Raman Spectroscopy vs HPLC: Efficiency in Pharmaceutical Testing
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Pharmaceutical Testing Technologies Background and Objectives
Pharmaceutical testing has evolved significantly over the past several decades, transitioning from basic chemical assays to sophisticated analytical methodologies. The pharmaceutical industry's testing requirements have become increasingly stringent due to regulatory demands, quality control necessities, and the growing complexity of drug formulations. Traditional testing methods like High-Performance Liquid Chromatography (HPLC) have long been the gold standard for pharmaceutical analysis, offering high sensitivity and reliability in quantitative determination of active pharmaceutical ingredients and impurities.
Raman spectroscopy, a vibrational spectroscopic technique based on inelastic scattering of monochromatic light, has emerged as a promising alternative in recent years. First discovered by C.V. Raman in 1928, this technology has seen remarkable advancements with the development of more powerful lasers, sensitive detectors, and sophisticated data processing algorithms. The integration of Raman spectroscopy into pharmaceutical testing workflows represents a significant technological shift with potential implications for efficiency, cost-effectiveness, and process optimization.
The evolution trajectory of pharmaceutical testing technologies indicates a clear trend toward non-destructive, rapid, and in-line capable methodologies. This shift aligns with the industry's growing emphasis on continuous manufacturing processes and real-time release testing paradigms. Regulatory bodies, including the FDA and EMA, have increasingly encouraged the adoption of Process Analytical Technology (PAT) frameworks, within which spectroscopic methods like Raman play a crucial role.
The primary objective of this technical research is to comprehensively evaluate the comparative efficiency of Raman spectroscopy versus HPLC in pharmaceutical testing applications. This assessment encompasses multiple dimensions of efficiency: analytical performance (sensitivity, specificity, accuracy, precision), operational efficiency (sample preparation requirements, analysis time, throughput capacity), economic considerations (equipment costs, operational expenses, return on investment), and implementation factors (training requirements, validation complexity, regulatory acceptance).
Additionally, this research aims to identify specific pharmaceutical testing scenarios where Raman spectroscopy may offer significant advantages over HPLC, as well as contexts where HPLC remains the superior choice. The potential for complementary implementation of both technologies within an integrated testing strategy will also be explored, recognizing that the optimal approach may involve strategic deployment of multiple analytical methodologies rather than wholesale replacement of established techniques.
Understanding the technological capabilities, limitations, and complementary aspects of these testing methodologies is essential for pharmaceutical manufacturers seeking to optimize their quality control processes while maintaining compliance with increasingly demanding regulatory standards.
Raman spectroscopy, a vibrational spectroscopic technique based on inelastic scattering of monochromatic light, has emerged as a promising alternative in recent years. First discovered by C.V. Raman in 1928, this technology has seen remarkable advancements with the development of more powerful lasers, sensitive detectors, and sophisticated data processing algorithms. The integration of Raman spectroscopy into pharmaceutical testing workflows represents a significant technological shift with potential implications for efficiency, cost-effectiveness, and process optimization.
The evolution trajectory of pharmaceutical testing technologies indicates a clear trend toward non-destructive, rapid, and in-line capable methodologies. This shift aligns with the industry's growing emphasis on continuous manufacturing processes and real-time release testing paradigms. Regulatory bodies, including the FDA and EMA, have increasingly encouraged the adoption of Process Analytical Technology (PAT) frameworks, within which spectroscopic methods like Raman play a crucial role.
The primary objective of this technical research is to comprehensively evaluate the comparative efficiency of Raman spectroscopy versus HPLC in pharmaceutical testing applications. This assessment encompasses multiple dimensions of efficiency: analytical performance (sensitivity, specificity, accuracy, precision), operational efficiency (sample preparation requirements, analysis time, throughput capacity), economic considerations (equipment costs, operational expenses, return on investment), and implementation factors (training requirements, validation complexity, regulatory acceptance).
Additionally, this research aims to identify specific pharmaceutical testing scenarios where Raman spectroscopy may offer significant advantages over HPLC, as well as contexts where HPLC remains the superior choice. The potential for complementary implementation of both technologies within an integrated testing strategy will also be explored, recognizing that the optimal approach may involve strategic deployment of multiple analytical methodologies rather than wholesale replacement of established techniques.
