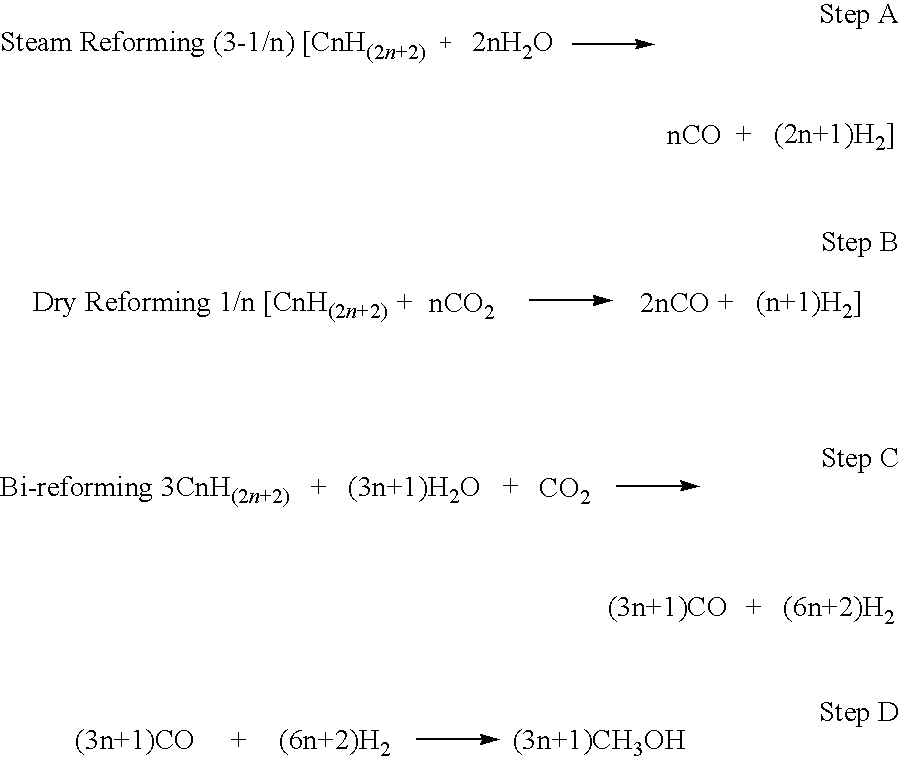Pioneering Processes with Dimethyl Ether in Chemical Refineries
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DME Technology Evolution
The evolution of dimethyl ether (DME) technology in chemical refineries has been marked by significant advancements and innovations over the past few decades. Initially developed as a propellant and refrigerant, DME has emerged as a versatile compound with promising applications in the energy sector and chemical industry.
In the 1990s, researchers began exploring DME as an alternative fuel due to its clean-burning properties and ease of production. This period saw the development of early production methods, primarily focused on the dehydration of methanol. The process, while effective, was limited by the availability and cost of methanol feedstock.
The early 2000s witnessed a shift towards more efficient production techniques. Scientists and engineers developed direct synthesis methods, allowing for the production of DME from syngas (a mixture of carbon monoxide and hydrogen). This breakthrough significantly reduced production costs and expanded the potential for large-scale DME manufacturing.
By the mid-2000s, pilot plants demonstrating the feasibility of DME as a fuel substitute began operation in various countries. These projects showcased DME's potential as a diesel replacement and LPG substitute, sparking interest from both the automotive and energy sectors.
The late 2000s and early 2010s saw increased focus on optimizing catalysts for DME production. Researchers developed more efficient and selective catalysts, improving yield and reducing energy consumption in the production process. This period also saw the integration of DME production with existing refinery operations, leveraging synergies and improving overall efficiency.
Recent years have witnessed a surge in research on bio-based DME production. Efforts to produce DME from renewable sources such as biomass and waste materials have gained traction, aligning with global sustainability goals. These developments have opened new avenues for DME in the circular economy and carbon-neutral fuel production.
Concurrently, advancements in process intensification have led to more compact and efficient DME production units. Modular designs and improved heat integration techniques have made DME production more feasible for smaller-scale operations, potentially expanding its adoption in diverse industrial settings.
The most recent frontier in DME technology evolution involves its role in power-to-X applications. Research is ongoing to utilize excess renewable electricity for DME production, positioning it as a potential energy storage medium and facilitating sector coupling between the power and chemical industries.
