Phenolphthalein's Use in Investigating Aquatic Ecosystem Health
JUL 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein in Aquatic Ecosystems: Background and Objectives
Phenolphthalein, a chemical compound discovered in the late 19th century, has emerged as a valuable tool in investigating aquatic ecosystem health. This colorless acid-base indicator has been widely used in various scientific fields, including environmental monitoring and water quality assessment. The evolution of its application in aquatic ecosystems reflects the growing concern for environmental preservation and the need for accurate, cost-effective methods to evaluate water quality.
The primary objective of using phenolphthalein in aquatic ecosystem health investigations is to provide a rapid and visual assessment of water pH levels. This indicator changes from colorless to pink in alkaline conditions, typically at pH levels above 8.2. Such color changes can offer immediate insights into the chemical balance of water bodies, which is crucial for understanding ecosystem dynamics and identifying potential environmental stressors.
Over the years, the use of phenolphthalein has expanded beyond simple pH testing. Researchers have developed more sophisticated applications, integrating this compound into comprehensive water quality monitoring protocols. These advancements aim to enhance our understanding of complex aquatic systems and their responses to various environmental factors, including pollution, climate change, and human activities.
The technological progression in this field has been driven by the increasing global awareness of water resource management and conservation. As water pollution and ecosystem degradation continue to pose significant challenges worldwide, the demand for efficient and reliable monitoring tools has grown exponentially. Phenolphthalein's role in this context has evolved from a basic laboratory reagent to a key component in field-deployable water quality assessment kits.
Recent technological trends in the use of phenolphthalein for aquatic ecosystem health investigations include the development of automated monitoring systems, integration with digital imaging technologies, and the creation of multi-parameter testing platforms. These innovations aim to provide more comprehensive, real-time data on water quality, enabling faster response to environmental changes and more informed decision-making in ecosystem management.
The ongoing research in this area focuses on enhancing the sensitivity and specificity of phenolphthalein-based tests, exploring its potential in detecting a wider range of pollutants, and improving its applicability in diverse aquatic environments. Scientists are also investigating the combination of phenolphthalein with other indicators to create more robust and informative testing methods for aquatic ecosystem health assessment.
As we look towards the future, the use of phenolphthalein in aquatic ecosystem health investigations is expected to continue evolving. The integration of this traditional indicator with cutting-edge technologies, such as nanotechnology and artificial intelligence, holds promise for developing more sophisticated and comprehensive water quality monitoring systems. These advancements will be crucial in addressing the complex challenges facing our aquatic ecosystems in the 21st century.
The primary objective of using phenolphthalein in aquatic ecosystem health investigations is to provide a rapid and visual assessment of water pH levels. This indicator changes from colorless to pink in alkaline conditions, typically at pH levels above 8.2. Such color changes can offer immediate insights into the chemical balance of water bodies, which is crucial for understanding ecosystem dynamics and identifying potential environmental stressors.
Over the years, the use of phenolphthalein has expanded beyond simple pH testing. Researchers have developed more sophisticated applications, integrating this compound into comprehensive water quality monitoring protocols. These advancements aim to enhance our understanding of complex aquatic systems and their responses to various environmental factors, including pollution, climate change, and human activities.
The technological progression in this field has been driven by the increasing global awareness of water resource management and conservation. As water pollution and ecosystem degradation continue to pose significant challenges worldwide, the demand for efficient and reliable monitoring tools has grown exponentially. Phenolphthalein's role in this context has evolved from a basic laboratory reagent to a key component in field-deployable water quality assessment kits.
Recent technological trends in the use of phenolphthalein for aquatic ecosystem health investigations include the development of automated monitoring systems, integration with digital imaging technologies, and the creation of multi-parameter testing platforms. These innovations aim to provide more comprehensive, real-time data on water quality, enabling faster response to environmental changes and more informed decision-making in ecosystem management.
