Quantum Interconnects in the Pharmaceutical Industry
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Interconnect Evolution and Objectives
Quantum interconnects represent a pivotal advancement in quantum computing technology, enabling the seamless transfer of quantum information between different quantum processing units. The evolution of quantum interconnects began in the early 2000s with rudimentary quantum state transfer mechanisms, primarily confined to laboratory settings with limited practical applications. By 2010, researchers had developed more sophisticated quantum channels capable of maintaining quantum coherence over short distances, marking a significant milestone in quantum networking capabilities.
The pharmaceutical industry's interest in quantum interconnects emerged around 2015, when computational limitations in drug discovery processes became increasingly apparent. Traditional computing methods struggled with simulating complex molecular interactions accurately, creating a bottleneck in pharmaceutical research and development pipelines. This technological gap catalyzed investment in quantum computing solutions, with quantum interconnects identified as a critical component for scaling quantum computational power to levels required for pharmaceutical applications.
Between 2018 and 2022, quantum interconnect technology witnessed accelerated development, with breakthroughs in quantum repeaters, quantum memory systems, and error correction protocols. These advancements significantly improved the fidelity and distance of quantum information transfer, making distributed quantum computing architectures increasingly viable for pharmaceutical research applications.
The primary objective of quantum interconnect technology in the pharmaceutical context is to enable distributed quantum computing networks capable of simulating molecular structures and interactions with unprecedented accuracy. This capability would dramatically accelerate drug discovery processes by allowing researchers to predict drug efficacy and side effects with greater precision before entering costly clinical trials.
Secondary objectives include developing fault-tolerant quantum communication channels resistant to decoherence, minimizing quantum information loss during transfer operations, and creating standardized protocols for quantum data exchange between heterogeneous quantum systems. These technical goals align with the pharmaceutical industry's need for reliable, scalable quantum computing resources that can be deployed across research facilities globally.
The long-term vision for quantum interconnects in pharmaceuticals extends beyond mere computational advantages. By 2030, the industry aims to establish quantum-enhanced drug discovery platforms where quantum processors specializing in different computational tasks can work in concert through robust interconnect technologies. This would potentially reduce drug development timelines from the current average of 10-15 years to under 5 years, representing a paradigm shift in pharmaceutical innovation capabilities.
Current technological trajectories suggest quantum interconnects will continue evolving toward higher coherence times, greater transmission distances, and improved error correction capabilities, ultimately enabling the quantum computing ecosystem necessary for next-generation pharmaceutical research and development.
The pharmaceutical industry's interest in quantum interconnects emerged around 2015, when computational limitations in drug discovery processes became increasingly apparent. Traditional computing methods struggled with simulating complex molecular interactions accurately, creating a bottleneck in pharmaceutical research and development pipelines. This technological gap catalyzed investment in quantum computing solutions, with quantum interconnects identified as a critical component for scaling quantum computational power to levels required for pharmaceutical applications.
Between 2018 and 2022, quantum interconnect technology witnessed accelerated development, with breakthroughs in quantum repeaters, quantum memory systems, and error correction protocols. These advancements significantly improved the fidelity and distance of quantum information transfer, making distributed quantum computing architectures increasingly viable for pharmaceutical research applications.
The primary objective of quantum interconnect technology in the pharmaceutical context is to enable distributed quantum computing networks capable of simulating molecular structures and interactions with unprecedented accuracy. This capability would dramatically accelerate drug discovery processes by allowing researchers to predict drug efficacy and side effects with greater precision before entering costly clinical trials.
Secondary objectives include developing fault-tolerant quantum communication channels resistant to decoherence, minimizing quantum information loss during transfer operations, and creating standardized protocols for quantum data exchange between heterogeneous quantum systems. These technical goals align with the pharmaceutical industry's need for reliable, scalable quantum computing resources that can be deployed across research facilities globally.
The long-term vision for quantum interconnects in pharmaceuticals extends beyond mere computational advantages. By 2030, the industry aims to establish quantum-enhanced drug discovery platforms where quantum processors specializing in different computational tasks can work in concert through robust interconnect technologies. This would potentially reduce drug development timelines from the current average of 10-15 years to under 5 years, representing a paradigm shift in pharmaceutical innovation capabilities.
