Photoelectrochemical Water Splitting: Enhancing light absorption efficiency.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEC Water Splitting Background and Objectives
Photoelectrochemical (PEC) water splitting represents a promising approach for sustainable hydrogen production, leveraging solar energy to directly convert water into hydrogen and oxygen. This technology has evolved significantly since its inception in 1972 when Fujishima and Honda first demonstrated the photocatalytic decomposition of water using titanium dioxide electrodes under ultraviolet light. Over the past five decades, research has progressively focused on enhancing efficiency, stability, and cost-effectiveness of PEC systems.
The fundamental principle of PEC water splitting involves the absorption of photons by semiconductor materials, generating electron-hole pairs that drive water oxidation and reduction reactions. However, the practical implementation faces significant challenges, particularly in light absorption efficiency. Most semiconductor materials either absorb only a narrow portion of the solar spectrum or suffer from rapid charge recombination, limiting overall system efficiency.
Current technological trends indicate a shift toward multi-junction photoelectrodes, nanostructured materials, and innovative surface modifications to enhance light harvesting capabilities. Research is increasingly focused on developing materials that can efficiently capture photons across the visible and near-infrared regions of the solar spectrum, which constitute approximately 95% of solar energy reaching Earth's surface.
The primary technical objective in enhancing light absorption efficiency involves developing photoelectrode materials with optimal band gaps (1.5-2.0 eV) that maximize solar spectrum utilization while maintaining sufficient electrochemical potential for water splitting. Secondary objectives include improving charge separation and transport, reducing surface recombination, and enhancing catalytic activity at the semiconductor-electrolyte interface.
Global research efforts are increasingly directed toward earth-abundant, non-toxic materials to ensure sustainability and scalability. Transition metal oxides, nitrides, and sulfides have emerged as promising candidates due to their tunable optical properties and relative stability in aqueous environments. Additionally, hybrid organic-inorganic systems and quantum-confined nanostructures are being explored for their unique optoelectronic properties.
The advancement of this technology aligns with broader energy transition goals, particularly the growing hydrogen economy. Achieving solar-to-hydrogen conversion efficiencies above 10% with system lifetimes exceeding 10,000 hours is widely considered the threshold for commercial viability. Current laboratory demonstrations have reached efficiencies of 5-8%, indicating significant progress but highlighting the need for continued innovation.
Understanding the historical context and evolutionary trajectory of PEC water splitting technology provides crucial insights for identifying promising research directions and establishing realistic technical milestones for enhancing light absorption efficiency in next-generation systems.
The fundamental principle of PEC water splitting involves the absorption of photons by semiconductor materials, generating electron-hole pairs that drive water oxidation and reduction reactions. However, the practical implementation faces significant challenges, particularly in light absorption efficiency. Most semiconductor materials either absorb only a narrow portion of the solar spectrum or suffer from rapid charge recombination, limiting overall system efficiency.
Current technological trends indicate a shift toward multi-junction photoelectrodes, nanostructured materials, and innovative surface modifications to enhance light harvesting capabilities. Research is increasingly focused on developing materials that can efficiently capture photons across the visible and near-infrared regions of the solar spectrum, which constitute approximately 95% of solar energy reaching Earth's surface.
The primary technical objective in enhancing light absorption efficiency involves developing photoelectrode materials with optimal band gaps (1.5-2.0 eV) that maximize solar spectrum utilization while maintaining sufficient electrochemical potential for water splitting. Secondary objectives include improving charge separation and transport, reducing surface recombination, and enhancing catalytic activity at the semiconductor-electrolyte interface.
Global research efforts are increasingly directed toward earth-abundant, non-toxic materials to ensure sustainability and scalability. Transition metal oxides, nitrides, and sulfides have emerged as promising candidates due to their tunable optical properties and relative stability in aqueous environments. Additionally, hybrid organic-inorganic systems and quantum-confined nanostructures are being explored for their unique optoelectronic properties.
The advancement of this technology aligns with broader energy transition goals, particularly the growing hydrogen economy. Achieving solar-to-hydrogen conversion efficiencies above 10% with system lifetimes exceeding 10,000 hours is widely considered the threshold for commercial viability. Current laboratory demonstrations have reached efficiencies of 5-8%, indicating significant progress but highlighting the need for continued innovation.
