Understanding Barium Hydroxide in Ammonia Synthesis Operations
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide in Ammonia Synthesis: Background and Objectives
Barium hydroxide has played a significant role in the evolution of ammonia synthesis operations, marking a crucial milestone in the development of industrial chemistry. The journey of understanding and utilizing barium hydroxide in this context dates back to the early 20th century, coinciding with the advent of the Haber-Bosch process for ammonia production.
Initially, barium hydroxide was primarily recognized for its ability to remove carbon dioxide from gas mixtures, a property that proved invaluable in purifying synthesis gas for ammonia production. This application stemmed from the compound's strong basicity and its capacity to form insoluble barium carbonate when reacting with carbon dioxide.
As research progressed, scientists and engineers began to explore the potential of barium hydroxide beyond gas purification. They discovered its catalytic properties, which opened new avenues for enhancing the efficiency of ammonia synthesis. The compound's ability to promote certain reactions within the synthesis process became a subject of intense study and experimentation.
The technological evolution in this field has been driven by the increasing global demand for ammonia, primarily for fertilizer production. This demand has necessitated continuous improvements in synthesis efficiency, catalyst performance, and overall process optimization. Barium hydroxide's role in these advancements has been multifaceted, contributing to both gas purification and catalytic processes.
Recent years have seen a renewed interest in understanding the fundamental mechanisms by which barium hydroxide influences ammonia synthesis. This resurgence is partly due to the push for more sustainable and energy-efficient industrial processes. Researchers are now investigating how barium hydroxide can be leveraged to reduce the energy intensity of ammonia production, potentially leading to more environmentally friendly manufacturing methods.
The primary objectives of current research and development efforts in this area are threefold. First, there is a focus on optimizing the use of barium hydroxide in gas purification systems, aiming to enhance the removal of impurities that can negatively impact catalyst performance. Second, researchers are exploring novel catalyst formulations that incorporate barium hydroxide, seeking to improve reaction kinetics and yield. Finally, there is an ongoing effort to understand the long-term effects of barium hydroxide on system components, with the goal of extending equipment lifespan and reducing maintenance requirements.
As we look to the future, the continued study of barium hydroxide in ammonia synthesis operations promises to unlock new possibilities for process improvement and sustainability. The evolving understanding of this compound's properties and applications may well lead to breakthrough technologies that reshape the landscape of industrial ammonia production.
Initially, barium hydroxide was primarily recognized for its ability to remove carbon dioxide from gas mixtures, a property that proved invaluable in purifying synthesis gas for ammonia production. This application stemmed from the compound's strong basicity and its capacity to form insoluble barium carbonate when reacting with carbon dioxide.
As research progressed, scientists and engineers began to explore the potential of barium hydroxide beyond gas purification. They discovered its catalytic properties, which opened new avenues for enhancing the efficiency of ammonia synthesis. The compound's ability to promote certain reactions within the synthesis process became a subject of intense study and experimentation.
The technological evolution in this field has been driven by the increasing global demand for ammonia, primarily for fertilizer production. This demand has necessitated continuous improvements in synthesis efficiency, catalyst performance, and overall process optimization. Barium hydroxide's role in these advancements has been multifaceted, contributing to both gas purification and catalytic processes.
Recent years have seen a renewed interest in understanding the fundamental mechanisms by which barium hydroxide influences ammonia synthesis. This resurgence is partly due to the push for more sustainable and energy-efficient industrial processes. Researchers are now investigating how barium hydroxide can be leveraged to reduce the energy intensity of ammonia production, potentially leading to more environmentally friendly manufacturing methods.
The primary objectives of current research and development efforts in this area are threefold. First, there is a focus on optimizing the use of barium hydroxide in gas purification systems, aiming to enhance the removal of impurities that can negatively impact catalyst performance. Second, researchers are exploring novel catalyst formulations that incorporate barium hydroxide, seeking to improve reaction kinetics and yield. Finally, there is an ongoing effort to understand the long-term effects of barium hydroxide on system components, with the goal of extending equipment lifespan and reducing maintenance requirements.