Current technological trajectories suggest quantum interconnects will continue evolving toward higher coherence times, greater transmission distances, and improved error correction capabilities, ultimately enabling the quantum computing ecosystem necessary for next-generation pharmaceutical research and development.
Pharmaceutical Industry Demand for Quantum Technologies
The pharmaceutical industry is experiencing a significant transformation driven by the need for more efficient drug discovery and development processes. Quantum technologies, particularly quantum computing and quantum interconnects, are emerging as potential game-changers in addressing the complex computational challenges faced by pharmaceutical companies. The industry's demand for quantum technologies stems from several critical needs that conventional computing systems struggle to address effectively.
Drug discovery processes currently require enormous computational resources to simulate molecular interactions and predict drug efficacy. Pharmaceutical companies typically spend 10-15 years and billions of dollars bringing a single drug to market, with high failure rates throughout the development pipeline. This inefficiency creates substantial demand for quantum technologies that can accelerate molecular modeling and simulation processes.
Quantum computing's ability to model quantum mechanical systems makes it uniquely suited for simulating molecular structures and interactions at unprecedented scales and accuracy. Major pharmaceutical companies including Merck, Biogen, and Pfizer have initiated quantum computing research programs, signaling growing industry recognition of quantum technologies' potential value.
The market for quantum technologies in pharmaceuticals is projected to grow significantly as the technology matures. Early adopters are primarily focusing on research partnerships with quantum technology providers rather than direct infrastructure investment, creating demand for quantum computing as a service (QCaaS) models tailored to pharmaceutical applications.
Beyond drug discovery, quantum technologies show promise in optimizing clinical trial designs, improving manufacturing processes, and enhancing supply chain management. The ability to process and analyze complex biological data sets represents another significant demand driver, particularly as personalized medicine becomes more prevalent in treatment approaches.
Regulatory considerations are also shaping demand patterns. As quantum technologies demonstrate potential to improve drug safety prediction and reduce adverse events, regulatory bodies are beginning to explore frameworks for validating quantum-enabled drug development processes, potentially creating additional incentives for adoption.
The pharmaceutical industry's demand for quantum interconnects specifically relates to the need for secure transmission of sensitive research data and the integration of quantum computing resources with existing high-performance computing infrastructure. As quantum advantage becomes achievable for specific pharmaceutical applications, demand for robust quantum interconnect technologies that can reliably link quantum processors while maintaining coherence will increase substantially.
Drug discovery processes currently require enormous computational resources to simulate molecular interactions and predict drug efficacy. Pharmaceutical companies typically spend 10-15 years and billions of dollars bringing a single drug to market, with high failure rates throughout the development pipeline. This inefficiency creates substantial demand for quantum technologies that can accelerate molecular modeling and simulation processes.
Quantum computing's ability to model quantum mechanical systems makes it uniquely suited for simulating molecular structures and interactions at unprecedented scales and accuracy. Major pharmaceutical companies including Merck, Biogen, and Pfizer have initiated quantum computing research programs, signaling growing industry recognition of quantum technologies' potential value.
The market for quantum technologies in pharmaceuticals is projected to grow significantly as the technology matures. Early adopters are primarily focusing on research partnerships with quantum technology providers rather than direct infrastructure investment, creating demand for quantum computing as a service (QCaaS) models tailored to pharmaceutical applications.
Beyond drug discovery, quantum technologies show promise in optimizing clinical trial designs, improving manufacturing processes, and enhancing supply chain management. The ability to process and analyze complex biological data sets represents another significant demand driver, particularly as personalized medicine becomes more prevalent in treatment approaches.
Regulatory considerations are also shaping demand patterns. As quantum technologies demonstrate potential to improve drug safety prediction and reduce adverse events, regulatory bodies are beginning to explore frameworks for validating quantum-enabled drug development processes, potentially creating additional incentives for adoption.
The pharmaceutical industry's demand for quantum interconnects specifically relates to the need for secure transmission of sensitive research data and the integration of quantum computing resources with existing high-performance computing infrastructure. As quantum advantage becomes achievable for specific pharmaceutical applications, demand for robust quantum interconnect technologies that can reliably link quantum processors while maintaining coherence will increase substantially.