Understanding the technological capabilities, limitations, and complementary aspects of these testing methodologies is essential for pharmaceutical manufacturers seeking to optimize their quality control processes while maintaining compliance with increasingly demanding regulatory standards.
Market Demand Analysis for Rapid Pharmaceutical Testing Methods
The pharmaceutical testing market is experiencing significant growth driven by increasing regulatory requirements, rising drug development activities, and growing demand for quality assurance in pharmaceutical products. The global pharmaceutical analytical testing outsourcing market was valued at approximately $6.1 billion in 2020 and is projected to reach $11.4 billion by 2028, growing at a CAGR of 8.2% during the forecast period.
Rapid testing methods have become particularly crucial as pharmaceutical companies face pressure to reduce time-to-market while maintaining stringent quality standards. Traditional testing methods like High-Performance Liquid Chromatography (HPLC) have been the gold standard for decades, but they often require extensive sample preparation, consume significant amounts of solvents, and can take hours to complete a single analysis.
Market research indicates that pharmaceutical companies are increasingly seeking faster, more cost-effective, and environmentally friendly testing alternatives. A survey of pharmaceutical quality control laboratories revealed that 78% consider testing turnaround time as a critical factor in their method selection process, while 65% reported concerns about the environmental impact of traditional testing methods.
Raman spectroscopy has emerged as a promising alternative, offering non-destructive, rapid analysis with minimal sample preparation. The market for Raman spectroscopy in pharmaceutical applications was valued at $258 million in 2021 and is expected to grow at a CAGR of 7.8% through 2027, reflecting the increasing adoption of this technology.
Key market drivers for rapid pharmaceutical testing methods include the growing biologics sector, which requires more sophisticated analytical techniques, and the rise of continuous manufacturing processes that demand real-time quality control. Additionally, regulatory bodies like the FDA and EMA are increasingly accepting alternative analytical methods that demonstrate equivalent or superior performance to traditional approaches.
Regional analysis shows North America leading the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the fastest growth due to increasing pharmaceutical manufacturing activities and regulatory harmonization efforts.
Customer needs assessment reveals that pharmaceutical companies prioritize methods that can be integrated into existing workflows, provide comprehensive chemical information, and offer potential for at-line or in-line process monitoring. Cost per analysis remains a significant consideration, with 72% of survey respondents indicating willingness to invest in new technologies if they demonstrate clear return on investment through reduced testing time and operational costs.
Rapid testing methods have become particularly crucial as pharmaceutical companies face pressure to reduce time-to-market while maintaining stringent quality standards. Traditional testing methods like High-Performance Liquid Chromatography (HPLC) have been the gold standard for decades, but they often require extensive sample preparation, consume significant amounts of solvents, and can take hours to complete a single analysis.
Market research indicates that pharmaceutical companies are increasingly seeking faster, more cost-effective, and environmentally friendly testing alternatives. A survey of pharmaceutical quality control laboratories revealed that 78% consider testing turnaround time as a critical factor in their method selection process, while 65% reported concerns about the environmental impact of traditional testing methods.
Raman spectroscopy has emerged as a promising alternative, offering non-destructive, rapid analysis with minimal sample preparation. The market for Raman spectroscopy in pharmaceutical applications was valued at $258 million in 2021 and is expected to grow at a CAGR of 7.8% through 2027, reflecting the increasing adoption of this technology.
Key market drivers for rapid pharmaceutical testing methods include the growing biologics sector, which requires more sophisticated analytical techniques, and the rise of continuous manufacturing processes that demand real-time quality control. Additionally, regulatory bodies like the FDA and EMA are increasingly accepting alternative analytical methods that demonstrate equivalent or superior performance to traditional approaches.
Regional analysis shows North America leading the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the fastest growth due to increasing pharmaceutical manufacturing activities and regulatory harmonization efforts.
Customer needs assessment reveals that pharmaceutical companies prioritize methods that can be integrated into existing workflows, provide comprehensive chemical information, and offer potential for at-line or in-line process monitoring. Cost per analysis remains a significant consideration, with 72% of survey respondents indicating willingness to invest in new technologies if they demonstrate clear return on investment through reduced testing time and operational costs.
Current State and Challenges in Analytical Testing Technologies
The analytical testing landscape in pharmaceuticals is currently dominated by two major technologies: High-Performance Liquid Chromatography (HPLC) and Raman Spectroscopy. HPLC has long been established as the gold standard for pharmaceutical analysis, offering high sensitivity, specificity, and quantitative accuracy. However, it requires extensive sample preparation, consumes significant amounts of solvents, and involves time-consuming procedures that limit throughput in quality control environments.