In the 1990s, researchers began exploring DME as an alternative fuel due to its clean-burning properties and ease of production. This period saw the development of early production methods, primarily focused on the dehydration of methanol. The process, while effective, was limited by the availability and cost of methanol feedstock.
The early 2000s witnessed a shift towards more efficient production techniques. Scientists and engineers developed direct synthesis methods, allowing for the production of DME from syngas (a mixture of carbon monoxide and hydrogen). This breakthrough significantly reduced production costs and expanded the potential for large-scale DME manufacturing.
By the mid-2000s, pilot plants demonstrating the feasibility of DME as a fuel substitute began operation in various countries. These projects showcased DME's potential as a diesel replacement and LPG substitute, sparking interest from both the automotive and energy sectors.
The late 2000s and early 2010s saw increased focus on optimizing catalysts for DME production. Researchers developed more efficient and selective catalysts, improving yield and reducing energy consumption in the production process. This period also saw the integration of DME production with existing refinery operations, leveraging synergies and improving overall efficiency.
Recent years have witnessed a surge in research on bio-based DME production. Efforts to produce DME from renewable sources such as biomass and waste materials have gained traction, aligning with global sustainability goals. These developments have opened new avenues for DME in the circular economy and carbon-neutral fuel production.
Concurrently, advancements in process intensification have led to more compact and efficient DME production units. Modular designs and improved heat integration techniques have made DME production more feasible for smaller-scale operations, potentially expanding its adoption in diverse industrial settings.
The most recent frontier in DME technology evolution involves its role in power-to-X applications. Research is ongoing to utilize excess renewable electricity for DME production, positioning it as a potential energy storage medium and facilitating sector coupling between the power and chemical industries.
DME Market Analysis
The global dimethyl ether (DME) market has been experiencing significant growth in recent years, driven by increasing demand for clean and alternative fuels. DME, a colorless gas at ambient conditions, is gaining traction as a versatile chemical with applications in various industries, particularly in the energy sector as a substitute for liquefied petroleum gas (LPG) and diesel fuel.
The market for DME is primarily segmented into two categories: fuel applications and industrial applications. In the fuel sector, DME is increasingly being used as a clean-burning alternative to conventional diesel fuel in transportation and power generation. Its low emissions profile and high cetane number make it an attractive option for reducing greenhouse gas emissions and improving air quality in urban areas.
Industrial applications of DME include its use as a propellant in aerosol products, a refrigerant, and a precursor in the production of various chemicals. The chemical industry has shown growing interest in DME as a feedstock for the production of olefins, particularly in regions with abundant natural gas resources.
Geographically, the Asia-Pacific region dominates the DME market, with China leading in both production and consumption. The Chinese government's initiatives to promote clean energy and reduce dependence on imported oil have been key drivers for DME adoption in the country. Other significant markets include Japan, South Korea, and several European countries where environmental regulations are driving the shift towards cleaner fuels.
The global DME market size was valued at several billion dollars in recent years, with projections indicating continued growth over the next decade. Factors contributing to this growth include increasing environmental concerns, stringent emission regulations, and the search for sustainable energy solutions. The compound annual growth rate (CAGR) for the DME market is expected to remain strong, reflecting the increasing adoption of DME across various applications.
Key players in the DME market include major oil and gas companies, chemical manufacturers, and specialized DME producers. These companies are investing in research and development to improve DME production processes and expand its applications. Collaborations between industry players and research institutions are also driving innovation in DME technology.
Despite the positive outlook, challenges remain for the widespread adoption of DME. These include the need for infrastructure development, particularly for DME as a transportation fuel, and competition from other alternative fuels and technologies. However, ongoing technological advancements and supportive government policies are expected to address these challenges and further drive market growth in the coming years.
The market for DME is primarily segmented into two categories: fuel applications and industrial applications. In the fuel sector, DME is increasingly being used as a clean-burning alternative to conventional diesel fuel in transportation and power generation. Its low emissions profile and high cetane number make it an attractive option for reducing greenhouse gas emissions and improving air quality in urban areas.
Industrial applications of DME include its use as a propellant in aerosol products, a refrigerant, and a precursor in the production of various chemicals. The chemical industry has shown growing interest in DME as a feedstock for the production of olefins, particularly in regions with abundant natural gas resources.