Current Quantum Interconnect Limitations in Pharma
Despite significant advancements in quantum computing technologies, quantum interconnects within pharmaceutical applications face substantial limitations that hinder their widespread implementation. The primary challenge lies in maintaining quantum coherence across distributed quantum systems. Current pharmaceutical quantum computing applications require stable quantum states for molecular modeling, yet environmental factors such as temperature fluctuations and electromagnetic interference in laboratory settings frequently disrupt quantum coherence, limiting computational reliability.
Scalability presents another significant barrier. While quantum interconnects show promise in controlled laboratory environments, scaling these systems to meet the computational demands of pharmaceutical research—particularly for complex protein folding simulations and drug interaction models—remains problematic. Current interconnect technologies struggle to maintain fidelity when linking more than a few dozen qubits, whereas pharmaceutical applications often require thousands of interconnected qubits for meaningful results.
Error correction mechanisms for quantum interconnects in pharmaceutical settings are still rudimentary. The error rates in quantum transmission channels exceed acceptable thresholds for precise molecular modeling, resulting in computational outcomes with questionable reliability for drug development processes. This limitation necessitates extensive classical verification of quantum results, negating much of the speed advantage quantum systems theoretically offer.
Integration challenges with existing pharmaceutical research infrastructure further complicate implementation. Most quantum interconnect systems require specialized environments incompatible with standard laboratory equipment. The interfaces between quantum systems and classical computing infrastructure—essential for data preparation and result interpretation in pharmaceutical research—remain cumbersome and inefficient, creating workflow bottlenecks.
Cost factors also significantly limit adoption. Current quantum interconnect technologies require expensive cryogenic cooling systems and specialized materials that make implementation prohibitively expensive for all but the largest pharmaceutical companies. The return on investment remains uncertain given the experimental nature of many quantum applications in drug discovery.
Technical expertise requirements present another barrier. The operation and maintenance of quantum interconnect systems demand highly specialized knowledge spanning quantum physics, materials science, and pharmaceutical informatics—a rare combination that creates staffing challenges for research teams. This expertise gap slows adoption and effective utilization of quantum technologies in pharmaceutical research environments.
Standardization issues further complicate the landscape, with competing quantum interconnect protocols limiting interoperability between different quantum computing platforms used across the pharmaceutical research ecosystem. This fragmentation impedes collaborative research efforts and creates vendor lock-in concerns for pharmaceutical companies considering significant investments in quantum infrastructure.
Scalability presents another significant barrier. While quantum interconnects show promise in controlled laboratory environments, scaling these systems to meet the computational demands of pharmaceutical research—particularly for complex protein folding simulations and drug interaction models—remains problematic. Current interconnect technologies struggle to maintain fidelity when linking more than a few dozen qubits, whereas pharmaceutical applications often require thousands of interconnected qubits for meaningful results.
Error correction mechanisms for quantum interconnects in pharmaceutical settings are still rudimentary. The error rates in quantum transmission channels exceed acceptable thresholds for precise molecular modeling, resulting in computational outcomes with questionable reliability for drug development processes. This limitation necessitates extensive classical verification of quantum results, negating much of the speed advantage quantum systems theoretically offer.
Integration challenges with existing pharmaceutical research infrastructure further complicate implementation. Most quantum interconnect systems require specialized environments incompatible with standard laboratory equipment. The interfaces between quantum systems and classical computing infrastructure—essential for data preparation and result interpretation in pharmaceutical research—remain cumbersome and inefficient, creating workflow bottlenecks.
Cost factors also significantly limit adoption. Current quantum interconnect technologies require expensive cryogenic cooling systems and specialized materials that make implementation prohibitively expensive for all but the largest pharmaceutical companies. The return on investment remains uncertain given the experimental nature of many quantum applications in drug discovery.
Technical expertise requirements present another barrier. The operation and maintenance of quantum interconnect systems demand highly specialized knowledge spanning quantum physics, materials science, and pharmaceutical informatics—a rare combination that creates staffing challenges for research teams. This expertise gap slows adoption and effective utilization of quantum technologies in pharmaceutical research environments.
Standardization issues further complicate the landscape, with competing quantum interconnect protocols limiting interoperability between different quantum computing platforms used across the pharmaceutical research ecosystem. This fragmentation impedes collaborative research efforts and creates vendor lock-in concerns for pharmaceutical companies considering significant investments in quantum infrastructure.