Raman Spectroscopy has emerged as a promising alternative, providing rapid, non-destructive analysis with minimal sample preparation. The technology has advanced significantly in recent years with the development of portable and handheld devices, enabling point-of-use testing capabilities that were previously impossible. Despite these advantages, Raman still faces challenges in sensitivity compared to HPLC, particularly for low concentration analytes.
A critical challenge in the current analytical testing landscape is the regulatory framework. While HPLC methods are well-established in pharmacopeias worldwide, Raman methods are still gaining regulatory acceptance. This creates a significant barrier for pharmaceutical companies considering adoption of Raman technology, as method validation and regulatory approval processes can be lengthy and resource-intensive.
Data management presents another substantial challenge. HPLC generates chromatographic data that analysts have decades of experience interpreting, while Raman produces complex spectral data requiring different expertise and interpretation approaches. The industry faces a knowledge gap in transitioning between these different data paradigms.
Geographically, analytical testing technology development shows distinct patterns. North America and Europe lead in HPLC innovation, with established companies continuously improving column technology and detection methods. Meanwhile, Asia-Pacific regions, particularly Japan and China, are making significant advances in Raman instrumentation, developing more sensitive and cost-effective devices.
Cost considerations remain a major factor influencing technology adoption. HPLC systems require ongoing expenses for columns, solvents, and maintenance, while Raman systems typically involve higher initial capital investment but lower operating costs. This economic dynamic affects how different markets and companies approach technology selection.
The integration of artificial intelligence and machine learning represents both an opportunity and challenge. These technologies can potentially enhance spectral interpretation for Raman and chromatographic data analysis for HPLC, but require specialized expertise that is currently in short supply within the pharmaceutical industry.
Raman Spectroscopy has emerged as a promising alternative, providing rapid, non-destructive analysis with minimal sample preparation. The technology has advanced significantly in recent years with the development of portable and handheld devices, enabling point-of-use testing capabilities that were previously impossible. Despite these advantages, Raman still faces challenges in sensitivity compared to HPLC, particularly for low concentration analytes.
A critical challenge in the current analytical testing landscape is the regulatory framework. While HPLC methods are well-established in pharmacopeias worldwide, Raman methods are still gaining regulatory acceptance. This creates a significant barrier for pharmaceutical companies considering adoption of Raman technology, as method validation and regulatory approval processes can be lengthy and resource-intensive.
Data management presents another substantial challenge. HPLC generates chromatographic data that analysts have decades of experience interpreting, while Raman produces complex spectral data requiring different expertise and interpretation approaches. The industry faces a knowledge gap in transitioning between these different data paradigms.
Geographically, analytical testing technology development shows distinct patterns. North America and Europe lead in HPLC innovation, with established companies continuously improving column technology and detection methods. Meanwhile, Asia-Pacific regions, particularly Japan and China, are making significant advances in Raman instrumentation, developing more sensitive and cost-effective devices.
Cost considerations remain a major factor influencing technology adoption. HPLC systems require ongoing expenses for columns, solvents, and maintenance, while Raman systems typically involve higher initial capital investment but lower operating costs. This economic dynamic affects how different markets and companies approach technology selection.
The integration of artificial intelligence and machine learning represents both an opportunity and challenge. These technologies can potentially enhance spectral interpretation for Raman and chromatographic data analysis for HPLC, but require specialized expertise that is currently in short supply within the pharmaceutical industry.
Comparative Analysis of Raman and HPLC Methodologies
01 Integration of Raman spectroscopy with HPLC for enhanced analysis
Combining Raman spectroscopy with High-Performance Liquid Chromatography (HPLC) creates powerful analytical systems that offer complementary data for compound identification and quantification. This integration allows for real-time monitoring of HPLC separations, providing both structural and chemical information simultaneously. The combined approach enhances detection sensitivity, specificity, and enables more comprehensive characterization of complex mixtures compared to either technique alone.- Integration of Raman spectroscopy with HPLC for enhanced analysis: Combining Raman spectroscopy with HPLC techniques creates powerful analytical systems that offer complementary data for complex sample analysis. This integration allows for simultaneous structural and compositional information, improving identification and quantification of compounds in mixtures. The combined approach enhances detection sensitivity, specificity, and provides real-time monitoring capabilities during chromatographic separation.