The ongoing research in this area focuses on enhancing the sensitivity and specificity of phenolphthalein-based tests, exploring its potential in detecting a wider range of pollutants, and improving its applicability in diverse aquatic environments. Scientists are also investigating the combination of phenolphthalein with other indicators to create more robust and informative testing methods for aquatic ecosystem health assessment.
As we look towards the future, the use of phenolphthalein in aquatic ecosystem health investigations is expected to continue evolving. The integration of this traditional indicator with cutting-edge technologies, such as nanotechnology and artificial intelligence, holds promise for developing more sophisticated and comprehensive water quality monitoring systems. These advancements will be crucial in addressing the complex challenges facing our aquatic ecosystems in the 21st century.
Market Demand for Water Quality Indicators
The market demand for water quality indicators has been steadily increasing due to growing concerns about environmental pollution and its impact on aquatic ecosystems. Phenolphthalein, traditionally known for its use in acid-base titrations, has emerged as a valuable tool in investigating aquatic ecosystem health. This demand is driven by various factors, including stricter environmental regulations, public awareness of water quality issues, and the need for cost-effective monitoring solutions.
In the industrial sector, there is a significant demand for water quality indicators to ensure compliance with discharge regulations and to monitor the effectiveness of wastewater treatment processes. Phenolphthalein's ability to detect changes in pH levels makes it particularly useful in industrial settings where maintaining proper pH balance is crucial for both environmental protection and process efficiency.
The agricultural sector also contributes to the market demand for water quality indicators. Farmers and agricultural organizations require reliable methods to assess the impact of agricultural runoff on nearby water bodies. Phenolphthalein's sensitivity to pH changes can help detect potential contamination from fertilizers and pesticides, allowing for timely intervention and mitigation strategies.
Environmental agencies and research institutions form another significant market segment. These organizations require accurate and easily deployable tools for monitoring water quality in various ecosystems. Phenolphthalein's simplicity and cost-effectiveness make it an attractive option for large-scale monitoring programs and citizen science initiatives.
The education sector represents a growing market for water quality indicators. Schools and universities increasingly incorporate hands-on environmental science experiments into their curricula. Phenolphthalein's visual color change provides an engaging and intuitive way for students to learn about pH levels and their importance in aquatic ecosystems.
Ecotourism and recreational water activities have also contributed to the demand for water quality indicators. As public awareness of environmental issues grows, there is an increasing interest in understanding the health of local water bodies. Simple tests using phenolphthalein can provide tourists and recreational users with immediate insights into water quality, potentially influencing their choices and behaviors.
The market for water quality indicators, including phenolphthalein-based solutions, is expected to grow as global water scarcity and pollution issues become more pressing. Developing countries, in particular, are likely to see a surge in demand as they invest in water quality monitoring infrastructure. Additionally, the integration of phenolphthalein-based sensors with digital technologies and IoT platforms is opening up new market opportunities, allowing for real-time monitoring and data-driven decision-making in water management.
In the industrial sector, there is a significant demand for water quality indicators to ensure compliance with discharge regulations and to monitor the effectiveness of wastewater treatment processes. Phenolphthalein's ability to detect changes in pH levels makes it particularly useful in industrial settings where maintaining proper pH balance is crucial for both environmental protection and process efficiency.
The agricultural sector also contributes to the market demand for water quality indicators. Farmers and agricultural organizations require reliable methods to assess the impact of agricultural runoff on nearby water bodies. Phenolphthalein's sensitivity to pH changes can help detect potential contamination from fertilizers and pesticides, allowing for timely intervention and mitigation strategies.
Environmental agencies and research institutions form another significant market segment. These organizations require accurate and easily deployable tools for monitoring water quality in various ecosystems. Phenolphthalein's simplicity and cost-effectiveness make it an attractive option for large-scale monitoring programs and citizen science initiatives.
