead, the market for muscimol-based pharmaceuticals is projected to expand substantially over the next decade. Advancements in synthetic biology and chemical engineering are expected to overcome production challenges, potentially leading to more cost-effective and scalable manufacturing processes. As clinical trials progress and new therapeutic applications are discovered, the market is likely to see the introduction of novel muscimol-based drugs targeting a wide range of neurological and psychiatric conditions.
The global market for muscimol-based pharmaceuticals is primarily segmented into research chemicals, potential therapeutic drugs, and diagnostic tools. Currently, the research chemical segment dominates the market, as muscimol is extensively used in neuroscience studies to investigate GABA receptor functions. However, the therapeutic drug segment is expected to witness the highest growth rate in the coming years.
Several factors are contributing to the expanding market demand for muscimol-based pharmaceuticals. The rising prevalence of neurological disorders, such as epilepsy and Parkinson's disease, is a key driver. Additionally, the growing interest in novel treatments for anxiety disorders and sleep disturbances has fueled research into muscimol's potential applications.
The pharmaceutical industry's shift towards precision medicine and targeted therapies has also boosted interest in muscimol-based drugs. As researchers gain a deeper understanding of the molecular mechanisms underlying various disorders, the unique properties of muscimol make it an attractive candidate for developing highly specific treatments.
Geographically, North America currently leads the muscimol-based pharmaceutical market, owing to its advanced healthcare infrastructure and significant investments in neuroscience research. Europe follows closely, with several academic institutions and pharmaceutical companies actively exploring muscimol's therapeutic potential. The Asia-Pacific region is expected to witness rapid growth in the coming years, driven by increasing healthcare expenditure and rising awareness of neurological disorders.
Despite the promising outlook, the market faces several challenges. Regulatory hurdles associated with psychoactive compounds and the need for extensive clinical trials to establish safety and efficacy profiles are significant barriers to market entry. Moreover, the limited availability of high-quality, pharmaceutical-grade muscimol poses challenges for large-scale production and commercialization.
Looking ahead, the market for muscimol-based pharmaceuticals is projected to expand substantially over the next decade. Advancements in synthetic biology and chemical engineering are expected to overcome production challenges, potentially leading to more cost-effective and scalable manufacturing processes. As clinical trials progress and new therapeutic applications are discovered, the market is likely to see the introduction of novel muscimol-based drugs targeting a wide range of neurological and psychiatric conditions.
Current Challenges in Muscimol Molecular Configuration
Despite significant advancements in muscimol research, several challenges persist in the field of muscimol molecular configuration. One of the primary obstacles is the complexity of muscimol's molecular structure and its interaction with GABA receptors. The precise binding mechanism and conformational changes during receptor activation remain incompletely understood, hindering the development of more effective and targeted therapies.
Another challenge lies in the synthesis and purification of muscimol and its derivatives. Current methods often yield low quantities or require complex, multi-step processes, making large-scale production for pharmaceutical applications difficult and costly. Additionally, the stability of muscimol under various physiological conditions poses a significant hurdle in formulation development and drug delivery.
The blood-brain barrier (BBB) presents a formidable challenge in muscimol-based drug development. While muscimol can cross the BBB to some extent, its passage is limited, reducing its potential efficacy in treating central nervous system disorders. Enhancing BBB penetration without compromising the compound's pharmacological properties remains a key area of focus for researchers.
Selectivity and specificity of muscimol action also present ongoing challenges. While muscimol is known for its potent GABA-A receptor agonist activity, achieving subtype selectivity within the GABA receptor family is crucial for minimizing side effects and optimizing therapeutic outcomes. The development of muscimol analogs with improved selectivity profiles is an active area of research, but progress has been slow due to the structural similarities among receptor subtypes.
Furthermore, the potential for tolerance and dependence associated with long-term muscimol use raises concerns in chronic treatment scenarios. Understanding the molecular mechanisms underlying these phenomena and developing strategies to mitigate them are essential for the successful clinical application of muscimol-based therapies.
Lastly, the regulatory landscape surrounding muscimol and its derivatives poses challenges for pharmaceutical development. As a naturally occurring psychoactive compound, muscimol faces stringent regulatory scrutiny, necessitating extensive safety and efficacy studies before potential approval for therapeutic use. Navigating these regulatory requirements while maintaining the compound's therapeutic potential remains a significant hurdle for researchers and pharmaceutical companies alike.