Existing Quantum Interconnect Implementations
01 Quantum interconnect architectures for quantum computing
Various architectures for quantum interconnects that enable communication between quantum computing components. These designs focus on creating reliable connections between quantum bits (qubits) while maintaining quantum coherence. The architectures include specialized waveguides, coupling mechanisms, and integrated circuit designs that facilitate quantum information transfer across different parts of quantum computing systems.- Quantum interconnect architectures: Various architectures for quantum interconnects that enable communication between quantum processing units. These architectures include optical interconnects, superconducting circuits, and hybrid systems that facilitate the transfer of quantum information while maintaining coherence. These designs address the challenges of connecting quantum bits across distances while minimizing decoherence and information loss.
- Quantum-optical interface technologies: Technologies that bridge quantum systems with optical communication networks, enabling long-distance quantum information transfer. These interfaces convert quantum states between different physical implementations, such as from solid-state qubits to photonic qubits. Key components include photonic crystal waveguides, optical resonators, and frequency conversion devices that maintain quantum coherence during state transfer.
- Superconducting quantum interconnect systems: Specialized interconnect systems using superconducting materials and circuits to link quantum processors. These systems leverage superconducting properties to maintain quantum coherence while transferring information between quantum elements. Designs include Josephson junction-based couplers, microwave resonators, and quantum buses that enable entanglement distribution across physically separated quantum processors.
- Fabrication methods for quantum interconnects: Advanced manufacturing techniques specifically developed for creating reliable quantum interconnect structures. These methods address the challenges of fabricating nanoscale features with precise alignment and minimal defects. Techniques include specialized lithography processes, selective material deposition, and novel integration approaches that preserve quantum properties during fabrication while enabling scalable production.
- Quantum network protocols and control systems: Software and control systems designed to manage quantum interconnects and enable reliable quantum communication networks. These protocols handle error correction, entanglement distribution, and synchronization between quantum nodes. The systems include classical control electronics that interface with quantum hardware, timing protocols for coordinated operations, and network management architectures that optimize quantum resource allocation across distributed systems.
02 Optical quantum interconnects
Optical-based quantum interconnect technologies that use photons to transfer quantum information. These systems employ photonic waveguides, optical fibers, and photonic integrated circuits to establish quantum connections. The technologies focus on minimizing decoherence and signal loss while maximizing fidelity in quantum state transfer, often incorporating specialized materials and structures to enhance photon-based quantum communication.Expand Specific Solutions03 Superconducting quantum interconnects
Superconducting technologies for quantum interconnects that operate at extremely low temperatures to maintain quantum coherence. These designs utilize superconducting materials and circuits to create low-loss quantum channels between qubits or quantum processors. The technologies include specialized Josephson junctions, resonators, and transmission lines that enable reliable quantum information transfer while minimizing thermal noise and decoherence effects.Expand Specific Solutions04 Quantum interconnect fabrication methods
Manufacturing techniques and processes specifically developed for creating quantum interconnects. These methods address the challenges of fabricating nanoscale quantum components with high precision and reliability. The approaches include specialized deposition techniques, etching processes, and material integration strategies that enable the creation of quantum interconnects with minimal defects and optimal quantum properties.Expand Specific Solutions05 Quantum network interconnect protocols
Protocols and control systems designed for managing quantum interconnects in networked environments. These technologies focus on the reliable transmission of quantum information across distributed quantum computing systems. The protocols address challenges such as quantum state preservation during transmission, entanglement distribution, and quantum error correction in interconnected quantum networks, enabling scalable quantum computing architectures.Expand Specific Solutions
Leading Quantum Technology Providers in Pharmaceuticals
Quantum Interconnects in the Pharmaceutical Industry is emerging as a transformative technology at the early development stage, with an estimated market potential of $2-5 billion by 2030. The competitive landscape features established tech giants (IBM, Google, Intel) developing foundational quantum hardware, alongside specialized quantum companies like SeeQC and Zapata Computing focusing on pharmaceutical applications. Major pharmaceutical players (Astellas Pharma, RemeGen, CureVac) are exploring quantum solutions for drug discovery and supply chain optimization. Academic institutions (MIT, Caltech, Dartmouth) are driving fundamental research, creating a collaborative ecosystem where technical maturity varies significantly across applications, with quantum simulation for molecular modeling showing the most near-term promise.
International Business Machines Corp.