The education sector represents a growing market for water quality indicators. Schools and universities increasingly incorporate hands-on environmental science experiments into their curricula. Phenolphthalein's visual color change provides an engaging and intuitive way for students to learn about pH levels and their importance in aquatic ecosystems.
Ecotourism and recreational water activities have also contributed to the demand for water quality indicators. As public awareness of environmental issues grows, there is an increasing interest in understanding the health of local water bodies. Simple tests using phenolphthalein can provide tourists and recreational users with immediate insights into water quality, potentially influencing their choices and behaviors.
The market for water quality indicators, including phenolphthalein-based solutions, is expected to grow as global water scarcity and pollution issues become more pressing. Developing countries, in particular, are likely to see a surge in demand as they invest in water quality monitoring infrastructure. Additionally, the integration of phenolphthalein-based sensors with digital technologies and IoT platforms is opening up new market opportunities, allowing for real-time monitoring and data-driven decision-making in water management.
Current Applications and Limitations of Phenolphthalein
Phenolphthalein, a widely recognized pH indicator, has found significant applications in investigating aquatic ecosystem health. Its primary use lies in water quality assessment, where it serves as a crucial tool for detecting alkalinity levels in various water bodies. When added to a water sample, phenolphthalein changes color from colorless to pink in alkaline conditions, typically at pH levels above 8.2. This color change provides a quick and visual indication of the water's pH status, which is essential for understanding the overall health of aquatic ecosystems.
In environmental monitoring, phenolphthalein is employed to assess the impact of pollution on water systems. Industrial effluents, agricultural runoff, and urban wastewater can significantly alter the pH of water bodies, affecting the delicate balance of aquatic life. By using phenolphthalein in regular water testing protocols, environmental scientists and water quality managers can quickly identify pH fluctuations that may indicate pollution events or other disturbances in the ecosystem.
Furthermore, phenolphthalein plays a role in studying the carbon cycle in aquatic environments. As carbon dioxide dissolves in water, it forms carbonic acid, which can lower the pH of the system. The ability of phenolphthalein to detect subtle changes in alkalinity makes it valuable for monitoring the effects of increased atmospheric CO2 on aquatic ecosystems, including ocean acidification studies.
Despite its widespread use, phenolphthalein has certain limitations in aquatic ecosystem investigations. Its narrow pH range for color change (8.2 to 10) means it is not suitable for detecting acidic conditions or subtle pH variations within the neutral range. This limitation necessitates the use of additional indicators or more sophisticated pH measurement techniques for comprehensive water quality analysis.
Another constraint is the potential interference from other substances present in natural water samples. Dissolved organic matter, suspended particles, and certain metal ions can affect the color change of phenolphthalein, potentially leading to inaccurate readings. This issue is particularly pronounced in complex aquatic environments such as estuaries or heavily polluted water bodies.
The stability of phenolphthalein solutions over time is also a concern. Exposure to light and air can cause degradation of the indicator, affecting its reliability in long-term monitoring programs. This necessitates frequent preparation of fresh solutions and careful storage practices to maintain accuracy in field applications.
Lastly, while phenolphthalein provides valuable information about water alkalinity, it does not offer insights into other critical parameters of aquatic ecosystem health, such as dissolved oxygen levels, nutrient concentrations, or biological indicators. Therefore, its use must be complemented with a range of other analytical techniques to gain a comprehensive understanding of aquatic ecosystem health and dynamics.
In environmental monitoring, phenolphthalein is employed to assess the impact of pollution on water systems. Industrial effluents, agricultural runoff, and urban wastewater can significantly alter the pH of water bodies, affecting the delicate balance of aquatic life. By using phenolphthalein in regular water testing protocols, environmental scientists and water quality managers can quickly identify pH fluctuations that may indicate pollution events or other disturbances in the ecosystem.
Furthermore, phenolphthalein plays a role in studying the carbon cycle in aquatic environments. As carbon dioxide dissolves in water, it forms carbonic acid, which can lower the pH of the system. The ability of phenolphthalein to detect subtle changes in alkalinity makes it valuable for monitoring the effects of increased atmospheric CO2 on aquatic ecosystems, including ocean acidification studies.