Another challenge lies in the synthesis and purification of muscimol and its derivatives. Current methods often yield low quantities or require complex, multi-step processes, making large-scale production for pharmaceutical applications difficult and costly. Additionally, the stability of muscimol under various physiological conditions poses a significant hurdle in formulation development and drug delivery.
The blood-brain barrier (BBB) presents a formidable challenge in muscimol-based drug development. While muscimol can cross the BBB to some extent, its passage is limited, reducing its potential efficacy in treating central nervous system disorders. Enhancing BBB penetration without compromising the compound's pharmacological properties remains a key area of focus for researchers.
Selectivity and specificity of muscimol action also present ongoing challenges. While muscimol is known for its potent GABA-A receptor agonist activity, achieving subtype selectivity within the GABA receptor family is crucial for minimizing side effects and optimizing therapeutic outcomes. The development of muscimol analogs with improved selectivity profiles is an active area of research, but progress has been slow due to the structural similarities among receptor subtypes.
Furthermore, the potential for tolerance and dependence associated with long-term muscimol use raises concerns in chronic treatment scenarios. Understanding the molecular mechanisms underlying these phenomena and developing strategies to mitigate them are essential for the successful clinical application of muscimol-based therapies.
Lastly, the regulatory landscape surrounding muscimol and its derivatives poses challenges for pharmaceutical development. As a naturally occurring psychoactive compound, muscimol faces stringent regulatory scrutiny, necessitating extensive safety and efficacy studies before potential approval for therapeutic use. Navigating these regulatory requirements while maintaining the compound's therapeutic potential remains a significant hurdle for researchers and pharmaceutical companies alike.
Current Approaches to Muscimol Molecular Optimization
01 Molecular structure and chemical properties
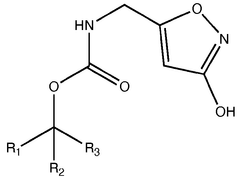
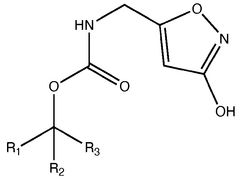
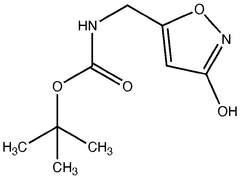
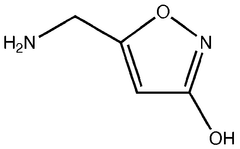
Muscimol is a psychoactive compound with the molecular formula C4H6N2O2. It has a zwitterionic structure in aqueous solution, containing both a protonated amino group and a deprotonated carboxylic acid group. The molecule is relatively small and polar, which contributes to its ability to cross the blood-brain barrier.- Molecular structure and chemical properties: Muscimol is a psychoactive compound with the molecular formula C4H6N2O2. It has a zwitterionic structure in aqueous solution, with a positively charged amino group and a negatively charged carboxyl group. The molecule is relatively small and polar, which contributes to its ability to cross the blood-brain barrier.
- Synthesis and production methods: Various methods have been developed for the synthesis of muscimol, including both chemical and biosynthetic approaches. Some techniques involve the use of precursor compounds derived from natural sources, while others rely on fully synthetic routes. Optimization of these processes aims to improve yield and purity.
- Pharmacological effects and mechanisms: Muscimol acts as a potent GABA receptor agonist, particularly at GABA-A receptors. Its molecular configuration allows it to bind to these receptors, leading to increased inhibitory neurotransmission in the central nervous system. This interaction is responsible for its sedative, hypnotic, and muscle relaxant effects.
- Analytical methods for detection and quantification: Various analytical techniques have been developed to detect and quantify muscimol in biological samples and pharmaceutical preparations. These methods often involve chromatographic separation coupled with mass spectrometry or other detection systems. The molecular configuration of muscimol influences its behavior in these analytical processes.
- Formulation and drug delivery strategies: The molecular configuration of muscimol impacts its formulation into pharmaceutical preparations. Researchers have explored various drug delivery strategies to optimize its bioavailability and therapeutic effects. These may include novel formulations, controlled release systems, or targeted delivery approaches that take advantage of muscimol's chemical properties.