- Advanced detection methods for improving HPLC efficiency: Novel detection methods incorporating Raman spectroscopy significantly improve HPLC efficiency by enabling label-free detection with high specificity. These methods utilize surface-enhanced Raman scattering (SERS) and other enhancement techniques to overcome sensitivity limitations. The advanced detection approaches allow for lower detection limits, faster analysis times, and improved discrimination between similar compounds in complex matrices.
- Miniaturization and automation of Raman-HPLC systems: Miniaturized and automated Raman-HPLC systems represent significant advancements in analytical technology. These compact systems integrate microfluidic components with specialized Raman detection cells, enabling high-throughput analysis with reduced sample volumes and solvent consumption. Automation of these systems improves reproducibility, reduces human error, and allows for continuous monitoring applications in various fields including pharmaceuticals and environmental analysis.
- Novel sample preparation techniques for Raman-HPLC analysis: Innovative sample preparation methods specifically designed for Raman-HPLC analysis enhance overall analytical efficiency. These techniques include specialized extraction procedures, pre-concentration methods, and sample clean-up protocols that improve signal quality and reduce interference. Advanced sample handling approaches optimize the compatibility between chromatographic separation and subsequent Raman spectroscopic detection, resulting in more reliable and sensitive analyses.
- Data processing algorithms for Raman-HPLC data interpretation: Sophisticated data processing algorithms specifically developed for Raman-HPLC data interpretation significantly improve analytical outcomes. These computational methods include chemometric approaches, machine learning algorithms, and multivariate statistical analyses that extract meaningful information from complex spectral and chromatographic datasets. Advanced software solutions enable automated peak identification, quantification, and visualization of results, facilitating faster and more accurate interpretation of analytical data.
02 Advanced detection methods for improving HPLC efficiency using Raman technology
Novel Raman-based detection methods significantly improve HPLC efficiency by enabling label-free detection with high specificity. These advanced detection systems incorporate specialized optics, enhanced signal processing algorithms, and optimized flow cell designs to maximize sensitivity while minimizing interference. Surface-enhanced Raman spectroscopy (SERS) techniques further amplify signals, allowing for detection of trace compounds that traditional HPLC detectors might miss, thereby increasing overall analytical efficiency.Expand Specific Solutions03 Miniaturization and automation of Raman-HPLC systems
Miniaturized and automated Raman-HPLC systems represent significant advancements in analytical technology. These compact systems integrate microfluidic components with specialized Raman probes to create portable, high-throughput analytical platforms. Automation features include computerized sample handling, data acquisition, and analysis algorithms that reduce operator intervention and increase reproducibility. These innovations make sophisticated analytical capabilities more accessible for field applications and routine laboratory use.Expand Specific Solutions04 Novel flow cell designs for Raman-enhanced HPLC
Specialized flow cell designs optimize the integration of Raman spectroscopy with HPLC systems. These innovative cells feature optimized optical geometries, materials with low background signals, and enhanced path lengths to maximize analyte detection. Some designs incorporate nanostructured surfaces to leverage surface-enhanced Raman effects, while others implement multiple detection windows for simultaneous measurements. These advancements significantly improve signal-to-noise ratios and detection limits in chromatographic separations.Expand Specific Solutions05 Data fusion and analysis methods for Raman-HPLC systems
Advanced data fusion and analysis methods are crucial for extracting maximum information from combined Raman-HPLC systems. These approaches include multivariate statistical techniques, machine learning algorithms, and chemometric methods that correlate spectroscopic and chromatographic data. Real-time data processing enables automated peak identification, quantification, and structural elucidation. These computational methods enhance the ability to analyze complex mixtures, identify unknown compounds, and perform quality control in pharmaceutical, environmental, and biomedical applications.Expand Specific Solutions
Key Industry Players in Analytical Instrumentation
Raman Spectroscopy versus HPLC in pharmaceutical testing presents a competitive landscape at a mature yet evolving stage. The market is experiencing steady growth, valued at approximately $1.5 billion with a projected CAGR of 7-8% through 2027. While HPLC remains the established gold standard with broader adoption, Raman spectroscopy is gaining traction due to its non-destructive, rapid analysis capabilities. Key players shaping this technological competition include Shimadzu Corp. and Horiba Ltd. offering comprehensive HPLC solutions, while Renishaw Plc, Thermo Scientific, and Koninklijke Philips NV lead Raman spectroscopy innovation. Pharmaceutical companies increasingly adopt hybrid approaches, leveraging complementary strengths of both technologies for comprehensive quality control and regulatory compliance.