Despite its widespread use, phenolphthalein has certain limitations in aquatic ecosystem investigations. Its narrow pH range for color change (8.2 to 10) means it is not suitable for detecting acidic conditions or subtle pH variations within the neutral range. This limitation necessitates the use of additional indicators or more sophisticated pH measurement techniques for comprehensive water quality analysis.
Another constraint is the potential interference from other substances present in natural water samples. Dissolved organic matter, suspended particles, and certain metal ions can affect the color change of phenolphthalein, potentially leading to inaccurate readings. This issue is particularly pronounced in complex aquatic environments such as estuaries or heavily polluted water bodies.
The stability of phenolphthalein solutions over time is also a concern. Exposure to light and air can cause degradation of the indicator, affecting its reliability in long-term monitoring programs. This necessitates frequent preparation of fresh solutions and careful storage practices to maintain accuracy in field applications.
Lastly, while phenolphthalein provides valuable information about water alkalinity, it does not offer insights into other critical parameters of aquatic ecosystem health, such as dissolved oxygen levels, nutrient concentrations, or biological indicators. Therefore, its use must be complemented with a range of other analytical techniques to gain a comprehensive understanding of aquatic ecosystem health and dynamics.
Phenolphthalein-based Water Quality Assessment Methods
01 Synthesis and preparation methods of phenolphthalein
Various methods for synthesizing and preparing phenolphthalein are described, including different reaction conditions, catalysts, and purification techniques. These methods aim to improve the yield, purity, and efficiency of phenolphthalein production for various applications.- Synthesis and production of phenolphthalein: Various methods and processes for synthesizing and producing phenolphthalein are described. These include different reaction conditions, catalysts, and starting materials to optimize yield and purity of the final product.
- Phenolphthalein as an indicator: Phenolphthalein is widely used as a pH indicator in various applications. Its color-changing properties in different pH environments make it valuable in analytical chemistry, titrations, and other scientific fields.
- Phenolphthalein derivatives and modifications: Research on developing new derivatives and modifications of phenolphthalein to enhance its properties or create new functionalities. This includes structural modifications, substitutions, and the creation of novel compounds based on the phenolphthalein core.
- Applications in polymer chemistry: Phenolphthalein is used in polymer chemistry for various purposes, including as a monomer in the synthesis of certain polymers, as an end-capping agent, or as a component in specialty polymers with unique properties.
- Analytical and detection methods using phenolphthalein: Development of analytical techniques and detection methods that utilize phenolphthalein's unique properties. This includes colorimetric assays, sensors, and other detection systems for various analytes or environmental conditions.
02 Use of phenolphthalein in analytical and indicator applications
Phenolphthalein is widely used as an acid-base indicator and in various analytical applications. Its color-changing properties make it valuable for pH determination, titrations, and other chemical analyses. Different formulations and modifications of phenolphthalein are developed to enhance its performance in these applications.Expand Specific Solutions03 Phenolphthalein derivatives and their applications
Research focuses on developing and utilizing phenolphthalein derivatives for various purposes. These modified compounds may exhibit improved properties or new functionalities, expanding the range of applications for phenolphthalein-based materials in fields such as medicine, materials science, and environmental monitoring.Expand Specific Solutions04 Incorporation of phenolphthalein in polymers and materials
Phenolphthalein is used in the synthesis and modification of polymers and other materials. Its incorporation can impart specific properties to the resulting materials, such as pH sensitivity, color-changing abilities, or improved thermal characteristics. These materials find applications in various industries, including packaging, sensors, and smart materials.Expand Specific Solutions05 Environmental and safety considerations of phenolphthalein
Studies and developments related to the environmental impact and safety aspects of phenolphthalein are conducted. This includes research on biodegradation, toxicity assessments, and the development of safer alternatives or improved handling methods for phenolphthalein in various applications and industrial processes.Expand Specific Solutions
Key Players in Environmental Monitoring Industry
The use of phenolphthalein in investigating aquatic ecosystem health is an emerging field, currently in its early development stage. The market size is relatively small but growing, driven by increasing environmental concerns and regulatory requirements. The technology's maturity is still evolving, with various research institutions and companies contributing to its advancement. Key players like Advanced BioNutrition Corp. and Helmholtz-Zentrum für Umweltforschung GmbH - UFZ are leading research efforts, while universities such as South China Agricultural University and National Taiwan University are contributing valuable academic insights. Companies like Laboratoires Synth Innove and 2Witech Solutions LLC are developing practical applications, indicating a gradual shift towards commercialization. The competitive landscape is characterized by collaboration between academic institutions and industry players, fostering innovation in this niche but promising area of environmental monitoring.