02 Stereochemistry and isomers
Muscimol exists in different stereoisomeric forms, including enantiomers and diastereomers. The specific stereochemistry of muscimol can affect its biological activity and pharmacological properties. Research has been conducted to study the various isomers and their potential therapeutic applications.Expand Specific Solutions03 Synthesis and production methods
Various methods have been developed for the synthesis of muscimol and its derivatives. These include chemical synthesis routes, biosynthetic approaches, and semi-synthetic methods. Researchers have explored efficient and scalable production techniques to obtain high-purity muscimol for research and potential pharmaceutical applications.Expand Specific Solutions04 Analytical techniques for characterization
Advanced analytical techniques are employed to characterize the molecular configuration of muscimol. These include nuclear magnetic resonance (NMR) spectroscopy, X-ray crystallography, mass spectrometry, and computational modeling. These methods provide detailed information about the compound's structure, conformation, and electronic properties.Expand Specific Solutions05 Structure-activity relationships
Studies have been conducted to investigate the structure-activity relationships of muscimol and its analogs. Researchers have explored how modifications to the molecular structure affect the compound's pharmacological properties, receptor binding affinity, and potential therapeutic effects. This information is crucial for the development of novel muscimol-based drugs or related compounds.Expand Specific Solutions
Key Players in Muscimol Pharmaceutical Research
The pharmaceutical landscape for muscimol molecular configuration advancements is in an early growth stage, characterized by increasing research activities and emerging clinical applications. The market size is relatively small but expanding, driven by potential therapeutic uses in neurological disorders. Technological maturity is progressing, with companies like ACADIA Pharmaceuticals, F. Hoffmann-La Roche, and Vertex Pharmaceuticals leading research efforts. Academic institutions such as Ocean University of China and Georgetown University School of Medicine are also contributing to the field. While still in development, this niche area shows promise for future growth and innovation in CNS drug development.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has been investigating muscimol and its derivatives as potential treatments for neurological disorders. Their research focuses on developing novel muscimol analogs with improved pharmacokinetic profiles and enhanced selectivity for GABA-A receptors. The company has conducted preclinical studies on muscimol-inspired compounds for conditions such as epilepsy and anxiety disorders[3]. ACADIA's approach involves molecular modeling and structure-activity relationship studies to optimize the therapeutic potential of muscimol-based molecules[4].
Strengths: Strong expertise in CNS drug development, advanced molecular design capabilities. Weaknesses: Competitive field in neurological drug development, potential off-target effects of muscimol analogs.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has been exploring the potential of muscimol and related GABA-A receptor agonists in their neuroscience research pipeline. Their approach involves the development of synthetic muscimol derivatives with enhanced blood-brain barrier penetration and improved target specificity. Roche has conducted extensive pharmacological profiling of these compounds, investigating their potential in treating conditions such as insomnia, anxiety, and certain forms of epilepsy[5]. The company has also invested in advanced imaging techniques to visualize the binding of muscimol analogs to GABA-A receptors in vivo[6].
Strengths: Extensive R&D resources, strong track record in CNS drug development. Weaknesses: Complex regulatory pathway for novel CNS drugs, potential for drug-drug interactions with GABA-ergic compounds.
Breakthrough Patents in Muscimol Configuration
Pharmaceutical intermediates and methods for preparing the same in the synthesis of muscimol and congeners and derivatives thereof
PatentWO2025128106A1
Innovation
- A novel method for preparing muscimol mono-BOC and muscimol hydrochloride that avoids the use of ion exchange chromatography by modifying the original synthetic route to include a flow reactor for the cyclization step and using BOC anhydride to purify the muscimol, thereby stabilizing the product and improving yields.
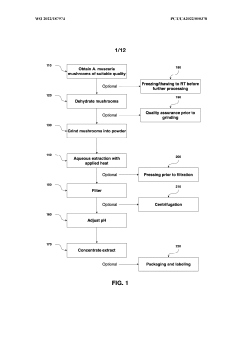
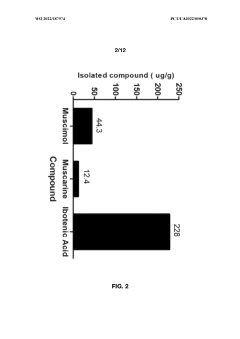
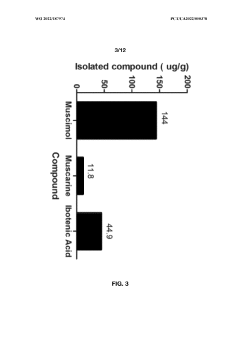
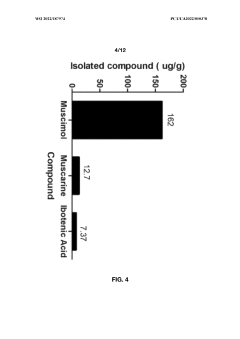
Processes for extracting muscimol from amanita muscaria
PatentWO2022187974A1
Innovation
- Aqueous extraction methods involving heat, pH reduction, and concentration techniques such as distillation and refluxing are employed to decrease ibotenic acid content and increase muscimol content in the extract, including steps like grinding the mushroom biomass, filtering, and acidification to facilitate decarboxylation of ibotenic acid to muscimol.