Koninklijke Philips NV
Technical Solution: Philips has developed an integrated pharmaceutical testing platform that leverages advanced Raman spectroscopy technology to address efficiency challenges in quality control processes. Their system incorporates proprietary optical designs that enhance signal collection efficiency, enabling faster analysis times compared to traditional HPLC methods. Philips' approach focuses on in-line process analytical technology (PAT) applications, where their Raman systems continuously monitor pharmaceutical manufacturing processes in real-time without sampling or preparation steps required by HPLC. Their technology employs specialized algorithms that can compensate for fluorescence interference, a common challenge when analyzing pharmaceutical compounds with Raman spectroscopy. Philips' pharmaceutical testing solutions include dedicated software packages with pre-built models for common pharmaceutical applications such as blend uniformity assessment, polymorph identification, and content uniformity testing. Their systems feature automated calibration and verification protocols that ensure consistent performance while minimizing operator intervention requirements compared to HPLC systems that require regular mobile phase preparation and column maintenance.
Strengths: Real-time continuous monitoring capabilities not possible with HPLC; non-destructive testing allowing sample preservation; minimal consumables costs compared to HPLC's ongoing solvent and column expenses; ability to analyze through containment systems for sterile applications. Weaknesses: Less established in regulated pharmaceutical environments compared to HPLC; more challenging quantitative analysis for complex mixtures; potential interference from environmental factors in manufacturing settings; requires specialized expertise for method development.
Renishaw Plc
Technical Solution: Renishaw has developed advanced Raman spectroscopy systems specifically designed for pharmaceutical testing applications. Their inVia™ Qontor® Raman microscope incorporates LiveTrack™ technology that maintains focus in real-time during sample analysis, enabling more reliable results when examining uneven or complex pharmaceutical samples. The system offers rapid chemical identification capabilities with spatial resolution down to sub-micron level, allowing for detailed analysis of drug formulations, polymorphs, and contaminants. Renishaw's pharmaceutical testing solutions integrate automated measurement sequences and multivariate analysis software that can process complex spectral data sets, providing comprehensive chemical fingerprinting without sample preparation or destruction. Their systems can detect and quantify active pharmaceutical ingredients (APIs) at concentrations as low as 0.1% in formulations, significantly faster than traditional HPLC methods which require extensive sample preparation and longer analysis times.
Strengths: Non-destructive testing allowing sample preservation; minimal to no sample preparation required; rapid analysis times (seconds to minutes versus HPLC's 10-30 minutes); ability to analyze through packaging materials; excellent for polymorph identification. Weaknesses: Lower sensitivity than HPLC for trace analysis; potential fluorescence interference from some pharmaceutical compounds; higher initial equipment investment; requires more specialized operator training.
Technical Innovations in Spectroscopic and Chromatographic Methods
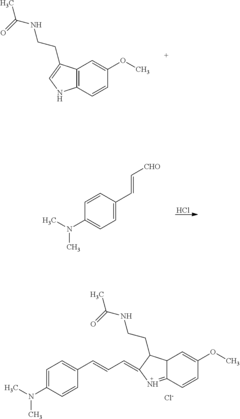
Method for rapidly measuring melatonin adulteration of chinese patent medicine or healthcare food
PatentActiveUS20140220698A1
Innovation
- A method involving the extraction of melatonin with ethyl acetate and the use of p-dimethylaminocinnamaldehyde as a color developing agent, which results in a blue-green color change when melatonin is present, allowing for rapid and accurate on-site detection without the need for expensive equipment.