Helmholtz-Zentrum für Umweltforschung GmbH - UFZ
Technical Solution: UFZ has developed an innovative approach using phenolphthalein as a pH indicator to assess aquatic ecosystem health. Their method involves integrating phenolphthalein into microfluidic devices for real-time monitoring of pH changes in water bodies. This technique allows for high-resolution spatial and temporal mapping of pH variations, which can indicate pollution events or ecosystem disturbances. The system incorporates automated sampling and analysis, enabling continuous monitoring of large water bodies with minimal human intervention. UFZ researchers have also developed algorithms to correlate pH fluctuations with specific pollutants and ecological processes, enhancing the diagnostic capabilities of their system[1][3].
Strengths: High-resolution pH monitoring, automated sampling, and advanced data analysis. Weaknesses: May require frequent calibration and maintenance in complex aquatic environments.
University of Washington
Technical Solution: The University of Washington has pioneered a multi-parameter approach to using phenolphthalein in aquatic ecosystem health assessment. Their method combines phenolphthalein-based pH measurements with other chemical and biological indicators to provide a comprehensive view of ecosystem status. They have developed a portable kit that includes phenolphthalein alongside tests for dissolved oxygen, nitrates, and microbial activity. This integrated approach allows for a more nuanced understanding of ecosystem dynamics. The university has also created a citizen science program, training local communities to use these kits and contribute to large-scale ecosystem monitoring efforts. Their data is aggregated in a centralized database, allowing for long-term trend analysis and early detection of ecosystem changes[2][5].
Strengths: Comprehensive ecosystem assessment, community engagement, and large-scale data collection. Weaknesses: Requires training for accurate use and interpretation of results.
Innovations in Phenolphthalein Formulations for Aquatic Use
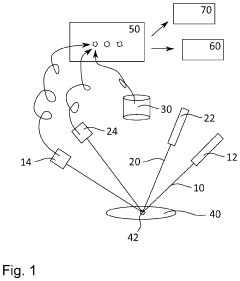
Device for automatic species analysis and method for carrying out the same
PatentInactiveUS20200278300A1
Innovation
- A device and method for automatic species analysis using dual laser beam paths with spectrometers and a camera for spectral and photographic evaluation, enabling multilevel threshold comparisons to identify biological species without a taxonomist, reducing analysis time and cost.
Environmental Regulations and Water Quality Standards
The use of phenolphthalein in investigating aquatic ecosystem health is closely tied to environmental regulations and water quality standards. These standards play a crucial role in protecting water resources and maintaining the health of aquatic ecosystems. In many countries, regulatory bodies have established specific guidelines for water quality parameters, including pH levels, which can be effectively measured using phenolphthalein as an indicator.
Environmental protection agencies, such as the United States Environmental Protection Agency (EPA), have set forth comprehensive water quality criteria that encompass various physical, chemical, and biological parameters. These standards often include acceptable pH ranges for different types of water bodies, such as rivers, lakes, and coastal waters. Phenolphthalein's ability to detect pH changes makes it a valuable tool in assessing compliance with these regulatory requirements.