Geographically, the Asia-Pacific region dominates the DME market, with China leading in both production and consumption. The Chinese government's initiatives to promote clean energy and reduce dependence on imported oil have been key drivers for DME adoption in the country. Other significant markets include Japan, South Korea, and several European countries where environmental regulations are driving the shift towards cleaner fuels.
The global DME market size was valued at several billion dollars in recent years, with projections indicating continued growth over the next decade. Factors contributing to this growth include increasing environmental concerns, stringent emission regulations, and the search for sustainable energy solutions. The compound annual growth rate (CAGR) for the DME market is expected to remain strong, reflecting the increasing adoption of DME across various applications.
Key players in the DME market include major oil and gas companies, chemical manufacturers, and specialized DME producers. These companies are investing in research and development to improve DME production processes and expand its applications. Collaborations between industry players and research institutions are also driving innovation in DME technology.
Despite the positive outlook, challenges remain for the widespread adoption of DME. These include the need for infrastructure development, particularly for DME as a transportation fuel, and competition from other alternative fuels and technologies. However, ongoing technological advancements and supportive government policies are expected to address these challenges and further drive market growth in the coming years.
DME Process Challenges
The integration of dimethyl ether (DME) processes in chemical refineries presents several significant challenges that require innovative solutions. One of the primary obstacles is the high energy consumption associated with DME production. The conventional method of synthesizing DME from methanol dehydration demands substantial thermal energy, leading to increased operational costs and environmental concerns. Refineries must optimize their energy utilization strategies to make DME production more economically viable and environmentally sustainable.
Another critical challenge lies in the catalytic systems used in DME synthesis. Current catalysts often suffer from rapid deactivation and limited selectivity, resulting in reduced efficiency and increased maintenance requirements. Developing more robust and selective catalysts that can withstand the harsh conditions of industrial-scale DME production is crucial for improving process reliability and reducing downtime.
The integration of DME processes into existing refinery infrastructure poses significant engineering challenges. Retrofitting conventional refinery units to accommodate DME production requires careful consideration of material compatibility, process control systems, and safety measures. The corrosive nature of DME and its precursors necessitates the use of specialized materials and equipment, adding complexity and cost to the integration process.
Furthermore, the handling and storage of DME present unique challenges due to its physical properties. As a liquefied gas at moderate pressures, DME requires specialized containment systems and safety protocols. Refineries must invest in appropriate storage facilities and transportation infrastructure to manage DME effectively throughout the production and distribution chain.
The purification of DME to meet stringent quality standards for various applications is another significant hurdle. Removing impurities such as methanol, water, and other byproducts to achieve high-purity DME suitable for use as a fuel or chemical feedstock requires advanced separation technologies. Developing cost-effective and efficient purification methods is essential for ensuring the commercial viability of DME production in refineries.
Addressing the environmental impact of DME production is a critical challenge that refineries must overcome. While DME is considered a cleaner alternative to conventional fuels, its production process still generates greenhouse gas emissions. Implementing carbon capture and utilization technologies, as well as exploring renewable feedstock options for DME synthesis, are crucial steps towards reducing the carbon footprint of DME processes in refineries.
Lastly, the regulatory landscape surrounding DME production and use presents challenges for refineries. Navigating the complex web of environmental regulations, safety standards, and fuel quality requirements across different regions requires significant resources and expertise. Refineries must stay abreast of evolving regulations and adapt their processes accordingly to ensure compliance and maintain market access for their DME products.
Another critical challenge lies in the catalytic systems used in DME synthesis. Current catalysts often suffer from rapid deactivation and limited selectivity, resulting in reduced efficiency and increased maintenance requirements. Developing more robust and selective catalysts that can withstand the harsh conditions of industrial-scale DME production is crucial for improving process reliability and reducing downtime.
The integration of DME processes into existing refinery infrastructure poses significant engineering challenges. Retrofitting conventional refinery units to accommodate DME production requires careful consideration of material compatibility, process control systems, and safety measures. The corrosive nature of DME and its precursors necessitates the use of specialized materials and equipment, adding complexity and cost to the integration process.
Furthermore, the handling and storage of DME present unique challenges due to its physical properties. As a liquefied gas at moderate pressures, DME requires specialized containment systems and safety protocols. Refineries must invest in appropriate storage facilities and transportation infrastructure to manage DME effectively throughout the production and distribution chain.