Understanding the historical context and evolutionary trajectory of PEC water splitting technology provides crucial insights for identifying promising research directions and establishing realistic technical milestones for enhancing light absorption efficiency in next-generation systems.
Market Analysis for Hydrogen Production Technologies
The global hydrogen market is experiencing significant growth, driven by increasing focus on clean energy solutions and decarbonization efforts across industries. Currently valued at approximately $130 billion, the hydrogen market is projected to reach $500 billion by 2030, with a compound annual growth rate exceeding 9.2% during the forecast period. Green hydrogen, produced through water electrolysis powered by renewable energy sources, represents the fastest-growing segment within this market.
Photoelectrochemical (PEC) water splitting technology, which directly converts solar energy into hydrogen fuel, occupies a promising niche within the broader hydrogen production landscape. While conventional electrolysis methods dominate the current market with over 70% share, PEC technology is gaining attention due to its potential for higher theoretical efficiency and reduced system complexity compared to separate photovoltaic-electrolysis systems.
Market demand for hydrogen spans multiple sectors, with industrial applications currently accounting for approximately 55% of consumption. However, transportation and energy storage applications are expected to grow at the fastest rates, with projected CAGRs of 25% and 22% respectively through 2030. This shift reflects the increasing adoption of hydrogen fuel cells in vehicles and grid-scale energy storage solutions.
Regionally, Asia-Pacific leads the hydrogen market with approximately 40% share, followed by Europe (30%) and North America (20%). China, Japan, and South Korea have established ambitious hydrogen roadmaps, while the European Union's Hydrogen Strategy targets 40GW of electrolyzer capacity by 2030. The United States is accelerating investments through initiatives like the Hydrogen Shot program, aiming to reduce clean hydrogen costs by 80% within the decade.
For PEC water splitting specifically, the market remains predominantly in the research and development phase, with limited commercial deployment. However, investment in this technology has grown by approximately 35% annually since 2018, reflecting increasing recognition of its potential to address efficiency limitations in conventional hydrogen production methods.
Key market drivers for enhanced light absorption efficiency in PEC systems include the need to reduce hydrogen production costs below $2/kg to achieve cost parity with fossil fuel-derived hydrogen, and the growing demand for integrated systems that can operate efficiently under real-world conditions. Additionally, policy support through carbon pricing mechanisms, renewable energy mandates, and direct subsidies for clean hydrogen production is creating favorable market conditions for advanced technologies like improved PEC systems.
Photoelectrochemical (PEC) water splitting technology, which directly converts solar energy into hydrogen fuel, occupies a promising niche within the broader hydrogen production landscape. While conventional electrolysis methods dominate the current market with over 70% share, PEC technology is gaining attention due to its potential for higher theoretical efficiency and reduced system complexity compared to separate photovoltaic-electrolysis systems.
Market demand for hydrogen spans multiple sectors, with industrial applications currently accounting for approximately 55% of consumption. However, transportation and energy storage applications are expected to grow at the fastest rates, with projected CAGRs of 25% and 22% respectively through 2030. This shift reflects the increasing adoption of hydrogen fuel cells in vehicles and grid-scale energy storage solutions.
Regionally, Asia-Pacific leads the hydrogen market with approximately 40% share, followed by Europe (30%) and North America (20%). China, Japan, and South Korea have established ambitious hydrogen roadmaps, while the European Union's Hydrogen Strategy targets 40GW of electrolyzer capacity by 2030. The United States is accelerating investments through initiatives like the Hydrogen Shot program, aiming to reduce clean hydrogen costs by 80% within the decade.
For PEC water splitting specifically, the market remains predominantly in the research and development phase, with limited commercial deployment. However, investment in this technology has grown by approximately 35% annually since 2018, reflecting increasing recognition of its potential to address efficiency limitations in conventional hydrogen production methods.
Key market drivers for enhanced light absorption efficiency in PEC systems include the need to reduce hydrogen production costs below $2/kg to achieve cost parity with fossil fuel-derived hydrogen, and the growing demand for integrated systems that can operate efficiently under real-world conditions. Additionally, policy support through carbon pricing mechanisms, renewable energy mandates, and direct subsidies for clean hydrogen production is creating favorable market conditions for advanced technologies like improved PEC systems.
