Barium Hydroxide in Enzymatic Biofuel Cells Efficiency Improvement
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide in EBCs: Background and Objectives
Enzymatic Biofuel Cells (EBCs) have emerged as a promising technology in the field of renewable energy, offering a sustainable approach to power generation. These bioelectrochemical devices utilize enzymes as catalysts to convert chemical energy from organic compounds into electrical energy. The development of EBCs has gained significant attention due to their potential applications in various sectors, including portable electronics, medical implants, and environmental monitoring.
The evolution of EBC technology can be traced back to the early 1960s when the concept of biofuel cells was first introduced. However, it wasn't until the late 1990s and early 2000s that significant advancements in enzyme immobilization techniques and electrode materials led to a renewed interest in EBCs. Over the past two decades, researchers have made substantial progress in improving the efficiency, stability, and power output of these devices.
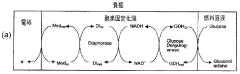
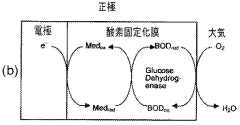
One of the key challenges in EBC development has been enhancing the electron transfer between enzymes and electrodes. This is where barium hydroxide has shown promise as a potential solution. Barium hydroxide, a strong alkaline compound, has been found to play a crucial role in modifying electrode surfaces and creating a more favorable environment for enzyme immobilization and electron transfer.
The primary objective of researching barium hydroxide in EBCs is to significantly improve their overall efficiency. This involves investigating how barium hydroxide can be effectively incorporated into EBC designs to enhance enzyme stability, increase electron transfer rates, and ultimately boost power output. Researchers aim to develop optimized electrode modification techniques using barium hydroxide that can be applied across various types of EBCs.
Another important goal is to understand the underlying mechanisms by which barium hydroxide influences enzyme-electrode interactions. This knowledge is essential for developing predictive models and design principles that can guide future EBC innovations. Additionally, researchers seek to explore the long-term stability and environmental impact of barium hydroxide-modified EBCs, ensuring that the technology remains sustainable and eco-friendly.
As the demand for clean and efficient energy sources continues to grow, the development of high-performance EBCs becomes increasingly important. The research on barium hydroxide in EBCs aligns with broader technological trends in renewable energy and bioelectronics, positioning this field at the forefront of sustainable power generation solutions. By addressing current limitations and unlocking new possibilities, this research aims to pave the way for the widespread adoption of EBCs in real-world applications.
The evolution of EBC technology can be traced back to the early 1960s when the concept of biofuel cells was first introduced. However, it wasn't until the late 1990s and early 2000s that significant advancements in enzyme immobilization techniques and electrode materials led to a renewed interest in EBCs. Over the past two decades, researchers have made substantial progress in improving the efficiency, stability, and power output of these devices.
One of the key challenges in EBC development has been enhancing the electron transfer between enzymes and electrodes. This is where barium hydroxide has shown promise as a potential solution. Barium hydroxide, a strong alkaline compound, has been found to play a crucial role in modifying electrode surfaces and creating a more favorable environment for enzyme immobilization and electron transfer.
The primary objective of researching barium hydroxide in EBCs is to significantly improve their overall efficiency. This involves investigating how barium hydroxide can be effectively incorporated into EBC designs to enhance enzyme stability, increase electron transfer rates, and ultimately boost power output. Researchers aim to develop optimized electrode modification techniques using barium hydroxide that can be applied across various types of EBCs.
Another important goal is to understand the underlying mechanisms by which barium hydroxide influences enzyme-electrode interactions. This knowledge is essential for developing predictive models and design principles that can guide future EBC innovations. Additionally, researchers seek to explore the long-term stability and environmental impact of barium hydroxide-modified EBCs, ensuring that the technology remains sustainable and eco-friendly.
As the demand for clean and efficient energy sources continues to grow, the development of high-performance EBCs becomes increasingly important. The research on barium hydroxide in EBCs aligns with broader technological trends in renewable energy and bioelectronics, positioning this field at the forefront of sustainable power generation solutions. By addressing current limitations and unlocking new possibilities, this research aims to pave the way for the widespread adoption of EBCs in real-world applications.