The purification of DME to meet stringent quality standards for various applications is another significant hurdle. Removing impurities such as methanol, water, and other byproducts to achieve high-purity DME suitable for use as a fuel or chemical feedstock requires advanced separation technologies. Developing cost-effective and efficient purification methods is essential for ensuring the commercial viability of DME production in refineries.
Addressing the environmental impact of DME production is a critical challenge that refineries must overcome. While DME is considered a cleaner alternative to conventional fuels, its production process still generates greenhouse gas emissions. Implementing carbon capture and utilization technologies, as well as exploring renewable feedstock options for DME synthesis, are crucial steps towards reducing the carbon footprint of DME processes in refineries.
Lastly, the regulatory landscape surrounding DME production and use presents challenges for refineries. Navigating the complex web of environmental regulations, safety standards, and fuel quality requirements across different regions requires significant resources and expertise. Refineries must stay abreast of evolving regulations and adapt their processes accordingly to ensure compliance and maintain market access for their DME products.
Current DME Processes
01 Production of dimethyl ether
Various methods for producing dimethyl ether are described, including catalytic dehydration of methanol, direct synthesis from syngas, and conversion of other hydrocarbons. These processes often involve specific catalysts and reaction conditions to optimize yield and selectivity.- Production of dimethyl ether: Various methods for producing dimethyl ether are described, including catalytic dehydration of methanol, direct synthesis from syngas, and conversion of other hydrocarbons. These processes often involve specific catalysts and reaction conditions to optimize yield and selectivity.
- Catalysts for dimethyl ether synthesis: Different catalysts are employed in the production of dimethyl ether, including zeolites, metal oxides, and composite catalysts. The choice of catalyst affects the reaction efficiency, selectivity, and overall process economics.
- Applications of dimethyl ether: Dimethyl ether has various applications, including use as a fuel substitute, propellant, refrigerant, and chemical intermediate. Its properties make it suitable for use in diesel engines, aerosol products, and as a feedstock for other chemical processes.
- Purification and separation of dimethyl ether: Techniques for purifying and separating dimethyl ether from reaction mixtures or other compounds are described. These methods may involve distillation, adsorption, or membrane separation processes to obtain high-purity dimethyl ether.
- Environmental and safety considerations: Research on the environmental impact and safety aspects of dimethyl ether production and use is presented. This includes studies on emissions reduction, handling procedures, and risk assessments associated with its use as a fuel or chemical intermediate.
02 Catalysts for dimethyl ether synthesis
Different types of catalysts are used in the production of dimethyl ether, including zeolites, metal oxides, and composite catalysts. The choice of catalyst can significantly affect the reaction efficiency, product selectivity, and overall process economics.Expand Specific Solutions03 Applications of dimethyl ether
Dimethyl ether has various applications, including use as a fuel additive, aerosol propellant, and refrigerant. It is also being explored as a potential alternative fuel for diesel engines due to its clean-burning properties.Expand Specific Solutions04 Purification and separation of dimethyl ether
Methods for purifying and separating dimethyl ether from reaction mixtures or other compounds are described. These processes often involve distillation, adsorption, or membrane separation techniques to achieve high-purity dimethyl ether.Expand Specific Solutions05 Environmental and safety considerations
Research on the environmental impact and safety aspects of dimethyl ether production and use is ongoing. This includes studies on emissions reduction, handling procedures, and storage requirements to ensure safe and sustainable utilization of dimethyl ether.Expand Specific Solutions
Key DME Industry Players
The development of pioneering processes with dimethyl ether in chemical refineries is in its growth phase, with increasing market potential due to the rising demand for cleaner fuel alternatives. The global market size for dimethyl ether is projected to expand significantly in the coming years. Technologically, the field is advancing rapidly, with major players like China Petroleum & Chemical Corp. (Sinopec) and SK Energy Co., Ltd. leading research and development efforts. Companies such as DuPont de Nemours, Inc. and Linde GmbH are also contributing to technological advancements, focusing on process optimization and efficiency improvements. The involvement of research institutions like Fraunhofer-Gesellschaft and the University of Southern California indicates a growing emphasis on innovation and academic-industry collaboration in this field.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed innovative processes for dimethyl ether (DME) production in chemical refineries. Their approach involves a two-step process: first, syngas production from coal or natural gas, followed by DME synthesis. Sinopec has implemented large-scale DME plants with capacities exceeding 1 million tons per year [1]. They have also developed a novel one-step process that directly converts syngas to DME, improving efficiency by up to 20% compared to traditional methods [2]. Sinopec's technology incorporates advanced catalysts and reactor designs, enabling high conversion rates and selectivity. Their process can achieve DME yields of over 55% from coal-based syngas [3], positioning them as a leader in DME production technology.