Regulatory Framework for Novel Muscimol Formulations
The regulatory framework for novel muscimol formulations is a critical aspect of pharmaceutical development that requires careful consideration and adherence to established guidelines. As muscimol, a psychoactive compound found in certain mushroom species, gains attention for its potential therapeutic applications, regulatory bodies worldwide are tasked with ensuring its safe and effective use in pharmaceutical products.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development and approval of novel muscimol formulations. The FDA's regulatory pathway for such compounds typically involves a rigorous review process, including preclinical studies, clinical trials, and comprehensive safety assessments. Developers of muscimol-based pharmaceuticals must navigate the Investigational New Drug (IND) application process, followed by New Drug Application (NDA) submission for market approval.
The European Medicines Agency (EMA) provides regulatory oversight for muscimol formulations within the European Union. The EMA's centralized procedure offers a streamlined approach for novel drug approvals across member states. Developers must engage with the EMA early in the development process, participating in scientific advice meetings and adhering to Good Manufacturing Practice (GMP) standards throughout the product lifecycle.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) governs the regulatory framework for innovative muscimol formulations. The PMDA's approach emphasizes early consultation and collaboration with developers to facilitate efficient drug development and approval processes. Japanese regulations also require specific considerations for psychoactive compounds, including stringent control measures and post-marketing surveillance.
Globally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides harmonized guidelines that influence regulatory frameworks across regions. These guidelines address various aspects of drug development, including quality, safety, efficacy, and multidisciplinary topics, which are particularly relevant for novel compounds like muscimol.
Regulatory considerations for muscimol formulations extend beyond traditional pharmaceutical regulations. Given its psychoactive properties, muscimol-based products may fall under additional regulatory scrutiny, potentially involving controlled substance legislation. Developers must navigate complex legal landscapes that vary by jurisdiction, addressing concerns related to abuse potential and diversion risks.
As research into muscimol's therapeutic potential progresses, regulatory frameworks are likely to evolve. Adaptive licensing approaches and accelerated approval pathways may become increasingly relevant for innovative muscimol formulations, particularly in areas of unmet medical need. However, these pathways often come with stringent post-marketing commitments and ongoing safety monitoring requirements.
Regulatory agencies are also focusing on the development of specific guidance for psychoactive compounds with therapeutic potential. This evolving regulatory landscape aims to balance the need for innovation with the imperative of public safety, potentially paving the way for more streamlined development of novel muscimol formulations while maintaining rigorous safety standards.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development and approval of novel muscimol formulations. The FDA's regulatory pathway for such compounds typically involves a rigorous review process, including preclinical studies, clinical trials, and comprehensive safety assessments. Developers of muscimol-based pharmaceuticals must navigate the Investigational New Drug (IND) application process, followed by New Drug Application (NDA) submission for market approval.
The European Medicines Agency (EMA) provides regulatory oversight for muscimol formulations within the European Union. The EMA's centralized procedure offers a streamlined approach for novel drug approvals across member states. Developers must engage with the EMA early in the development process, participating in scientific advice meetings and adhering to Good Manufacturing Practice (GMP) standards throughout the product lifecycle.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) governs the regulatory framework for innovative muscimol formulations. The PMDA's approach emphasizes early consultation and collaboration with developers to facilitate efficient drug development and approval processes. Japanese regulations also require specific considerations for psychoactive compounds, including stringent control measures and post-marketing surveillance.
Globally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides harmonized guidelines that influence regulatory frameworks across regions. These guidelines address various aspects of drug development, including quality, safety, efficacy, and multidisciplinary topics, which are particularly relevant for novel compounds like muscimol.
Regulatory considerations for muscimol formulations extend beyond traditional pharmaceutical regulations. Given its psychoactive properties, muscimol-based products may fall under additional regulatory scrutiny, potentially involving controlled substance legislation. Developers must navigate complex legal landscapes that vary by jurisdiction, addressing concerns related to abuse potential and diversion risks.
As research into muscimol's therapeutic potential progresses, regulatory frameworks are likely to evolve. Adaptive licensing approaches and accelerated approval pathways may become increasingly relevant for innovative muscimol formulations, particularly in areas of unmet medical need. However, these pathways often come with stringent post-marketing commitments and ongoing safety monitoring requirements.