Current Challenges in Light Absorption Efficiency
Despite significant advancements in photoelectrochemical (PEC) water splitting technology, light absorption efficiency remains a critical bottleneck limiting overall system performance. Current photoanode materials struggle to effectively capture the full solar spectrum, with most semiconductor materials only absorbing a narrow wavelength range. For instance, titanium dioxide (TiO2), a widely used photoanode material, primarily absorbs in the UV region which constitutes less than 5% of the solar spectrum, severely limiting practical hydrogen production rates.
Band gap engineering presents another significant challenge. The ideal photocatalyst requires a band gap narrow enough to absorb visible light (approximately 1.8-2.2 eV) while maintaining sufficient band edge positions to drive water splitting reactions. Materials with appropriate band gaps often suffer from rapid charge recombination or poor stability in aqueous environments, creating a complex optimization problem that has yet to be fully resolved.
Surface reflection losses further diminish light absorption efficiency, with planar semiconductor surfaces reflecting up to 30% of incident light depending on the refractive index mismatch at interfaces. This reflection represents a substantial loss mechanism that directly impacts quantum efficiency and solar-to-hydrogen conversion rates.
Light penetration depth limitations also pose significant challenges, particularly for nanostructured electrodes. While nanostructuring can increase surface area for catalytic reactions, it often creates complex light scattering patterns that may reduce overall light absorption in deeper regions of the electrode. The trade-off between surface area and light penetration remains poorly optimized in many current designs.
Competing absorption and scattering processes within the electrode materials further complicate efficiency improvements. Impurities, defects, and grain boundaries can serve as recombination centers that reduce the number of photogenerated carriers available for water splitting reactions. These loss mechanisms are particularly problematic in earth-abundant materials being developed as alternatives to expensive noble metal catalysts.
Stability under illumination presents another critical challenge, as many materials with promising light absorption properties suffer from photocorrosion or degradation during operation. This photoinduced instability often forces researchers to compromise between optimal light absorption and long-term durability, limiting practical implementation of otherwise promising materials.
The complex interplay between light absorption, charge separation, and catalytic activity creates a multivariable optimization problem that has proven difficult to solve with current materials and architectures. Addressing these challenges requires interdisciplinary approaches spanning materials science, optics, electrochemistry, and surface engineering.
Band gap engineering presents another significant challenge. The ideal photocatalyst requires a band gap narrow enough to absorb visible light (approximately 1.8-2.2 eV) while maintaining sufficient band edge positions to drive water splitting reactions. Materials with appropriate band gaps often suffer from rapid charge recombination or poor stability in aqueous environments, creating a complex optimization problem that has yet to be fully resolved.
Surface reflection losses further diminish light absorption efficiency, with planar semiconductor surfaces reflecting up to 30% of incident light depending on the refractive index mismatch at interfaces. This reflection represents a substantial loss mechanism that directly impacts quantum efficiency and solar-to-hydrogen conversion rates.
Light penetration depth limitations also pose significant challenges, particularly for nanostructured electrodes. While nanostructuring can increase surface area for catalytic reactions, it often creates complex light scattering patterns that may reduce overall light absorption in deeper regions of the electrode. The trade-off between surface area and light penetration remains poorly optimized in many current designs.
Competing absorption and scattering processes within the electrode materials further complicate efficiency improvements. Impurities, defects, and grain boundaries can serve as recombination centers that reduce the number of photogenerated carriers available for water splitting reactions. These loss mechanisms are particularly problematic in earth-abundant materials being developed as alternatives to expensive noble metal catalysts.
Stability under illumination presents another critical challenge, as many materials with promising light absorption properties suffer from photocorrosion or degradation during operation. This photoinduced instability often forces researchers to compromise between optimal light absorption and long-term durability, limiting practical implementation of otherwise promising materials.
The complex interplay between light absorption, charge separation, and catalytic activity creates a multivariable optimization problem that has proven difficult to solve with current materials and architectures. Addressing these challenges requires interdisciplinary approaches spanning materials science, optics, electrochemistry, and surface engineering.