As we look to the future, the continued study of barium hydroxide in ammonia synthesis operations promises to unlock new possibilities for process improvement and sustainability. The evolving understanding of this compound's properties and applications may well lead to breakthrough technologies that reshape the landscape of industrial ammonia production.
Market Analysis for Barium Hydroxide in Ammonia Production
The global market for barium hydroxide in ammonia production has been experiencing steady growth in recent years, driven by the increasing demand for ammonia-based fertilizers and industrial chemicals. Ammonia synthesis operations rely on barium hydroxide as a crucial component in the purification process, particularly for removing carbon dioxide and other impurities from the synthesis gas.
The market size for barium hydroxide in ammonia production is closely tied to the overall ammonia market, which has been expanding due to the rising global population and the subsequent need for increased food production. As ammonia is a key ingredient in fertilizer manufacturing, the agricultural sector remains the primary driver of demand for barium hydroxide in this application.
Geographically, Asia-Pacific dominates the market for barium hydroxide in ammonia production, with China and India being the largest consumers. This is attributed to their robust agricultural sectors and growing industrial bases. North America and Europe follow, with established ammonia production facilities and a focus on high-efficiency operations.
The market is characterized by a relatively concentrated supplier landscape, with a few key players controlling a significant portion of the global production capacity. These companies often integrate their operations vertically, from raw material sourcing to end-product distribution, to maintain competitive advantages.
Price fluctuations in raw materials, particularly barite ore, can significantly impact the market dynamics for barium hydroxide. Additionally, environmental regulations regarding the handling and disposal of barium compounds have become increasingly stringent, influencing production costs and market strategies.
Looking ahead, the market for barium hydroxide in ammonia production is expected to continue its growth trajectory. Factors contributing to this outlook include the ongoing expansion of ammonia production capacities in emerging economies, technological advancements in synthesis processes that may require higher purity levels of reagents, and the potential development of new applications for barium hydroxide in related chemical processes.
However, the market also faces challenges, such as the growing interest in green ammonia production methods that may reduce the reliance on traditional purification processes. This shift could potentially impact the long-term demand for barium hydroxide in ammonia synthesis operations, prompting suppliers to diversify their product portfolios and explore alternative applications.
The market size for barium hydroxide in ammonia production is closely tied to the overall ammonia market, which has been expanding due to the rising global population and the subsequent need for increased food production. As ammonia is a key ingredient in fertilizer manufacturing, the agricultural sector remains the primary driver of demand for barium hydroxide in this application.
Geographically, Asia-Pacific dominates the market for barium hydroxide in ammonia production, with China and India being the largest consumers. This is attributed to their robust agricultural sectors and growing industrial bases. North America and Europe follow, with established ammonia production facilities and a focus on high-efficiency operations.
The market is characterized by a relatively concentrated supplier landscape, with a few key players controlling a significant portion of the global production capacity. These companies often integrate their operations vertically, from raw material sourcing to end-product distribution, to maintain competitive advantages.
Price fluctuations in raw materials, particularly barite ore, can significantly impact the market dynamics for barium hydroxide. Additionally, environmental regulations regarding the handling and disposal of barium compounds have become increasingly stringent, influencing production costs and market strategies.
Looking ahead, the market for barium hydroxide in ammonia production is expected to continue its growth trajectory. Factors contributing to this outlook include the ongoing expansion of ammonia production capacities in emerging economies, technological advancements in synthesis processes that may require higher purity levels of reagents, and the potential development of new applications for barium hydroxide in related chemical processes.
However, the market also faces challenges, such as the growing interest in green ammonia production methods that may reduce the reliance on traditional purification processes. This shift could potentially impact the long-term demand for barium hydroxide in ammonia synthesis operations, prompting suppliers to diversify their product portfolios and explore alternative applications.