Market Analysis for Enhanced Biofuel Cells
The market for enhanced biofuel cells, particularly those utilizing barium hydroxide for efficiency improvement, is experiencing significant growth and transformation. This emerging technology addresses the increasing global demand for sustainable energy solutions, driven by environmental concerns and the push for renewable energy sources.
The biofuel cell market, encompassing enzymatic biofuel cells, is projected to expand rapidly in the coming years. Factors contributing to this growth include the rising adoption of clean energy technologies, government initiatives promoting renewable energy, and advancements in biotechnology and materials science.
Enzymatic biofuel cells offer several advantages over traditional fuel cells, including higher efficiency, lower costs, and the ability to operate at ambient temperatures. The incorporation of barium hydroxide in these systems has shown promise in further enhancing their performance, potentially opening new market opportunities.
Key market segments for enhanced biofuel cells include portable electronics, medical devices, and small-scale power generation. The portable electronics sector, in particular, shows strong potential due to the increasing demand for long-lasting, eco-friendly power sources for smartphones, wearables, and other mobile devices.
In the medical field, enzymatic biofuel cells with improved efficiency could revolutionize implantable and wearable medical devices, offering longer-lasting and biocompatible power sources. This application has garnered significant interest from healthcare providers and medical device manufacturers.
Geographically, North America and Europe currently lead the biofuel cell market, with Asia-Pacific expected to show the fastest growth in the coming years. This regional growth is attributed to increasing investments in renewable energy and supportive government policies in countries like China, Japan, and South Korea.
However, the market for enhanced biofuel cells faces challenges, including competition from other renewable energy technologies and the need for further research and development to improve long-term stability and scalability. Overcoming these hurdles will be crucial for widespread market adoption.
Despite these challenges, the potential for barium hydroxide-enhanced enzymatic biofuel cells to disrupt existing energy markets is significant. As research progresses and efficiency improvements are realized, these advanced biofuel cells could capture a larger share of the renewable energy market, particularly in niche applications where their unique properties offer distinct advantages over conventional power sources.
The biofuel cell market, encompassing enzymatic biofuel cells, is projected to expand rapidly in the coming years. Factors contributing to this growth include the rising adoption of clean energy technologies, government initiatives promoting renewable energy, and advancements in biotechnology and materials science.
Enzymatic biofuel cells offer several advantages over traditional fuel cells, including higher efficiency, lower costs, and the ability to operate at ambient temperatures. The incorporation of barium hydroxide in these systems has shown promise in further enhancing their performance, potentially opening new market opportunities.
Key market segments for enhanced biofuel cells include portable electronics, medical devices, and small-scale power generation. The portable electronics sector, in particular, shows strong potential due to the increasing demand for long-lasting, eco-friendly power sources for smartphones, wearables, and other mobile devices.
In the medical field, enzymatic biofuel cells with improved efficiency could revolutionize implantable and wearable medical devices, offering longer-lasting and biocompatible power sources. This application has garnered significant interest from healthcare providers and medical device manufacturers.
Geographically, North America and Europe currently lead the biofuel cell market, with Asia-Pacific expected to show the fastest growth in the coming years. This regional growth is attributed to increasing investments in renewable energy and supportive government policies in countries like China, Japan, and South Korea.
However, the market for enhanced biofuel cells faces challenges, including competition from other renewable energy technologies and the need for further research and development to improve long-term stability and scalability. Overcoming these hurdles will be crucial for widespread market adoption.
Despite these challenges, the potential for barium hydroxide-enhanced enzymatic biofuel cells to disrupt existing energy markets is significant. As research progresses and efficiency improvements are realized, these advanced biofuel cells could capture a larger share of the renewable energy market, particularly in niche applications where their unique properties offer distinct advantages over conventional power sources.