State-of-the-Art Light Harvesting Strategies
01 Nanostructured materials for enhanced light absorption
Nanostructured materials such as quantum dots, nanowires, and nanoparticles can significantly improve light absorption efficiency in photoelectrochemical water splitting systems. These materials offer increased surface area, tunable bandgaps, and enhanced light harvesting capabilities across the solar spectrum. The nanoscale dimensions allow for better charge separation and reduced recombination rates, leading to improved quantum efficiency and overall water splitting performance.- Nanostructured materials for enhanced light absorption: Nanostructured materials such as quantum dots, nanowires, and nanoparticles can significantly improve light absorption efficiency in photoelectrochemical water splitting systems. These materials offer increased surface area, tunable bandgaps, and enhanced light harvesting capabilities across the solar spectrum. The nanoscale dimensions allow for better charge separation and reduced recombination rates, leading to improved quantum efficiency and overall water splitting performance.
- Doping and heterostructure engineering: Doping semiconductor materials with metal or non-metal elements and creating heterostructures can enhance light absorption efficiency by modifying the band structure and extending the absorption range into visible light. These approaches reduce the bandgap energy, improve charge carrier mobility, and create built-in electric fields that facilitate charge separation. Heterostructures combining multiple materials with complementary properties can achieve broader spectral absorption and more efficient charge transfer.
- Plasmonic enhancement strategies: Incorporating plasmonic metal nanostructures into photoelectrochemical water splitting systems can enhance light absorption through localized surface plasmon resonance effects. These metallic nanostructures concentrate electromagnetic fields, extend light path lengths, and create hot electrons that can participate in the water splitting reaction. Strategic placement of plasmonic materials near semiconductor interfaces maximizes light harvesting while facilitating efficient charge transfer.
- Novel photocatalyst compositions: Development of novel photocatalyst compositions including perovskites, metal organic frameworks, and composite materials can significantly improve light absorption efficiency. These materials feature optimized band positions, enhanced stability under operating conditions, and tailored surface properties for improved catalytic activity. Multi-component systems can be designed to absorb complementary portions of the solar spectrum, enabling more complete utilization of incident light energy.
- Surface modification and co-catalyst integration: Surface modification techniques and integration of co-catalysts can enhance light absorption efficiency by reducing surface reflection, improving charge separation, and accelerating reaction kinetics. Treatments such as facet engineering, defect management, and surface passivation minimize recombination losses at interfaces. Strategic deposition of oxygen and hydrogen evolution co-catalysts creates efficient reaction sites while maintaining optimal light absorption properties of the base material.
02 Semiconductor photocatalyst modifications
Various modifications to semiconductor photocatalysts can enhance light absorption efficiency. These include doping with metal or non-metal elements, creating heterojunctions between different semiconductors, and surface modifications. Such approaches can narrow the bandgap, extend light absorption into the visible range, improve charge carrier mobility, and reduce recombination rates, resulting in higher photoelectrochemical water splitting efficiency.Expand Specific Solutions03 Plasmonic enhancement strategies
Plasmonic materials, particularly noble metal nanostructures, can be incorporated into photoelectrochemical systems to enhance light absorption through localized surface plasmon resonance effects. These materials concentrate electromagnetic fields, extend light absorption range, and improve charge separation. Plasmonic enhancement strategies include core-shell structures, decorated photoanodes, and integrated plasmonic-semiconductor systems that significantly boost water splitting efficiency.Expand Specific Solutions04 Hierarchical electrode architectures
Hierarchical electrode architectures with multi-scale structural features optimize light absorption by combining macro, micro, and nano-scale elements. These structures provide light trapping effects, multiple scattering pathways, and gradient refractive indices that minimize reflection losses. The hierarchical design also facilitates efficient mass transport of reactants and products while maintaining high surface area for reactions, significantly improving photoelectrochemical performance.Expand Specific Solutions05 Z-scheme and tandem cell configurations
Z-scheme and tandem cell configurations utilize multiple absorber materials with complementary absorption spectra to harvest a broader range of solar radiation. These systems connect two or more photoactive components in series or parallel arrangements to overcome the limitations of single-absorber systems. By strategically combining materials with different bandgaps, these configurations achieve higher theoretical solar-to-hydrogen conversion efficiencies and improved light utilization across the solar spectrum.Expand Specific Solutions
Leading Research Groups and Industrial Players
Photoelectrochemical water splitting technology is currently in the growth phase of its industry development, with a rapidly expanding global market projected to reach $12-15 billion by 2030. The technology maturity varies across different approaches, with significant advancements in light absorption efficiency being made by both academic institutions and industry players. Leading research entities like California Institute of Technology, University of Tokyo, and National University of Singapore are developing novel photocatalysts, while companies such as SABIC Global Technologies and Alliance for Sustainable Energy are focusing on scalable implementations. The competitive landscape features strong collaboration between academic institutions and industry partners, with Asian universities (particularly from China and South Korea) demonstrating increasing research output alongside established Western institutions.