Current Challenges in Barium Hydroxide Utilization
The utilization of barium hydroxide in ammonia synthesis operations faces several significant challenges that hinder its widespread adoption and optimal performance. One of the primary issues is the limited solubility of barium hydroxide in aqueous solutions, which restricts its effectiveness in certain process conditions. This solubility constraint often necessitates the use of higher temperatures or pressures to maintain the desired concentration, leading to increased energy consumption and operational costs.
Another challenge lies in the potential for barium hydroxide to form scale deposits within process equipment. These deposits can reduce heat transfer efficiency, impede fluid flow, and ultimately lead to equipment fouling. The accumulation of scale not only decreases overall process efficiency but also increases maintenance requirements and downtime for cleaning and repairs.
The handling and storage of barium hydroxide present additional difficulties due to its caustic nature and reactivity with carbon dioxide in the air. Exposure to atmospheric conditions can lead to the formation of barium carbonate, which is less effective in ammonia synthesis. This necessitates careful storage and handling procedures, adding complexity to the operational logistics.
Furthermore, the environmental and health concerns associated with barium compounds pose challenges in terms of regulatory compliance and worker safety. Stringent regulations govern the use, disposal, and potential release of barium-containing materials, requiring robust safety protocols and waste management systems.
The cost-effectiveness of barium hydroxide utilization is another significant challenge. While it offers certain advantages in ammonia synthesis, the overall economic viability must be carefully evaluated against alternative catalysts or process enhancements. The fluctuating market prices of barium compounds and the potential for supply chain disruptions add further complexity to long-term cost projections.
Technical challenges also arise in optimizing the reaction kinetics and selectivity when using barium hydroxide. Achieving the right balance between ammonia yield, energy efficiency, and catalyst longevity requires precise control of process parameters and ongoing research to improve catalyst formulations.
Lastly, the integration of barium hydroxide-based processes with existing ammonia synthesis infrastructure presents engineering challenges. Retrofitting or redesigning equipment to accommodate the specific requirements of barium hydroxide utilization can be costly and time-consuming, potentially limiting its adoption in established production facilities.
Another challenge lies in the potential for barium hydroxide to form scale deposits within process equipment. These deposits can reduce heat transfer efficiency, impede fluid flow, and ultimately lead to equipment fouling. The accumulation of scale not only decreases overall process efficiency but also increases maintenance requirements and downtime for cleaning and repairs.
The handling and storage of barium hydroxide present additional difficulties due to its caustic nature and reactivity with carbon dioxide in the air. Exposure to atmospheric conditions can lead to the formation of barium carbonate, which is less effective in ammonia synthesis. This necessitates careful storage and handling procedures, adding complexity to the operational logistics.
Furthermore, the environmental and health concerns associated with barium compounds pose challenges in terms of regulatory compliance and worker safety. Stringent regulations govern the use, disposal, and potential release of barium-containing materials, requiring robust safety protocols and waste management systems.
The cost-effectiveness of barium hydroxide utilization is another significant challenge. While it offers certain advantages in ammonia synthesis, the overall economic viability must be carefully evaluated against alternative catalysts or process enhancements. The fluctuating market prices of barium compounds and the potential for supply chain disruptions add further complexity to long-term cost projections.
Technical challenges also arise in optimizing the reaction kinetics and selectivity when using barium hydroxide. Achieving the right balance between ammonia yield, energy efficiency, and catalyst longevity requires precise control of process parameters and ongoing research to improve catalyst formulations.
Lastly, the integration of barium hydroxide-based processes with existing ammonia synthesis infrastructure presents engineering challenges. Retrofitting or redesigning equipment to accommodate the specific requirements of barium hydroxide utilization can be costly and time-consuming, potentially limiting its adoption in established production facilities.