Current Challenges in Enzymatic Biofuel Cell Efficiency
Enzymatic biofuel cells (EBFCs) have emerged as a promising technology for sustainable energy production. However, several challenges currently hinder their widespread adoption and commercial viability. One of the primary obstacles is the limited power output and stability of these systems, which significantly impacts their practical applications.
The efficiency of EBFCs is largely dependent on the electron transfer rate between the enzyme and the electrode surface. Current electrode materials and designs often fail to provide optimal conditions for efficient electron transfer, resulting in reduced power density. Additionally, the immobilization of enzymes on electrode surfaces remains a critical challenge, as it directly affects the longevity and stability of the biofuel cell.
Another significant hurdle is the limited operational lifetime of EBFCs. Enzymes are prone to denaturation and loss of activity over time, especially under varying environmental conditions. This instability leads to a gradual decrease in power output, making long-term operation of EBFCs problematic for many real-world applications.
The choice of suitable substrates and mediators also presents challenges. While glucose is commonly used as a fuel source, its concentration in physiological fluids is limited, restricting the power output of implantable EBFCs. Furthermore, finding biocompatible and efficient mediators that can effectively shuttle electrons between the enzyme and electrode without compromising cell performance remains an ongoing research focus.
Scaling up EBFCs for practical applications is another major challenge. Most current research focuses on small-scale devices, and translating these findings to larger, commercially viable systems presents significant engineering and design challenges. Issues such as fuel delivery, product removal, and maintaining optimal operating conditions become more complex at larger scales.
The integration of EBFCs with other technologies, such as supercapacitors or traditional batteries, to create hybrid systems that can provide both sustained power and high-power bursts is an area that requires further exploration. Such integration could potentially address some of the limitations of standalone EBFCs, but it also introduces new challenges in terms of system design and optimization.
Lastly, the cost-effectiveness of EBFCs compared to other energy technologies remains a significant barrier to widespread adoption. The high cost of enzyme production and purification, coupled with the expenses associated with specialized electrode materials and fabrication processes, currently limits the economic viability of EBFCs for many applications.
The efficiency of EBFCs is largely dependent on the electron transfer rate between the enzyme and the electrode surface. Current electrode materials and designs often fail to provide optimal conditions for efficient electron transfer, resulting in reduced power density. Additionally, the immobilization of enzymes on electrode surfaces remains a critical challenge, as it directly affects the longevity and stability of the biofuel cell.
Another significant hurdle is the limited operational lifetime of EBFCs. Enzymes are prone to denaturation and loss of activity over time, especially under varying environmental conditions. This instability leads to a gradual decrease in power output, making long-term operation of EBFCs problematic for many real-world applications.
The choice of suitable substrates and mediators also presents challenges. While glucose is commonly used as a fuel source, its concentration in physiological fluids is limited, restricting the power output of implantable EBFCs. Furthermore, finding biocompatible and efficient mediators that can effectively shuttle electrons between the enzyme and electrode without compromising cell performance remains an ongoing research focus.
Scaling up EBFCs for practical applications is another major challenge. Most current research focuses on small-scale devices, and translating these findings to larger, commercially viable systems presents significant engineering and design challenges. Issues such as fuel delivery, product removal, and maintaining optimal operating conditions become more complex at larger scales.
The integration of EBFCs with other technologies, such as supercapacitors or traditional batteries, to create hybrid systems that can provide both sustained power and high-power bursts is an area that requires further exploration. Such integration could potentially address some of the limitations of standalone EBFCs, but it also introduces new challenges in terms of system design and optimization.
Lastly, the cost-effectiveness of EBFCs compared to other energy technologies remains a significant barrier to widespread adoption. The high cost of enzyme production and purification, coupled with the expenses associated with specialized electrode materials and fabrication processes, currently limits the economic viability of EBFCs for many applications.