Alliance for Sustainable Energy LLC
Technical Solution: The Alliance for Sustainable Energy, which manages the National Renewable Energy Laboratory (NREL), has developed comprehensive approaches to enhance light absorption efficiency in photoelectrochemical water splitting. Their technology focuses on multi-junction semiconductor architectures that strategically partition the solar spectrum among different absorber layers to maximize photon utilization[2]. NREL researchers have pioneered the development of III-V semiconductor photoelectrodes with precisely engineered bandgaps and surface treatments that achieve record solar-to-hydrogen conversion efficiencies exceeding 16%. Their innovation includes the development of transparent conducting oxide overlayers with nanopatterned surfaces that serve dual functions as antireflective coatings and efficient charge transport layers[6]. Additionally, they've created advanced modeling tools that predict optical absorption and carrier transport in complex photoelectrode geometries, enabling rational design of light-trapping structures. Recent work has focused on earth-abundant materials like copper oxide and iron oxide with surface modification strategies that extend their visible light response while improving charge separation efficiency.
Strengths: Industry-leading device fabrication capabilities; excellent integration of computational modeling with experimental validation; strong focus on practical implementation and scale-up. Weaknesses: Some high-efficiency systems rely on expensive III-V semiconductors; challenges in maintaining performance under variable illumination conditions; durability issues with some earth-abundant material systems.
University of Tokyo
Technical Solution: The University of Tokyo has developed innovative approaches to enhance light absorption efficiency in photoelectrochemical water splitting through advanced materials engineering. Their researchers have created vertically aligned titanium dioxide nanotube arrays with precisely controlled dimensions that significantly increase the optical path length while maintaining efficient charge transport properties[3]. These structures demonstrate superior light trapping through multiple internal reflections within the nanotube architecture. The Tokyo team has also pioneered the development of Z-scheme heterojunction systems using graphene as an electron mediator between complementary semiconductors, enabling efficient visible light harvesting across broader spectral ranges. Their recent breakthrough involves plasmonic-enhanced photoelectrodes incorporating gold nanorods with tunable aspect ratios that create localized electromagnetic field enhancements at specific wavelengths, dramatically increasing absorption coefficients in otherwise weak absorbing regions[7]. Additionally, they've developed innovative surface modification strategies using molecular sensitizers that extend the absorption spectrum of wide-bandgap semiconductors into the visible region while simultaneously passivating surface recombination sites.
Strengths: Exceptional nanomaterials synthesis capabilities; strong integration of photonic principles with electrochemistry; excellent control over interfacial properties. Weaknesses: Some approaches utilize precious metals that increase costs; challenges in maintaining long-term stability of organic sensitizers; potential mass transport limitations in highly structured electrodes.
Key Innovations in Photocatalyst Design
Photoelectrode for improved photoelectrochemical performance and manufacturing method
PatentActiveKR1020240032316A
Innovation
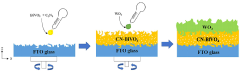
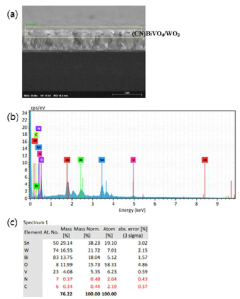
- A photoelectrode structure comprising a fluorine-doped tin oxide substrate with layers of bismuth vanadate and tungsten oxide, where bismuth vanadate includes pores and is topped with a graphite-type carbon nitride layer, enhancing light absorption in long wavelength ranges through Z-scheme heterojunction.