Technical Solution: IBM has developed quantum interconnect technologies specifically targeting pharmaceutical applications through their Quantum Network. Their approach focuses on creating stable quantum links between computing nodes using superconducting qubits and microwave resonators. IBM's quantum interconnect technology enables distributed quantum computing for complex molecular simulations, allowing pharmaceutical researchers to model drug interactions at unprecedented scales. Their Quantum System Two architecture incorporates modular quantum processors connected via specialized quantum communication channels that maintain quantum coherence across physically separated quantum processing units. This architecture specifically addresses pharmaceutical computational needs by enabling larger-scale quantum simulations of molecular structures and drug-protein interactions than would be possible on isolated quantum processors. IBM has demonstrated practical applications in drug discovery workflows where quantum interconnects allow seamless integration between classical high-performance computing resources and quantum processing units, creating hybrid computational environments optimized for pharmaceutical research.
Strengths: Industry-leading quantum coherence times in their interconnect technology; established partnerships with major pharmaceutical companies providing real-world validation; comprehensive software stack that simplifies adoption. Weaknesses: Their superconducting qubit approach requires extremely low temperatures, increasing operational complexity and cost; still faces scalability challenges for production-level pharmaceutical applications.
SeeQC, Inc.
Technical Solution: SeeQC has pioneered a unique approach to quantum interconnects for pharmaceutical applications through their Digital Quantum Computing (DQC) architecture. Their technology integrates classical and quantum processing using superconducting Single Flux Quantum (SFQ) digital logic, creating efficient quantum-classical interfaces critical for pharmaceutical computational workflows. SeeQC's quantum interconnect solution employs chip-scale cryogenic classical control electronics co-located with quantum processing elements, minimizing latency and signal degradation between quantum and classical domains. This architecture is particularly valuable for pharmaceutical applications as it enables more efficient implementation of quantum algorithms for drug discovery by reducing the overhead associated with room-temperature control electronics. Their multi-chip module approach allows quantum processing elements to be interconnected while maintaining quantum coherence, enabling scalable quantum systems that can address the computational complexity of pharmaceutical molecular modeling. SeeQC has demonstrated their technology's application in quantum chemistry simulations relevant to drug discovery, showing potential for accelerating pharmaceutical development pipelines.
Strengths: Integrated cryogenic control electronics reduce system complexity and improve scalability; chip-scale approach enables more practical deployment in pharmaceutical research settings; lower power requirements than competing technologies. Weaknesses: Relatively new technology with fewer demonstrated pharmaceutical use cases; requires specialized manufacturing capabilities; still working to achieve the quantum volume necessary for complex pharmaceutical applications.
Key Quantum Coherence and Entanglement Innovations
Process of constructing oxidation-reduction nanomedicine quantum dots room temperature quantum bit networks
PatentActiveUS8304758B2
Innovation
- A process involving a droplet-quantum crystal lattice epitaxy bottom-up self-assembly approach is used to create molecular mono-layered redox nanomedicine quantum dot room temperature superconductor qubit networks by converting aromatic structures, single electrons, and single photons into controlled NOT and Majority quantum cellular automates, with specific pharmaceuticals like verapamil and adenosine triphosphate, under controlled orthogonal protocols.
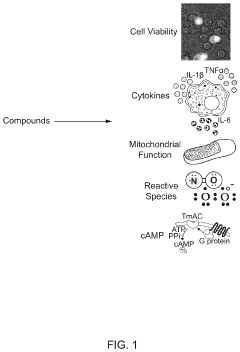
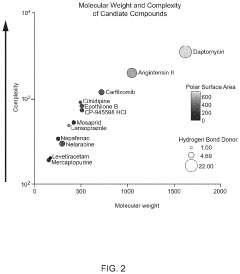
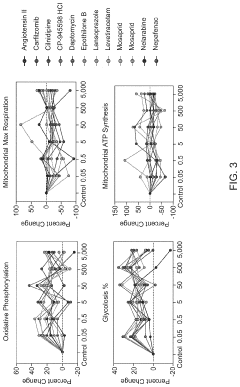
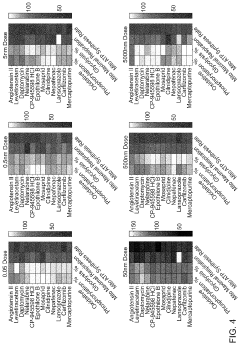
Platform for mapping compounds for pre-clinical effectiveness to predict clinical effectiveness for treating conditions
PatentPendingUS20230086047A1
Innovation
- A discovery platform using machine learning algorithms to predict cellular responses to pharmaceutical compounds through high-throughput, multi-endpoint functional in vitro assays, identifying significant correlations between physiochemical features and cellular responses, and generating predictive models for therapeutic outcomes.