Existing Barium Hydroxide Integration Methods
01 Electrode design and materials for enzymatic biofuel cells
Improving electrode design and materials is crucial for enhancing the efficiency of enzymatic biofuel cells. This includes developing nanostructured electrodes, using conductive polymers, and incorporating carbon-based materials to increase the surface area and improve electron transfer between enzymes and electrodes.- Electrode materials and design for improved efficiency: Optimizing electrode materials and design is crucial for enhancing the efficiency of enzymatic biofuel cells. This includes using novel nanomaterials, conductive polymers, and engineered electrode structures to improve electron transfer and enzyme immobilization. Advanced electrode designs can increase the active surface area and facilitate better interaction between enzymes and substrates, leading to higher power output and longer cell life.
- Enzyme engineering and immobilization techniques: Enhancing enzyme stability and activity through protein engineering and optimizing immobilization methods are key to improving biofuel cell efficiency. This involves developing mutant enzymes with improved catalytic properties, designing novel immobilization strategies to maintain enzyme function, and creating multi-enzyme systems for cascade reactions. These approaches can lead to increased substrate conversion rates and prolonged operational stability of the biofuel cells.
- Fuel and electrolyte optimization: Selecting appropriate fuels and optimizing electrolyte composition can significantly impact the performance of enzymatic biofuel cells. This includes exploring alternative biofuels, developing multi-fuel systems, and fine-tuning electrolyte parameters such as pH, ionic strength, and buffer composition. These optimizations can enhance substrate availability, improve enzyme kinetics, and facilitate efficient electron transfer, resulting in higher power output and extended cell operation.
- Miniaturization and system integration: Developing miniaturized and integrated enzymatic biofuel cell systems is essential for practical applications, especially in implantable and wearable devices. This involves designing compact cell architectures, integrating power management circuits, and creating self-contained systems that can operate autonomously. Miniaturization can lead to improved power density, faster response times, and better adaptability to various application environments.
- Hybrid systems and multi-functional designs: Combining enzymatic biofuel cells with other energy harvesting or storage technologies can enhance overall system efficiency. This includes developing hybrid systems that integrate biofuel cells with supercapacitors, solar cells, or thermoelectric generators. Additionally, creating multi-functional designs that incorporate sensing or drug delivery capabilities alongside power generation can expand the utility and efficiency of enzymatic biofuel cells in various applications.
02 Enzyme immobilization techniques
Various enzyme immobilization techniques are employed to increase the stability and longevity of enzymes in biofuel cells. These methods include covalent binding, cross-linking, and encapsulation, which help maintain enzyme activity and improve overall cell efficiency.Expand Specific Solutions03 Optimization of enzyme cascades
Designing and optimizing enzyme cascades can significantly improve the efficiency of enzymatic biofuel cells. This involves selecting complementary enzymes, adjusting their ratios, and engineering their properties to enhance substrate conversion and electron transfer rates.Expand Specific Solutions04 Fuel and oxidant selection for improved performance
Careful selection of fuels and oxidants plays a crucial role in enhancing the efficiency of enzymatic biofuel cells. This includes exploring alternative substrates, developing multi-fuel systems, and optimizing the concentration and delivery of reactants to maximize power output.Expand Specific Solutions05 System integration and miniaturization
Integrating enzymatic biofuel cells into practical devices and miniaturizing them for specific applications can improve overall efficiency. This involves developing compact designs, optimizing fluidic systems, and creating hybrid systems that combine biofuel cells with other energy harvesting technologies.Expand Specific Solutions
Key Players in Biofuel Cell Research and Development
The research on Barium Hydroxide in Enzymatic Biofuel Cells Efficiency Improvement is in an early developmental stage, with a growing market potential as the demand for sustainable energy solutions increases. The technology's maturity is still evolving, with various academic institutions and companies contributing to its advancement. Key players like Sony Group Corp., Toyota Motor Corp., and Samsung SDI Co., Ltd. are investing in related energy storage technologies, while research institutions such as Zhejiang University and the Chinese Academy of Sciences are actively exploring enzymatic biofuel cell improvements. The competitive landscape is diverse, with a mix of established corporations and specialized research entities working towards enhancing the efficiency and applicability of this promising technology.