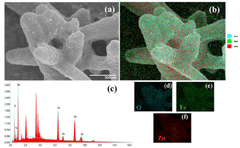
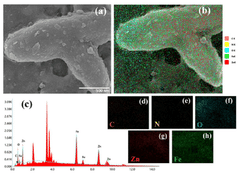
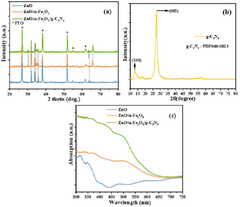
ZnO photoanode prepared using a double heterojunction with α-Fe2O3/g-C3N4 composite to enhance photoelectrochemical water splitting and surface light absorption
PatentActiveKR1020240048980A
Innovation
- A ZnO photoanode with a double heterojunction structure comprising α-Fe2O3 nanoparticles and g-C3N4 nanosheets is developed, where α-Fe2O3 is doped onto ZnO nanorods, and g-C3N4 nanosheets are loaded via impregnation, optimizing their amounts and thicknesses for improved charge separation and transfer.
Scalability and Cost Analysis
The scalability and cost considerations of photoelectrochemical (PEC) water splitting systems present significant challenges for widespread commercial adoption. Current laboratory-scale demonstrations, while promising in terms of light absorption efficiency, face substantial barriers when transitioning to industrial scales. The capital expenditure for PEC systems remains prohibitively high, estimated at $10-15/W compared to conventional hydrogen production methods at $1-2/W, primarily due to expensive semiconductor materials and noble metal catalysts.
Material costs constitute approximately 60-70% of total system expenses, with high-purity semiconductors like III-V compounds commanding prices of $1000-5000 per square meter. Alternative materials such as metal oxides offer cost advantages ($50-200 per square meter) but typically deliver lower efficiencies, creating a critical performance-cost tradeoff. Catalyst materials, particularly platinum and iridium-based compounds, contribute significantly to costs while facing supply chain vulnerabilities due to their scarcity.
Manufacturing scalability presents another dimension of challenges. Current fabrication techniques for high-efficiency photoelectrodes, including atomic layer deposition and molecular beam epitaxy, are inherently batch processes with limited throughput. The transition to continuous manufacturing processes remains technically challenging, with estimates suggesting a 5-10x cost reduction potential if achieved. Recent advances in roll-to-roll processing for certain photoelectrode materials show promise but require further development.
System lifetime and durability directly impact economic viability through amortized costs. Current PEC systems typically demonstrate stability for 100-1000 hours under laboratory conditions, whereas commercial viability requires 50,000+ hours of operation. Degradation mechanisms increase maintenance costs and reduce hydrogen production efficiency over time, significantly affecting levelized cost calculations.
Energy return on investment (EROI) analysis indicates that current PEC systems require 1-2 years of operation to recover the energy invested in their production. This metric must improve to below 6 months for competitive positioning against conventional hydrogen production methods. Recent techno-economic analyses suggest that achieving solar-to-hydrogen efficiencies above 15% with system costs below $100/m² and lifetimes exceeding 10 years would enable hydrogen production at $2-3/kg, approaching cost parity with fossil-based methods.
Economies of scale could potentially reduce costs by 30-50% through standardized manufacturing and supply chain optimization, but require significant initial investment. Public-private partnerships and targeted research funding focused on scalable materials and manufacturing processes represent critical pathways toward commercial viability of PEC water splitting technology.
Material costs constitute approximately 60-70% of total system expenses, with high-purity semiconductors like III-V compounds commanding prices of $1000-5000 per square meter. Alternative materials such as metal oxides offer cost advantages ($50-200 per square meter) but typically deliver lower efficiencies, creating a critical performance-cost tradeoff. Catalyst materials, particularly platinum and iridium-based compounds, contribute significantly to costs while facing supply chain vulnerabilities due to their scarcity.
Manufacturing scalability presents another dimension of challenges. Current fabrication techniques for high-efficiency photoelectrodes, including atomic layer deposition and molecular beam epitaxy, are inherently batch processes with limited throughput. The transition to continuous manufacturing processes remains technically challenging, with estimates suggesting a 5-10x cost reduction potential if achieved. Recent advances in roll-to-roll processing for certain photoelectrode materials show promise but require further development.
System lifetime and durability directly impact economic viability through amortized costs. Current PEC systems typically demonstrate stability for 100-1000 hours under laboratory conditions, whereas commercial viability requires 50,000+ hours of operation. Degradation mechanisms increase maintenance costs and reduce hydrogen production efficiency over time, significantly affecting levelized cost calculations.
Energy return on investment (EROI) analysis indicates that current PEC systems require 1-2 years of operation to recover the energy invested in their production. This metric must improve to below 6 months for competitive positioning against conventional hydrogen production methods. Recent techno-economic analyses suggest that achieving solar-to-hydrogen efficiencies above 15% with system costs below $100/m² and lifetimes exceeding 10 years would enable hydrogen production at $2-3/kg, approaching cost parity with fossil-based methods.