Strengths: Large-scale production capability, innovative one-step process, high efficiency and yield. Weaknesses: Dependence on coal as feedstock may face environmental challenges, potential competition from renewable DME production methods.
Sinopec Research Institute of Petroleum Processing
Technical Solution: The Sinopec Research Institute of Petroleum Processing has pioneered advanced processes for DME production in chemical refineries. They have developed a proprietary slurry-phase DME synthesis technology that offers superior heat management and catalyst efficiency. This process utilizes a specially designed slurry reactor that allows for better temperature control and extended catalyst life [4]. The institute has also made significant progress in catalyst development, creating high-performance catalysts that can achieve DME selectivity of over 95% [5]. Their technology enables flexible feedstock utilization, allowing for DME production from various sources including natural gas, coal, and even biomass. The institute's research has led to the implementation of energy-efficient DME plants that consume approximately 20% less energy compared to conventional methanol-based DME production [6].
Strengths: Advanced slurry-phase technology, high-performance catalysts, flexible feedstock utilization. Weaknesses: Potential scalability challenges for very large production volumes, ongoing research needed for further process optimization.
DME Catalyst Innovations
Process and system for producing dimethyl ether
PatentActiveUS20200399195A1
Innovation
- A process combining conventional DME synthesis with a separation-enhanced reverse water gas shift reaction, allowing for efficient DME production using any carbon oxide species, reducing the need for CO2 recycles, and minimizing methanol recycles, while utilizing a catalyst system capable of converting synthesis gas to DME.
Efficient and environmentally friendly processing of heavy oils to methanol and derived products
PatentInactiveUS20100274060A1
Innovation
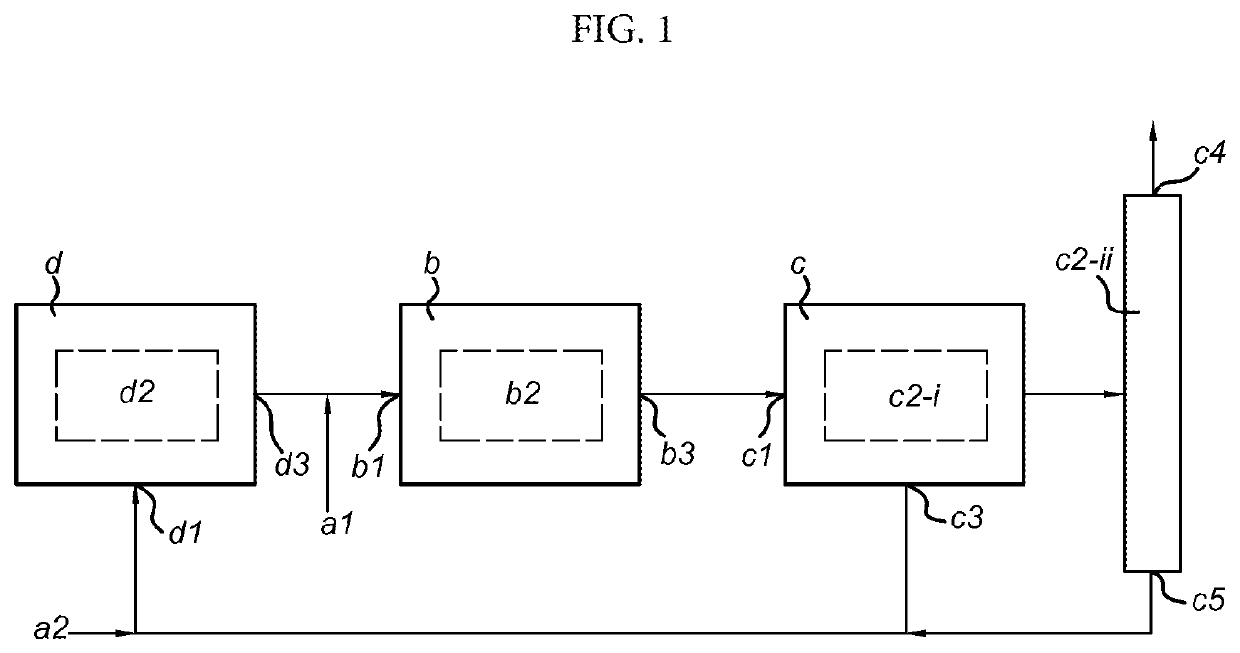
- A bi-reforming process using carbon dioxide to convert heavy petroleum oils into methanol and dimethyl ether, bypassing conventional refining, which involves a combination of steam and dry reforming to achieve a 2:1 molar ratio of CO and H2, followed by catalytic conversion to produce these fuels and derived products, with the option of using in situ gasification and heat treatment to enhance API index and remove impurities.