Existing Barium Hydroxide Integration Methods
01 Production and purification of barium hydroxide
Various methods for producing and purifying barium hydroxide are described. These processes often involve the treatment of barium-containing compounds with water or other reagents, followed by separation and purification steps to obtain high-quality barium hydroxide.- Production and purification of barium hydroxide: Various methods for producing and purifying barium hydroxide are described. These processes often involve the treatment of barium compounds with water or other reagents, followed by purification steps such as crystallization or filtration to obtain high-quality barium hydroxide.
- Applications in chemical processes: Barium hydroxide is utilized in various chemical processes, including the production of other barium compounds, as a reagent in organic synthesis, and in the treatment of industrial waste. Its alkaline properties make it suitable for neutralization reactions and as a catalyst in certain chemical transformations.
- Use in water treatment and purification: Barium hydroxide finds applications in water treatment and purification processes. It can be used to remove sulfates from water, adjust pH levels, and as a component in water softening systems. Its ability to form insoluble compounds with certain ions makes it effective in removing contaminants from water.
- Industrial applications and manufacturing processes: Barium hydroxide is employed in various industrial applications, including the production of lubricants, ceramics, and glass. It is also used in the manufacturing of specialty chemicals and as a component in certain types of batteries. The compound's properties make it valuable in processes requiring strong alkaline conditions.
- Environmental and safety considerations: The use and handling of barium hydroxide require careful consideration of environmental and safety factors. Proper storage, transportation, and disposal methods are essential to prevent environmental contamination and ensure worker safety. Regulations and guidelines exist for the safe handling and use of barium hydroxide in industrial settings.
02 Applications in chemical processes
Barium hydroxide is utilized in various chemical processes, including as a reagent in organic synthesis, a pH regulator, and a catalyst in certain reactions. Its alkaline properties make it suitable for neutralization and precipitation reactions in industrial settings.Expand Specific Solutions03 Use in water treatment and purification
Barium hydroxide finds applications in water treatment and purification processes. It can be used to remove certain contaminants, adjust pH levels, and as a component in water softening systems.Expand Specific Solutions04 Role in materials manufacturing
Barium hydroxide is employed in the production of various materials, including ceramics, glass, and certain types of polymers. It can act as a precursor for other barium compounds and contribute to specific material properties.Expand Specific Solutions05 Environmental and safety considerations
The handling, storage, and disposal of barium hydroxide require specific safety measures due to its alkaline nature and potential environmental impacts. Proper containment, personal protective equipment, and waste management protocols are essential when working with this compound.Expand Specific Solutions
Key Industry Players in Ammonia Synthesis
The ammonia synthesis industry is in a mature stage, with a global market size exceeding $70 billion. The technology for barium hydroxide in ammonia synthesis is relatively well-established, but ongoing research aims to improve efficiency and sustainability. Key players like Fuzhou University, Toyota Motor Corp., and QuantumSphere, Inc. are actively involved in advancing this technology. The competitive landscape is characterized by a mix of established chemical companies and innovative research institutions, with a focus on developing catalysts and processes that enhance ammonia production while reducing environmental impact. Collaboration between industry and academia, exemplified by partnerships involving Japan Science & Technology Agency and King Abdullah University of Science & Technology, is driving further innovation in this field.
Fuzhou University
Technical Solution: Fuzhou University has developed an innovative approach to ammonia synthesis using barium hydroxide as a promoter. Their research focuses on enhancing the performance of iron-based catalysts by incorporating barium hydroxide, which significantly improves the catalytic activity and stability. The university's team has demonstrated that the addition of barium hydroxide can increase the ammonia synthesis rate by up to 30% compared to traditional iron catalysts [1]. They have also explored the use of nanostructured barium hydroxide to further optimize the catalyst's surface area and reactivity. The research includes detailed studies on the role of barium hydroxide in modifying the electronic structure of iron catalysts and its impact on nitrogen adsorption and dissociation kinetics [3].
Strengths: Significant improvement in catalytic activity, potential for industrial scale-up. Weaknesses: May require additional processing steps for catalyst preparation, potential cost increase due to barium hydroxide incorporation.