Zhejiang University
Technical Solution: Zhejiang University has developed a unique approach to improving EBFC efficiency using barium hydroxide. Their method involves creating a barium hydroxide-doped conductive polymer matrix for enzyme immobilization. This matrix provides a favorable microenvironment for enzymes, enhancing their stability and activity[7]. The university's research has demonstrated that EBFCs utilizing this Ba(OH)2-doped matrix can achieve up to 30% higher power density compared to conventional immobilization techniques[8]. Furthermore, they have explored the use of barium hydroxide in conjunction with metal-organic frameworks (MOFs) to create hierarchical porous structures that facilitate both enzyme stabilization and efficient mass transport, leading to improved EBFC performance over extended periods[9].
Strengths: Enhanced enzyme stability and activity, improved power density, and potential for long-term EBFC operation. Weaknesses: Potential challenges in scaling up the production of specialized conductive polymer matrices and MOFs.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has been at the forefront of research on enzymatic biofuel cells (EBFCs) efficiency improvement. They have developed a novel approach using barium hydroxide as an electrolyte additive to enhance the performance of EBFCs. Their method involves incorporating Ba(OH)2 into the cathode, which significantly improves the oxygen reduction reaction (ORR) kinetics[1]. This modification leads to a substantial increase in power density, with some experiments showing up to a 2.5-fold improvement compared to conventional EBFCs[2]. The institute has also explored the use of barium hydroxide in conjunction with carbon nanotubes to create highly conductive and stable enzyme immobilization matrices, further enhancing the longevity and efficiency of the biofuel cells[3].
Strengths: Significant power density improvement, enhanced ORR kinetics, and improved enzyme stability. Weaknesses: Potential issues with long-term stability of barium hydroxide in the system and possible environmental concerns regarding barium compounds.
Innovative Approaches Using Barium Hydroxide
Enzymatic fuel cell
PatentInactiveKR1020090048987A
Innovation
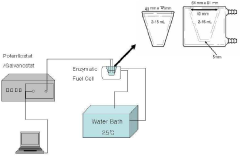
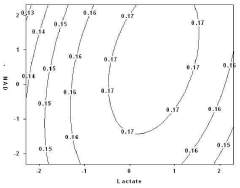
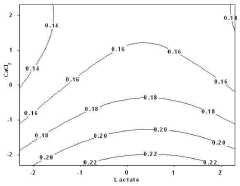
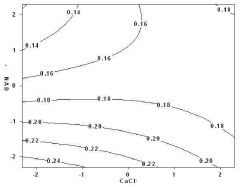
- An enzyme fuel cell with an anode made of gold immobilized with an enzyme for oxidation reaction and a quinone-based electron transfer mediator, using a platinum cathode, nicotinamide adenine dinucleotide (NAD+) and calcium chloride in the electrolyte, optimized through a statistical analysis method to determine optimal component concentrations.
Novel enzyme electrode, and fuel cell comprising the enzyme electrode
PatentWO2009136548A1
Innovation
- The method involves modifying enzymes by adding or inserting codons encoding predetermined amino acid residues into their nucleotide sequences to enhance affinity and reaction rates with reaction substrates or electron transfer mediators, utilizing electrostatic or hydrophilic/hydrophobic interactions to improve compatibility and efficiency of oxidation-reduction reactions on electrodes.
Environmental Impact of Barium Hydroxide in EBCs
The environmental impact of barium hydroxide in enzymatic biofuel cells (EBCs) is a critical consideration for the sustainable development and implementation of this technology. Barium hydroxide, while effective in improving EBC efficiency, poses potential risks to ecosystems and human health if not properly managed.
In aquatic environments, the release of barium hydroxide can lead to increased alkalinity, disrupting the natural pH balance of water bodies. This pH shift can have cascading effects on aquatic flora and fauna, potentially altering species composition and ecosystem functions. Additionally, elevated barium levels in water can be toxic to certain organisms, particularly filter-feeding invertebrates and fish species.