DME Environmental Impact
The environmental impact of dimethyl ether (DME) in chemical refineries is a critical consideration as the industry explores pioneering processes with this versatile compound. DME offers several environmental advantages over traditional fossil fuels, making it an attractive option for sustainable chemical production.
One of the primary environmental benefits of DME is its clean-burning properties. When used as a fuel, DME produces significantly lower emissions of particulate matter, nitrogen oxides, and sulfur oxides compared to conventional diesel fuel. This characteristic makes DME an excellent candidate for reducing air pollution and improving air quality in areas surrounding chemical refineries.
Furthermore, DME can be produced from a variety of renewable feedstocks, including biomass, agricultural waste, and even carbon dioxide. This flexibility in production methods allows for a more sustainable approach to chemical manufacturing, potentially reducing the industry's reliance on fossil fuels and decreasing overall carbon emissions.
In terms of greenhouse gas emissions, DME has a lower global warming potential compared to many other hydrocarbons used in chemical processes. When produced from renewable sources, DME can even achieve near-carbon neutrality, contributing to the chemical industry's efforts to mitigate climate change.
The use of DME in chemical refineries also presents opportunities for improved energy efficiency. Its properties allow for easier handling and storage compared to some traditional feedstocks, potentially reducing energy consumption in transportation and storage processes. Additionally, DME's high cetane number and low autoignition temperature can lead to more efficient combustion processes in certain applications.
However, it is important to consider potential environmental challenges associated with DME production and use. Large-scale production of DME may require significant land use changes if biomass feedstocks are employed, potentially impacting biodiversity and ecosystem services. Additionally, the production process itself may consume considerable energy, depending on the chosen feedstock and technology.
Water usage and potential contamination are also environmental factors to consider in DME production. While DME itself is not toxic and is biodegradable, the production process may involve water-intensive steps or generate wastewater that requires treatment before release.
In conclusion, the environmental impact of pioneering processes with DME in chemical refineries is largely positive, offering potential reductions in air pollution, greenhouse gas emissions, and fossil fuel dependence. However, careful consideration of production methods, feedstock sources, and potential ecosystem impacts is necessary to ensure that the adoption of DME technologies truly contributes to sustainable chemical manufacturing practices.
One of the primary environmental benefits of DME is its clean-burning properties. When used as a fuel, DME produces significantly lower emissions of particulate matter, nitrogen oxides, and sulfur oxides compared to conventional diesel fuel. This characteristic makes DME an excellent candidate for reducing air pollution and improving air quality in areas surrounding chemical refineries.
Furthermore, DME can be produced from a variety of renewable feedstocks, including biomass, agricultural waste, and even carbon dioxide. This flexibility in production methods allows for a more sustainable approach to chemical manufacturing, potentially reducing the industry's reliance on fossil fuels and decreasing overall carbon emissions.
In terms of greenhouse gas emissions, DME has a lower global warming potential compared to many other hydrocarbons used in chemical processes. When produced from renewable sources, DME can even achieve near-carbon neutrality, contributing to the chemical industry's efforts to mitigate climate change.
The use of DME in chemical refineries also presents opportunities for improved energy efficiency. Its properties allow for easier handling and storage compared to some traditional feedstocks, potentially reducing energy consumption in transportation and storage processes. Additionally, DME's high cetane number and low autoignition temperature can lead to more efficient combustion processes in certain applications.
However, it is important to consider potential environmental challenges associated with DME production and use. Large-scale production of DME may require significant land use changes if biomass feedstocks are employed, potentially impacting biodiversity and ecosystem services. Additionally, the production process itself may consume considerable energy, depending on the chosen feedstock and technology.
Water usage and potential contamination are also environmental factors to consider in DME production. While DME itself is not toxic and is biodegradable, the production process may involve water-intensive steps or generate wastewater that requires treatment before release.