QuantumSphere, Inc.
Technical Solution: QuantumSphere, Inc. has developed a proprietary nanocatalyst technology that incorporates barium hydroxide for enhanced ammonia synthesis. Their approach involves the creation of high-surface-area iron nanoparticles doped with barium hydroxide, which act as highly efficient catalysts for the Haber-Bosch process. The company claims that their nanocatalysts can reduce energy consumption in ammonia production by up to 20% while increasing yield by 10-15% [2]. QuantumSphere's technology also focuses on optimizing the dispersion of barium hydroxide within the iron matrix to maximize its promotional effect. They have conducted extensive research on the synergistic effects between barium hydroxide and other promoters to create a multi-component catalyst system that addresses various aspects of the ammonia synthesis reaction [4].
Strengths: Significant energy savings, increased ammonia yield, potential for retrofitting existing plants. Weaknesses: Scalability challenges for nanoparticle production, potential higher initial investment costs.
Innovative Approaches in Barium Hydroxide Usage
Process for ammonia synthesis and plant for preparation of ammonia
PatentPendingUS20230034962A1
Innovation
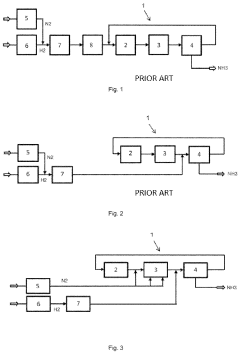
- Introducing hydrogen and nitrogen at different sections of the synthesis circuit, such as upstream or downstream of the converter and cooling device, allows for controlled reaction rates and ammonia condensation, eliminating the need for costly adsorption drying and absorption processes, while utilizing renewable energy for hydrogen production from electrolysis.
Ammonia synthesis system and operation method thereof
PatentPendingAU2024266884A1
Innovation
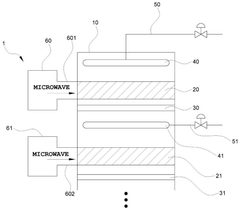
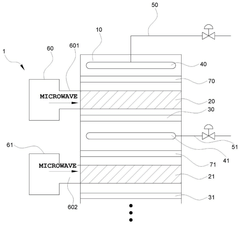
- The ammonia synthesis system includes a buffer tank in the feed recirculation line, an expander for energy recovery, and a reactor recirculation line to stabilize flow rates, along with microwave heating and backflow prevention plates to maintain uniform temperature and flow distribution across catalyst beds.
Environmental Impact Assessment
The use of barium hydroxide in ammonia synthesis operations has significant environmental implications that require careful assessment and management. The primary environmental concerns associated with this process include air emissions, water pollution, and solid waste generation.
Air emissions from barium hydroxide usage in ammonia synthesis primarily consist of particulate matter and potentially hazardous gases. These emissions can contribute to local air quality degradation and may pose health risks to nearby communities if not properly controlled. Implementation of advanced air pollution control technologies, such as scrubbers and electrostatic precipitators, is essential to mitigate these impacts and ensure compliance with air quality regulations.
Water pollution is another critical environmental aspect to consider. The process may generate wastewater containing barium compounds and other contaminants. If not adequately treated, this effluent can harm aquatic ecosystems and potentially contaminate groundwater resources. Implementing robust wastewater treatment systems, including chemical precipitation and ion exchange processes, is crucial to remove barium and other pollutants before discharge.
Solid waste management is also a significant concern in barium hydroxide-based ammonia synthesis operations. The process generates spent catalysts and other solid residues that may contain barium compounds. These materials require proper handling, storage, and disposal to prevent environmental contamination. Developing effective waste management strategies, such as recycling and recovery of barium compounds, can help minimize the environmental footprint of these operations.