Economies of scale could potentially reduce costs by 30-50% through standardized manufacturing and supply chain optimization, but require significant initial investment. Public-private partnerships and targeted research funding focused on scalable materials and manufacturing processes represent critical pathways toward commercial viability of PEC water splitting technology.
Environmental Impact and Sustainability Assessment
Photoelectrochemical water splitting systems offer significant environmental benefits compared to conventional hydrogen production methods, primarily through their potential to reduce greenhouse gas emissions. When powered by renewable energy sources, these systems can produce hydrogen with near-zero carbon footprint, unlike steam methane reforming which accounts for approximately 95% of current hydrogen production and generates substantial CO2 emissions. A comprehensive life cycle assessment reveals that photoelectrochemical systems could reduce carbon emissions by up to 80-90% compared to fossil fuel-based hydrogen production methods.
The sustainability advantages extend beyond carbon reduction. These systems utilize water as the primary feedstock, an abundant resource when compared to precious metals or rare earth elements required for other clean energy technologies. However, water quality and availability remain important considerations, particularly in water-stressed regions. Purification processes for input water may add to the overall environmental footprint, though significantly less than the environmental impact of extracting and processing fossil fuels.
Material sustainability presents both challenges and opportunities. Current high-efficiency photoelectrochemical systems often rely on scarce materials such as platinum group metals for catalysts and rare semiconductor materials. Research into earth-abundant alternatives like iron oxide, copper oxide, and carbon-based materials shows promising directions for improving sustainability. The durability of these systems also affects their environmental profile, with longer-lasting components reducing the frequency of replacement and associated resource consumption.
Land use requirements for scaled photoelectrochemical systems must be evaluated against competing needs. Distributed small-scale systems integrated into existing infrastructure could minimize additional land requirements, while large-scale centralized facilities would need careful siting considerations. The potential for co-location with water treatment facilities offers synergistic benefits that could enhance overall sustainability.
End-of-life management represents another critical environmental consideration. Developing effective recycling protocols for semiconductor materials, catalysts, and other components will be essential for closing the material loop and minimizing waste. Current research indicates that up to 85% of precious metals from these systems could potentially be recovered and reused, significantly improving their lifetime environmental profile.
When integrated with renewable energy sources, photoelectrochemical water splitting systems can contribute to grid stability by providing energy storage solutions, further enhancing the environmental benefits of renewable energy deployment. This integration capability positions the technology as a potential cornerstone in sustainable energy transitions across various sectors.
The sustainability advantages extend beyond carbon reduction. These systems utilize water as the primary feedstock, an abundant resource when compared to precious metals or rare earth elements required for other clean energy technologies. However, water quality and availability remain important considerations, particularly in water-stressed regions. Purification processes for input water may add to the overall environmental footprint, though significantly less than the environmental impact of extracting and processing fossil fuels.
Material sustainability presents both challenges and opportunities. Current high-efficiency photoelectrochemical systems often rely on scarce materials such as platinum group metals for catalysts and rare semiconductor materials. Research into earth-abundant alternatives like iron oxide, copper oxide, and carbon-based materials shows promising directions for improving sustainability. The durability of these systems also affects their environmental profile, with longer-lasting components reducing the frequency of replacement and associated resource consumption.
Land use requirements for scaled photoelectrochemical systems must be evaluated against competing needs. Distributed small-scale systems integrated into existing infrastructure could minimize additional land requirements, while large-scale centralized facilities would need careful siting considerations. The potential for co-location with water treatment facilities offers synergistic benefits that could enhance overall sustainability.
End-of-life management represents another critical environmental consideration. Developing effective recycling protocols for semiconductor materials, catalysts, and other components will be essential for closing the material loop and minimizing waste. Current research indicates that up to 85% of precious metals from these systems could potentially be recovered and reused, significantly improving their lifetime environmental profile.
When integrated with renewable energy sources, photoelectrochemical water splitting systems can contribute to grid stability by providing energy storage solutions, further enhancing the environmental benefits of renewable energy deployment. This integration capability positions the technology as a potential cornerstone in sustainable energy transitions across various sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!