Regulatory Framework for Quantum Computing in Healthcare
The regulatory landscape for quantum computing in healthcare and pharmaceutical applications represents a complex and evolving framework that stakeholders must navigate carefully. Currently, quantum technologies in pharmaceutical research operate within a regulatory vacuum, as most existing healthcare regulations were established before quantum computing's emergence. The FDA, EMA, and other global regulatory bodies are beginning to recognize the need for specialized frameworks addressing quantum-enabled drug discovery, quantum-secured patient data systems, and quantum-enhanced clinical trials.
Key regulatory considerations include data privacy implications, as quantum computing could potentially break current encryption standards protecting sensitive patient information. The EU's GDPR and the US HIPAA regulations will require significant updates to address quantum-specific vulnerabilities and capabilities. Several regulatory agencies have established working groups to develop guidelines for validating quantum algorithms used in drug discovery and clinical decision-making processes.
Pharmaceutical companies implementing quantum interconnect technologies face a dual regulatory challenge: demonstrating both the safety of quantum-derived pharmaceutical products and the security of quantum systems handling sensitive data. The FDA's emerging framework for Software as a Medical Device (SaMD) provides some guidance, but remains insufficient for quantum-specific applications that may operate fundamentally differently from classical computing systems.
International harmonization efforts are underway through organizations like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which recently established a quantum computing interest group. This group aims to develop standardized approaches for validating quantum algorithms in pharmaceutical research and establishing appropriate audit trails for quantum-assisted drug discovery processes.
Regulatory sandboxes have emerged as a promising approach, allowing pharmaceutical companies to test quantum interconnect technologies in controlled environments with regulatory oversight but reduced compliance burdens. The UK's MHRA and Singapore's Health Sciences Authority have pioneered such programs specifically for quantum applications in healthcare, providing valuable models for other jurisdictions.
As quantum technologies mature, experts anticipate a shift toward performance-based regulatory frameworks that focus on outcomes rather than prescriptive technical requirements. This approach would allow for continued innovation while ensuring patient safety and data security remain paramount considerations in the rapidly evolving quantum pharmaceutical landscape.
Key regulatory considerations include data privacy implications, as quantum computing could potentially break current encryption standards protecting sensitive patient information. The EU's GDPR and the US HIPAA regulations will require significant updates to address quantum-specific vulnerabilities and capabilities. Several regulatory agencies have established working groups to develop guidelines for validating quantum algorithms used in drug discovery and clinical decision-making processes.
Pharmaceutical companies implementing quantum interconnect technologies face a dual regulatory challenge: demonstrating both the safety of quantum-derived pharmaceutical products and the security of quantum systems handling sensitive data. The FDA's emerging framework for Software as a Medical Device (SaMD) provides some guidance, but remains insufficient for quantum-specific applications that may operate fundamentally differently from classical computing systems.
International harmonization efforts are underway through organizations like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which recently established a quantum computing interest group. This group aims to develop standardized approaches for validating quantum algorithms in pharmaceutical research and establishing appropriate audit trails for quantum-assisted drug discovery processes.
Regulatory sandboxes have emerged as a promising approach, allowing pharmaceutical companies to test quantum interconnect technologies in controlled environments with regulatory oversight but reduced compliance burdens. The UK's MHRA and Singapore's Health Sciences Authority have pioneered such programs specifically for quantum applications in healthcare, providing valuable models for other jurisdictions.
As quantum technologies mature, experts anticipate a shift toward performance-based regulatory frameworks that focus on outcomes rather than prescriptive technical requirements. This approach would allow for continued innovation while ensuring patient safety and data security remain paramount considerations in the rapidly evolving quantum pharmaceutical landscape.