Regulatory agencies are also focusing on the development of specific guidance for psychoactive compounds with therapeutic potential. This evolving regulatory landscape aims to balance the need for innovation with the imperative of public safety, potentially paving the way for more streamlined development of novel muscimol formulations while maintaining rigorous safety standards.
Pharmacokinetic Considerations for Muscimol Derivatives
The pharmacokinetic profile of muscimol and its derivatives plays a crucial role in determining their therapeutic potential and safety. Muscimol, a potent GABA-A receptor agonist, exhibits unique pharmacokinetic properties that influence its efficacy and potential side effects. Understanding these characteristics is essential for developing improved muscimol-based pharmaceuticals.
Absorption of muscimol is relatively rapid, with peak plasma concentrations typically reached within 1-2 hours after oral administration. However, the bioavailability of muscimol can be limited due to its polar nature, which hinders passive diffusion across biological membranes. This has led researchers to explore various strategies to enhance absorption, including the development of lipophilic prodrugs and novel drug delivery systems.
Distribution of muscimol throughout the body is influenced by its hydrophilic nature. While it can cross the blood-brain barrier to some extent, the efficiency of central nervous system penetration is not optimal. This has prompted investigations into chemical modifications and delivery techniques to improve brain targeting and increase therapeutic efficacy for neurological disorders.
Metabolism of muscimol primarily occurs in the liver through oxidative deamination, resulting in the formation of inactive metabolites. The relatively rapid metabolism contributes to its short half-life, which can limit its duration of action. Efforts to prolong the therapeutic effects of muscimol have focused on developing derivatives with improved metabolic stability or controlled-release formulations.
Excretion of muscimol and its metabolites occurs primarily through renal elimination. The rapid clearance of muscimol from the body necessitates frequent dosing to maintain therapeutic levels, which can be problematic for patient compliance and increase the risk of side effects. Researchers are exploring strategies to modify the excretion profile and extend the drug's half-life.
Pharmacokinetic considerations for muscimol derivatives also include potential drug-drug interactions, particularly with other CNS-active compounds. The impact of genetic polymorphisms on muscimol metabolism and transport is another area of investigation, as individual variations can significantly affect drug response and safety profiles.
Advances in muscimol molecular configuration have led to the development of novel derivatives with improved pharmacokinetic properties. These include compounds with enhanced lipophilicity for better absorption and brain penetration, as well as derivatives designed to resist rapid metabolism. Additionally, researchers are exploring the potential of nanocarrier systems and targeted delivery approaches to optimize the pharmacokinetic profile of muscimol-based therapeutics.
Absorption of muscimol is relatively rapid, with peak plasma concentrations typically reached within 1-2 hours after oral administration. However, the bioavailability of muscimol can be limited due to its polar nature, which hinders passive diffusion across biological membranes. This has led researchers to explore various strategies to enhance absorption, including the development of lipophilic prodrugs and novel drug delivery systems.
Distribution of muscimol throughout the body is influenced by its hydrophilic nature. While it can cross the blood-brain barrier to some extent, the efficiency of central nervous system penetration is not optimal. This has prompted investigations into chemical modifications and delivery techniques to improve brain targeting and increase therapeutic efficacy for neurological disorders.
Metabolism of muscimol primarily occurs in the liver through oxidative deamination, resulting in the formation of inactive metabolites. The relatively rapid metabolism contributes to its short half-life, which can limit its duration of action. Efforts to prolong the therapeutic effects of muscimol have focused on developing derivatives with improved metabolic stability or controlled-release formulations.
Excretion of muscimol and its metabolites occurs primarily through renal elimination. The rapid clearance of muscimol from the body necessitates frequent dosing to maintain therapeutic levels, which can be problematic for patient compliance and increase the risk of side effects. Researchers are exploring strategies to modify the excretion profile and extend the drug's half-life.
Pharmacokinetic considerations for muscimol derivatives also include potential drug-drug interactions, particularly with other CNS-active compounds. The impact of genetic polymorphisms on muscimol metabolism and transport is another area of investigation, as individual variations can significantly affect drug response and safety profiles.
Advances in muscimol molecular configuration have led to the development of novel derivatives with improved pharmacokinetic properties. These include compounds with enhanced lipophilicity for better absorption and brain penetration, as well as derivatives designed to resist rapid metabolism. Additionally, researchers are exploring the potential of nanocarrier systems and targeted delivery approaches to optimize the pharmacokinetic profile of muscimol-based therapeutics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!