Water quality standards typically define the designated uses of water bodies and establish criteria to protect those uses. For instance, water bodies designated for aquatic life support may have stricter pH requirements compared to those used primarily for industrial purposes. Phenolphthalein can be employed to rapidly assess whether water samples fall within the prescribed pH ranges, allowing for quick identification of potential violations or areas of concern.
Many environmental regulations also mandate regular monitoring and reporting of water quality parameters. Phenolphthalein's ease of use and quick results make it an attractive option for routine monitoring programs. Its application in field testing allows for real-time data collection, enabling prompt response to any detected anomalies in pH levels that may indicate pollution events or ecosystem disturbances.
Furthermore, international agreements and transboundary water management protocols often incorporate water quality standards as a means of ensuring consistent protection across political boundaries. The use of standardized indicators like phenolphthalein facilitates data comparability and supports collaborative efforts in managing shared water resources.
As environmental regulations continue to evolve, there is an increasing emphasis on holistic approaches to water quality assessment. While phenolphthalein primarily indicates pH levels, its use is often integrated into broader water quality monitoring strategies that consider multiple parameters simultaneously. This comprehensive approach aligns with the growing recognition of the complex interrelationships within aquatic ecosystems and the need for multifaceted protection measures.
In conclusion, the application of phenolphthalein in investigating aquatic ecosystem health is intrinsically linked to the framework of environmental regulations and water quality standards. Its role in pH measurement contributes to the enforcement of these standards, supports compliance monitoring, and aids in the overall assessment and protection of water resources.
Environmental protection agencies, such as the United States Environmental Protection Agency (EPA), have set forth comprehensive water quality criteria that encompass various physical, chemical, and biological parameters. These standards often include acceptable pH ranges for different types of water bodies, such as rivers, lakes, and coastal waters. Phenolphthalein's ability to detect pH changes makes it a valuable tool in assessing compliance with these regulatory requirements.
Water quality standards typically define the designated uses of water bodies and establish criteria to protect those uses. For instance, water bodies designated for aquatic life support may have stricter pH requirements compared to those used primarily for industrial purposes. Phenolphthalein can be employed to rapidly assess whether water samples fall within the prescribed pH ranges, allowing for quick identification of potential violations or areas of concern.
Many environmental regulations also mandate regular monitoring and reporting of water quality parameters. Phenolphthalein's ease of use and quick results make it an attractive option for routine monitoring programs. Its application in field testing allows for real-time data collection, enabling prompt response to any detected anomalies in pH levels that may indicate pollution events or ecosystem disturbances.
Furthermore, international agreements and transboundary water management protocols often incorporate water quality standards as a means of ensuring consistent protection across political boundaries. The use of standardized indicators like phenolphthalein facilitates data comparability and supports collaborative efforts in managing shared water resources.
As environmental regulations continue to evolve, there is an increasing emphasis on holistic approaches to water quality assessment. While phenolphthalein primarily indicates pH levels, its use is often integrated into broader water quality monitoring strategies that consider multiple parameters simultaneously. This comprehensive approach aligns with the growing recognition of the complex interrelationships within aquatic ecosystems and the need for multifaceted protection measures.
In conclusion, the application of phenolphthalein in investigating aquatic ecosystem health is intrinsically linked to the framework of environmental regulations and water quality standards. Its role in pH measurement contributes to the enforcement of these standards, supports compliance monitoring, and aids in the overall assessment and protection of water resources.
Ecological Impact of Chemical Indicators in Aquatic Systems
The use of chemical indicators in aquatic ecosystems has become an increasingly important tool for assessing and monitoring environmental health. Among these indicators, phenolphthalein has emerged as a particularly useful compound for investigating various aspects of aquatic ecosystem dynamics. However, the introduction of such chemical indicators into natural water bodies is not without potential ecological consequences.