Data Security and IP Protection in Quantum Pharma Applications
The integration of quantum technologies in pharmaceutical research introduces unprecedented data security challenges. As quantum interconnects enable faster drug discovery and molecular modeling, they simultaneously create new vulnerabilities in protecting valuable intellectual property. Traditional encryption methods become increasingly vulnerable to quantum computing attacks, necessitating quantum-resistant cryptographic solutions specifically tailored for pharmaceutical applications.
Pharmaceutical companies typically invest billions in R&D, making their molecular data, drug formulations, and clinical trial results prime targets for cyber espionage. Quantum interconnects, while enhancing computational capabilities, also expand the attack surface through quantum channels that may be susceptible to novel interception techniques. The industry must address these vulnerabilities while maintaining compliance with stringent regulatory frameworks like HIPAA, GDPR, and FDA requirements.
Quantum Key Distribution (QKD) represents a promising solution for securing pharmaceutical data exchanges. By leveraging quantum properties like entanglement and superposition, QKD creates theoretically unhackable communication channels. Several pharmaceutical giants have begun implementing QKD pilots to protect their most sensitive research data, particularly in cross-border collaborations where IP theft risks are heightened.
Post-quantum cryptography (PQC) adoption is accelerating within the pharmaceutical sector, with lattice-based and hash-based algorithms showing particular promise for protecting long-term data assets. These approaches offer protection against both classical and quantum attacks, crucial for pharmaceutical IP that may retain value for decades. Industry consortia are developing standardized PQC frameworks specifically addressing pharmaceutical use cases.
Homomorphic encryption presents another vital technology, allowing computational operations on encrypted pharmaceutical data without exposing the underlying information. This enables secure multi-party computation across research institutions while maintaining proprietary protection of molecular structures and biological mechanisms. Early implementations have demonstrated successful secure collaboration on protein folding simulations between competing pharmaceutical entities.
Zero-knowledge proofs are emerging as essential tools for verifiable data sharing in pharmaceutical quantum applications. These cryptographic protocols allow one party to prove possession of certain information without revealing the information itself, facilitating secure validation of research findings while protecting methodological IP. This approach is particularly valuable in regulatory submissions where transparency must be balanced with proprietary protection.
The pharmaceutical industry is increasingly adopting hardware-based security solutions, including quantum random number generators and tamper-resistant quantum processors. These physical safeguards complement cryptographic approaches by creating trusted execution environments for the most sensitive quantum computations involving proprietary molecular designs and simulation parameters.
Pharmaceutical companies typically invest billions in R&D, making their molecular data, drug formulations, and clinical trial results prime targets for cyber espionage. Quantum interconnects, while enhancing computational capabilities, also expand the attack surface through quantum channels that may be susceptible to novel interception techniques. The industry must address these vulnerabilities while maintaining compliance with stringent regulatory frameworks like HIPAA, GDPR, and FDA requirements.
Quantum Key Distribution (QKD) represents a promising solution for securing pharmaceutical data exchanges. By leveraging quantum properties like entanglement and superposition, QKD creates theoretically unhackable communication channels. Several pharmaceutical giants have begun implementing QKD pilots to protect their most sensitive research data, particularly in cross-border collaborations where IP theft risks are heightened.
Post-quantum cryptography (PQC) adoption is accelerating within the pharmaceutical sector, with lattice-based and hash-based algorithms showing particular promise for protecting long-term data assets. These approaches offer protection against both classical and quantum attacks, crucial for pharmaceutical IP that may retain value for decades. Industry consortia are developing standardized PQC frameworks specifically addressing pharmaceutical use cases.
Homomorphic encryption presents another vital technology, allowing computational operations on encrypted pharmaceutical data without exposing the underlying information. This enables secure multi-party computation across research institutions while maintaining proprietary protection of molecular structures and biological mechanisms. Early implementations have demonstrated successful secure collaboration on protein folding simulations between competing pharmaceutical entities.
Zero-knowledge proofs are emerging as essential tools for verifiable data sharing in pharmaceutical quantum applications. These cryptographic protocols allow one party to prove possession of certain information without revealing the information itself, facilitating secure validation of research findings while protecting methodological IP. This approach is particularly valuable in regulatory submissions where transparency must be balanced with proprietary protection.
The pharmaceutical industry is increasingly adopting hardware-based security solutions, including quantum random number generators and tamper-resistant quantum processors. These physical safeguards complement cryptographic approaches by creating trusted execution environments for the most sensitive quantum computations involving proprietary molecular designs and simulation parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!