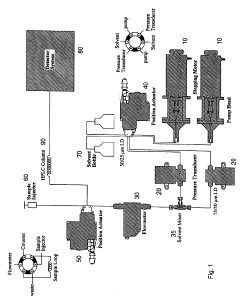
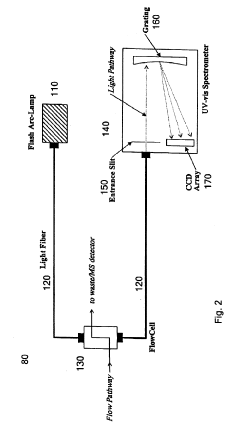
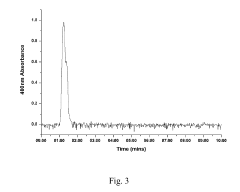
High performance liquid chromatography with UV-visible detection
PatentActiveUS20190265212A1
Innovation
- A high-performance liquid chromatography apparatus featuring a detector device with a flash lamp, etched silicon grating, and charge-coupled device array for simultaneous wavelength detection, along with linear stepping pumps for efficient pumping, reducing the overall mass and size of the system.
Regulatory Compliance and Validation Requirements
Regulatory compliance represents a critical consideration when evaluating analytical technologies for pharmaceutical testing. Both Raman spectroscopy and High-Performance Liquid Chromatography (HPLC) must adhere to stringent regulatory frameworks established by authorities such as the FDA, EMA, and ICH. However, their validation requirements and compliance pathways differ significantly, influencing implementation decisions across the pharmaceutical industry.
For HPLC, well-established regulatory guidelines exist through USP <621> and ICH Q2(R1), providing clear validation protocols for specificity, linearity, accuracy, precision, range, and robustness. The technology benefits from decades of regulatory precedent, making compliance pathways predictable and well-documented. Most regulatory bodies recognize HPLC as a gold standard method, facilitating smoother approval processes for pharmaceutical products utilizing this analytical approach.
Raman spectroscopy, while gaining acceptance, faces more complex validation challenges. The technology must satisfy requirements outlined in USP <1120> for near-infrared spectroscopy, which can be applied to Raman methods by extension. PAT (Process Analytical Technology) initiatives by regulatory agencies have increasingly supported spectroscopic methods, but validation protocols remain less standardized compared to chromatographic techniques.
Method transfer considerations also differ substantially between these technologies. HPLC method transfer between laboratories requires extensive system suitability testing and comparative analysis to ensure equivalent performance across different instruments and locations. Conversely, Raman spectroscopy demands robust chemometric model transfer protocols and frequent recalibration when implementing across multiple manufacturing sites.
Documentation requirements present another significant distinction. HPLC methods typically generate chromatograms and quantitative reports that directly correlate to analyte concentrations. Raman spectroscopy produces complex spectral data requiring sophisticated chemometric models for interpretation, necessitating additional validation of the mathematical algorithms and data processing techniques employed.
For continuous manufacturing environments, Raman spectroscopy offers advantages through PAT frameworks, with the FDA's guidance on Process Validation supporting real-time release testing. However, implementing Raman as a release method requires comprehensive method validation and often comparative studies against established HPLC methods to demonstrate equivalence or superiority.
The cost of regulatory compliance differs markedly between these technologies. While HPLC validation follows well-trodden paths with predictable timelines and resource requirements, Raman methods often require more extensive initial validation efforts, though they may offer streamlined compliance monitoring once implemented.
For HPLC, well-established regulatory guidelines exist through USP <621> and ICH Q2(R1), providing clear validation protocols for specificity, linearity, accuracy, precision, range, and robustness. The technology benefits from decades of regulatory precedent, making compliance pathways predictable and well-documented. Most regulatory bodies recognize HPLC as a gold standard method, facilitating smoother approval processes for pharmaceutical products utilizing this analytical approach.
Raman spectroscopy, while gaining acceptance, faces more complex validation challenges. The technology must satisfy requirements outlined in USP <1120> for near-infrared spectroscopy, which can be applied to Raman methods by extension. PAT (Process Analytical Technology) initiatives by regulatory agencies have increasingly supported spectroscopic methods, but validation protocols remain less standardized compared to chromatographic techniques.
Method transfer considerations also differ substantially between these technologies. HPLC method transfer between laboratories requires extensive system suitability testing and comparative analysis to ensure equivalent performance across different instruments and locations. Conversely, Raman spectroscopy demands robust chemometric model transfer protocols and frequent recalibration when implementing across multiple manufacturing sites.
Documentation requirements present another significant distinction. HPLC methods typically generate chromatograms and quantitative reports that directly correlate to analyte concentrations. Raman spectroscopy produces complex spectral data requiring sophisticated chemometric models for interpretation, necessitating additional validation of the mathematical algorithms and data processing techniques employed.