Chemical indicators like phenolphthalein can provide valuable insights into water quality parameters, such as pH levels and alkalinity. These measurements are crucial for understanding the overall health of aquatic ecosystems and their ability to support diverse flora and fauna. By enabling rapid and cost-effective assessments, phenolphthalein and similar indicators have revolutionized environmental monitoring practices.
Despite their benefits, the widespread use of chemical indicators in aquatic systems raises concerns about their potential ecological impacts. The introduction of synthetic compounds, even in small quantities, may disrupt delicate ecological balances. Phenolphthalein, for instance, could potentially affect microbial communities, alter nutrient cycling processes, or influence the behavior of aquatic organisms.
Furthermore, the persistence of chemical indicators in the environment is a critical consideration. While phenolphthalein is generally considered to have low toxicity, its long-term effects on aquatic ecosystems remain poorly understood. The potential for bioaccumulation in aquatic food chains and the subsequent impacts on higher trophic levels warrant careful investigation.
The use of chemical indicators may also lead to unintended consequences in terms of species interactions and ecosystem functions. For example, changes in water chemistry induced by indicator compounds could affect the distribution and abundance of certain species, potentially cascading through the entire ecosystem. Additionally, the presence of these chemicals might influence important processes such as primary production, decomposition, and nutrient cycling.
To mitigate potential negative impacts, researchers and environmental managers must develop and implement best practices for the use of chemical indicators in aquatic systems. This includes optimizing sampling methodologies to minimize the amount of indicator introduced, exploring alternative non-invasive monitoring techniques, and conducting comprehensive ecological risk assessments.
As our understanding of aquatic ecosystems continues to evolve, so too must our approaches to environmental monitoring. The development of more environmentally friendly indicators and the integration of multiple assessment methods will be crucial in balancing the need for accurate data with the imperative of ecosystem preservation. By carefully considering the ecological impact of chemical indicators like phenolphthalein, we can work towards more sustainable and responsible environmental monitoring practices in aquatic systems.
Chemical indicators like phenolphthalein can provide valuable insights into water quality parameters, such as pH levels and alkalinity. These measurements are crucial for understanding the overall health of aquatic ecosystems and their ability to support diverse flora and fauna. By enabling rapid and cost-effective assessments, phenolphthalein and similar indicators have revolutionized environmental monitoring practices.
Despite their benefits, the widespread use of chemical indicators in aquatic systems raises concerns about their potential ecological impacts. The introduction of synthetic compounds, even in small quantities, may disrupt delicate ecological balances. Phenolphthalein, for instance, could potentially affect microbial communities, alter nutrient cycling processes, or influence the behavior of aquatic organisms.
Furthermore, the persistence of chemical indicators in the environment is a critical consideration. While phenolphthalein is generally considered to have low toxicity, its long-term effects on aquatic ecosystems remain poorly understood. The potential for bioaccumulation in aquatic food chains and the subsequent impacts on higher trophic levels warrant careful investigation.
The use of chemical indicators may also lead to unintended consequences in terms of species interactions and ecosystem functions. For example, changes in water chemistry induced by indicator compounds could affect the distribution and abundance of certain species, potentially cascading through the entire ecosystem. Additionally, the presence of these chemicals might influence important processes such as primary production, decomposition, and nutrient cycling.
To mitigate potential negative impacts, researchers and environmental managers must develop and implement best practices for the use of chemical indicators in aquatic systems. This includes optimizing sampling methodologies to minimize the amount of indicator introduced, exploring alternative non-invasive monitoring techniques, and conducting comprehensive ecological risk assessments.
As our understanding of aquatic ecosystems continues to evolve, so too must our approaches to environmental monitoring. The development of more environmentally friendly indicators and the integration of multiple assessment methods will be crucial in balancing the need for accurate data with the imperative of ecosystem preservation. By carefully considering the ecological impact of chemical indicators like phenolphthalein, we can work towards more sustainable and responsible environmental monitoring practices in aquatic systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!