In conclusion, the environmental impact of pioneering processes with DME in chemical refineries is largely positive, offering potential reductions in air pollution, greenhouse gas emissions, and fossil fuel dependence. However, careful consideration of production methods, feedstock sources, and potential ecosystem impacts is necessary to ensure that the adoption of DME technologies truly contributes to sustainable chemical manufacturing practices.
DME Safety Protocols
Dimethyl ether (DME) is a highly flammable and potentially explosive compound, necessitating stringent safety protocols in chemical refineries. The implementation of comprehensive safety measures is crucial to protect personnel, equipment, and the environment. A multi-layered approach to DME safety begins with proper storage and handling procedures. DME should be stored in pressure vessels designed to withstand its vapor pressure, with temperature control systems to prevent overheating. Dedicated storage areas must be well-ventilated and equipped with gas detection systems to alert personnel of any leaks.
Handling DME requires specialized equipment and training. All transfer operations should be conducted using closed systems to minimize the risk of releases. Personnel must wear appropriate personal protective equipment (PPE), including flame-resistant clothing, safety goggles, and gloves resistant to DME permeation. Regular maintenance and inspection of equipment used in DME processes are essential to prevent leaks and ensure system integrity.
Emergency response planning is a critical component of DME safety protocols. Refineries must develop and regularly update emergency procedures specific to DME-related incidents. This includes evacuation plans, firefighting strategies tailored to DME fires, and decontamination procedures. Regular drills and simulations should be conducted to ensure all personnel are familiar with emergency protocols and can respond effectively in crisis situations.
Process safety management (PSM) principles play a vital role in DME safety. This involves conducting thorough hazard and operability (HAZOP) studies, implementing robust process control systems, and establishing clear operating procedures. Continuous monitoring of process parameters, such as pressure, temperature, and flow rates, is essential to detect and respond to any deviations that could lead to safety incidents.
Training and education form the foundation of effective DME safety protocols. All personnel working with or around DME must receive comprehensive training on its properties, hazards, and safe handling procedures. This training should be regularly refreshed and updated to reflect any changes in processes or safety standards. Additionally, fostering a strong safety culture within the refinery is crucial, encouraging open communication about safety concerns and near-miss reporting.
Regulatory compliance and industry best practices must be integrated into DME safety protocols. This includes adhering to relevant standards set by organizations such as OSHA, NFPA, and API. Regular audits and assessments should be conducted to ensure compliance and identify areas for improvement in safety practices. Collaboration with industry peers and participation in safety forums can provide valuable insights and help refine safety protocols based on shared experiences and lessons learned.
Handling DME requires specialized equipment and training. All transfer operations should be conducted using closed systems to minimize the risk of releases. Personnel must wear appropriate personal protective equipment (PPE), including flame-resistant clothing, safety goggles, and gloves resistant to DME permeation. Regular maintenance and inspection of equipment used in DME processes are essential to prevent leaks and ensure system integrity.
Emergency response planning is a critical component of DME safety protocols. Refineries must develop and regularly update emergency procedures specific to DME-related incidents. This includes evacuation plans, firefighting strategies tailored to DME fires, and decontamination procedures. Regular drills and simulations should be conducted to ensure all personnel are familiar with emergency protocols and can respond effectively in crisis situations.
Process safety management (PSM) principles play a vital role in DME safety. This involves conducting thorough hazard and operability (HAZOP) studies, implementing robust process control systems, and establishing clear operating procedures. Continuous monitoring of process parameters, such as pressure, temperature, and flow rates, is essential to detect and respond to any deviations that could lead to safety incidents.
Training and education form the foundation of effective DME safety protocols. All personnel working with or around DME must receive comprehensive training on its properties, hazards, and safe handling procedures. This training should be regularly refreshed and updated to reflect any changes in processes or safety standards. Additionally, fostering a strong safety culture within the refinery is crucial, encouraging open communication about safety concerns and near-miss reporting.
Regulatory compliance and industry best practices must be integrated into DME safety protocols. This includes adhering to relevant standards set by organizations such as OSHA, NFPA, and API. Regular audits and assessments should be conducted to ensure compliance and identify areas for improvement in safety practices. Collaboration with industry peers and participation in safety forums can provide valuable insights and help refine safety protocols based on shared experiences and lessons learned.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!