For continuous manufacturing environments, Raman spectroscopy offers advantages through PAT frameworks, with the FDA's guidance on Process Validation supporting real-time release testing. However, implementing Raman as a release method requires comprehensive method validation and often comparative studies against established HPLC methods to demonstrate equivalence or superiority.
The cost of regulatory compliance differs markedly between these technologies. While HPLC validation follows well-trodden paths with predictable timelines and resource requirements, Raman methods often require more extensive initial validation efforts, though they may offer streamlined compliance monitoring once implemented.
Cost-Benefit Analysis of Implementation Strategies
Implementing either Raman spectroscopy or HPLC in pharmaceutical testing requires careful financial consideration beyond the initial equipment investment. The capital expenditure for a high-quality Raman spectrometer typically ranges from $30,000 to $100,000, while HPLC systems generally cost between $25,000 and $80,000. However, these initial costs represent only a fraction of the total implementation expense.
Operational costs reveal significant differences between the technologies. HPLC requires ongoing purchases of mobile phases, columns, and reference standards, averaging $15-25 per sample. Additionally, HPLC systems demand regular maintenance, with annual service contracts costing approximately 10-15% of the initial investment. Conversely, Raman spectroscopy offers substantially lower per-sample costs, typically under $5, with minimal consumables required.
Training requirements also impact the cost-benefit equation. HPLC operators generally need 2-4 weeks of specialized training, while Raman spectroscopy operators can achieve proficiency in 1-2 weeks. This difference translates to reduced labor costs and faster implementation timelines for Raman technology.
Time efficiency calculations demonstrate that Raman analysis typically completes in 1-5 minutes per sample without preparation time, while HPLC requires 10-60 minutes plus sample preparation. For facilities processing high sample volumes, this efficiency difference can yield substantial labor savings, estimated at $50,000-100,000 annually for facilities analyzing 50+ samples daily.
Return on investment (ROI) timelines differ significantly between the technologies. HPLC systems typically achieve ROI in 3-5 years, while Raman systems may reach ROI in 1.5-3 years, depending on sample volume. Pharmaceutical companies processing over 100 samples daily may see ROI from Raman technology in under 18 months.
Scalability considerations favor Raman spectroscopy for growing operations. Adding capacity with HPLC requires proportional increases in equipment, space, and personnel. Conversely, Raman systems can often handle increased throughput with minimal additional investment, particularly when implementing automated sampling systems.
Risk mitigation strategies should include phased implementation approaches. Many facilities benefit from maintaining both technologies, using Raman for rapid screening and HPLC for confirmation and regulatory documentation, maximizing efficiency while ensuring compliance with established pharmaceutical testing protocols.
Operational costs reveal significant differences between the technologies. HPLC requires ongoing purchases of mobile phases, columns, and reference standards, averaging $15-25 per sample. Additionally, HPLC systems demand regular maintenance, with annual service contracts costing approximately 10-15% of the initial investment. Conversely, Raman spectroscopy offers substantially lower per-sample costs, typically under $5, with minimal consumables required.
Training requirements also impact the cost-benefit equation. HPLC operators generally need 2-4 weeks of specialized training, while Raman spectroscopy operators can achieve proficiency in 1-2 weeks. This difference translates to reduced labor costs and faster implementation timelines for Raman technology.
Time efficiency calculations demonstrate that Raman analysis typically completes in 1-5 minutes per sample without preparation time, while HPLC requires 10-60 minutes plus sample preparation. For facilities processing high sample volumes, this efficiency difference can yield substantial labor savings, estimated at $50,000-100,000 annually for facilities analyzing 50+ samples daily.
Return on investment (ROI) timelines differ significantly between the technologies. HPLC systems typically achieve ROI in 3-5 years, while Raman systems may reach ROI in 1.5-3 years, depending on sample volume. Pharmaceutical companies processing over 100 samples daily may see ROI from Raman technology in under 18 months.
Scalability considerations favor Raman spectroscopy for growing operations. Adding capacity with HPLC requires proportional increases in equipment, space, and personnel. Conversely, Raman systems can often handle increased throughput with minimal additional investment, particularly when implementing automated sampling systems.
Risk mitigation strategies should include phased implementation approaches. Many facilities benefit from maintaining both technologies, using Raman for rapid screening and HPLC for confirmation and regulatory documentation, maximizing efficiency while ensuring compliance with established pharmaceutical testing protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!