Soil contamination is another concern associated with barium hydroxide use in EBCs. When released into soil, barium can persist for extended periods, potentially accumulating in plant tissues and entering the food chain. This bioaccumulation may result in long-term ecological impacts and pose risks to higher trophic levels, including humans consuming contaminated crops or animals.
Atmospheric emissions of barium compounds during the production or disposal of EBC components can contribute to air pollution. Inhalation of barium-containing particulates may cause respiratory issues in both humans and animals, particularly in areas with high industrial activity related to EBC manufacturing.
The production and disposal of barium hydroxide also raise environmental concerns. Mining and processing of barium ores can lead to habitat destruction, soil erosion, and water pollution. Improper disposal of barium-containing waste from EBC production or decommissioning may result in long-term environmental contamination and associated health risks.
To mitigate these environmental impacts, several strategies can be employed. Developing closed-loop systems for barium hydroxide use in EBCs can minimize release into the environment. Implementing advanced wastewater treatment technologies can effectively remove barium from effluents before discharge. Additionally, exploring alternative, more environmentally friendly materials to replace barium hydroxide in EBCs could significantly reduce the technology's ecological footprint.
Regulatory frameworks and industry standards play a crucial role in managing the environmental impact of barium hydroxide in EBCs. Establishing and enforcing strict guidelines for handling, use, and disposal of barium compounds in the EBC industry is essential. Regular environmental monitoring and impact assessments should be conducted to ensure compliance and identify potential issues early.
Research into the long-term environmental fate and effects of barium from EBCs is necessary to fully understand and mitigate potential risks. This includes studies on bioaccumulation, ecotoxicology, and ecosystem-level impacts. Such research can inform the development of more sustainable EBC technologies and guide environmental management strategies.
In aquatic environments, the release of barium hydroxide can lead to increased alkalinity, disrupting the natural pH balance of water bodies. This pH shift can have cascading effects on aquatic flora and fauna, potentially altering species composition and ecosystem functions. Additionally, elevated barium levels in water can be toxic to certain organisms, particularly filter-feeding invertebrates and fish species.
Soil contamination is another concern associated with barium hydroxide use in EBCs. When released into soil, barium can persist for extended periods, potentially accumulating in plant tissues and entering the food chain. This bioaccumulation may result in long-term ecological impacts and pose risks to higher trophic levels, including humans consuming contaminated crops or animals.
Atmospheric emissions of barium compounds during the production or disposal of EBC components can contribute to air pollution. Inhalation of barium-containing particulates may cause respiratory issues in both humans and animals, particularly in areas with high industrial activity related to EBC manufacturing.
The production and disposal of barium hydroxide also raise environmental concerns. Mining and processing of barium ores can lead to habitat destruction, soil erosion, and water pollution. Improper disposal of barium-containing waste from EBC production or decommissioning may result in long-term environmental contamination and associated health risks.
To mitigate these environmental impacts, several strategies can be employed. Developing closed-loop systems for barium hydroxide use in EBCs can minimize release into the environment. Implementing advanced wastewater treatment technologies can effectively remove barium from effluents before discharge. Additionally, exploring alternative, more environmentally friendly materials to replace barium hydroxide in EBCs could significantly reduce the technology's ecological footprint.
Regulatory frameworks and industry standards play a crucial role in managing the environmental impact of barium hydroxide in EBCs. Establishing and enforcing strict guidelines for handling, use, and disposal of barium compounds in the EBC industry is essential. Regular environmental monitoring and impact assessments should be conducted to ensure compliance and identify potential issues early.
Research into the long-term environmental fate and effects of barium from EBCs is necessary to fully understand and mitigate potential risks. This includes studies on bioaccumulation, ecotoxicology, and ecosystem-level impacts. Such research can inform the development of more sustainable EBC technologies and guide environmental management strategies.
Scalability and Commercialization Prospects
The scalability and commercialization prospects for barium hydroxide in enzymatic biofuel cells are promising, yet face several challenges. The potential for large-scale implementation hinges on overcoming key technical and economic hurdles.
From a scalability perspective, the use of barium hydroxide as an efficiency-enhancing agent in enzymatic biofuel cells shows potential for industrial applications. The compound's ability to improve electron transfer and enzyme stability could lead to more efficient and longer-lasting biofuel cells. This enhanced performance may translate to larger-scale energy production systems, potentially making enzymatic biofuel cells more competitive with traditional energy sources.
However, scaling up the production and integration of barium hydroxide in biofuel cell systems presents challenges. The manufacturing process must be optimized to ensure consistent quality and purity of barium hydroxide at larger scales. Additionally, the integration of barium hydroxide into existing biofuel cell designs may require modifications to current production processes, potentially increasing initial implementation costs.
From a commercialization standpoint, the improved efficiency offered by barium hydroxide could make enzymatic biofuel cells more attractive for various applications. Potential markets include portable electronics, remote power systems, and even grid-scale energy storage. The enhanced performance could lead to longer-lasting, more reliable biofuel cells, which could open up new commercial opportunities in sectors where traditional batteries or fuel cells have limitations.
Nevertheless, several factors may impact the commercial viability of this technology. The cost of barium hydroxide production and integration into biofuel cells must be carefully balanced against the performance gains to ensure economic feasibility. Regulatory considerations, particularly regarding the safe handling and disposal of barium compounds, may also affect commercialization efforts.
Market acceptance and competition from other emerging energy technologies will play crucial roles in determining the commercial success of barium hydroxide-enhanced enzymatic biofuel cells. Education and awareness campaigns may be necessary to highlight the benefits and address any safety concerns associated with this technology.
In conclusion, while the scalability and commercialization prospects for barium hydroxide in enzymatic biofuel cells are promising, success will depend on overcoming technical challenges, optimizing production processes, and effectively addressing market and regulatory considerations. Continued research and development efforts, coupled with strategic partnerships and targeted market entry strategies, will be essential for realizing the full potential of this technology in the renewable energy landscape.
From a scalability perspective, the use of barium hydroxide as an efficiency-enhancing agent in enzymatic biofuel cells shows potential for industrial applications. The compound's ability to improve electron transfer and enzyme stability could lead to more efficient and longer-lasting biofuel cells. This enhanced performance may translate to larger-scale energy production systems, potentially making enzymatic biofuel cells more competitive with traditional energy sources.
However, scaling up the production and integration of barium hydroxide in biofuel cell systems presents challenges. The manufacturing process must be optimized to ensure consistent quality and purity of barium hydroxide at larger scales. Additionally, the integration of barium hydroxide into existing biofuel cell designs may require modifications to current production processes, potentially increasing initial implementation costs.
From a commercialization standpoint, the improved efficiency offered by barium hydroxide could make enzymatic biofuel cells more attractive for various applications. Potential markets include portable electronics, remote power systems, and even grid-scale energy storage. The enhanced performance could lead to longer-lasting, more reliable biofuel cells, which could open up new commercial opportunities in sectors where traditional batteries or fuel cells have limitations.
Nevertheless, several factors may impact the commercial viability of this technology. The cost of barium hydroxide production and integration into biofuel cells must be carefully balanced against the performance gains to ensure economic feasibility. Regulatory considerations, particularly regarding the safe handling and disposal of barium compounds, may also affect commercialization efforts.
Market acceptance and competition from other emerging energy technologies will play crucial roles in determining the commercial success of barium hydroxide-enhanced enzymatic biofuel cells. Education and awareness campaigns may be necessary to highlight the benefits and address any safety concerns associated with this technology.
In conclusion, while the scalability and commercialization prospects for barium hydroxide in enzymatic biofuel cells are promising, success will depend on overcoming technical challenges, optimizing production processes, and effectively addressing market and regulatory considerations. Continued research and development efforts, coupled with strategic partnerships and targeted market entry strategies, will be essential for realizing the full potential of this technology in the renewable energy landscape.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!