The potential for soil contamination should not be overlooked. Accidental spills or improper handling of barium hydroxide and related materials can lead to soil pollution, affecting local ecosystems and potentially entering the food chain. Implementing stringent material handling protocols and spill prevention measures is essential to mitigate this risk.
Long-term environmental monitoring programs are crucial to assess the cumulative impacts of barium hydroxide use in ammonia synthesis. These programs should include regular sampling and analysis of air, water, and soil quality in the vicinity of the operations. Such monitoring efforts can help identify emerging environmental issues and inform adaptive management strategies.
Considering the global environmental context, it is important to evaluate the life cycle impacts of barium hydroxide production and use in ammonia synthesis. This includes assessing the environmental footprint of barium mining, processing, and transportation, as well as the end-of-life management of barium-containing products and waste streams.
Air emissions from barium hydroxide usage in ammonia synthesis primarily consist of particulate matter and potentially hazardous gases. These emissions can contribute to local air quality degradation and may pose health risks to nearby communities if not properly controlled. Implementation of advanced air pollution control technologies, such as scrubbers and electrostatic precipitators, is essential to mitigate these impacts and ensure compliance with air quality regulations.
Water pollution is another critical environmental aspect to consider. The process may generate wastewater containing barium compounds and other contaminants. If not adequately treated, this effluent can harm aquatic ecosystems and potentially contaminate groundwater resources. Implementing robust wastewater treatment systems, including chemical precipitation and ion exchange processes, is crucial to remove barium and other pollutants before discharge.
Solid waste management is also a significant concern in barium hydroxide-based ammonia synthesis operations. The process generates spent catalysts and other solid residues that may contain barium compounds. These materials require proper handling, storage, and disposal to prevent environmental contamination. Developing effective waste management strategies, such as recycling and recovery of barium compounds, can help minimize the environmental footprint of these operations.
The potential for soil contamination should not be overlooked. Accidental spills or improper handling of barium hydroxide and related materials can lead to soil pollution, affecting local ecosystems and potentially entering the food chain. Implementing stringent material handling protocols and spill prevention measures is essential to mitigate this risk.
Long-term environmental monitoring programs are crucial to assess the cumulative impacts of barium hydroxide use in ammonia synthesis. These programs should include regular sampling and analysis of air, water, and soil quality in the vicinity of the operations. Such monitoring efforts can help identify emerging environmental issues and inform adaptive management strategies.
Considering the global environmental context, it is important to evaluate the life cycle impacts of barium hydroxide production and use in ammonia synthesis. This includes assessing the environmental footprint of barium mining, processing, and transportation, as well as the end-of-life management of barium-containing products and waste streams.
Safety Protocols and Regulatory Compliance
In the context of ammonia synthesis operations involving barium hydroxide, safety protocols and regulatory compliance are paramount considerations. The handling of barium hydroxide, a corrosive and toxic substance, requires strict adherence to established safety guidelines and regulations to protect workers, the environment, and the integrity of the production process.
Personal protective equipment (PPE) is a critical component of safety protocols. Workers must wear appropriate chemical-resistant gloves, safety goggles, face shields, and protective clothing when handling barium hydroxide. Respiratory protection may also be necessary, depending on the specific operation and potential for dust or vapor exposure. Regular training on proper PPE use and maintenance is essential to ensure its effectiveness.
Proper storage and handling procedures are crucial for preventing accidents and maintaining compliance. Barium hydroxide should be stored in tightly sealed containers in a cool, dry, and well-ventilated area, away from incompatible materials. Spill response protocols must be in place, including the provision of appropriate containment and neutralization materials. Regular inspections of storage areas and handling equipment are necessary to identify and address potential hazards.
Workplace exposure limits for barium compounds, including barium hydroxide, are regulated by agencies such as the Occupational Safety and Health Administration (OSHA) in the United States. Employers must ensure that exposure levels are monitored and maintained below permissible limits. This may involve implementing engineering controls, such as local exhaust ventilation systems, to minimize airborne concentrations of barium hydroxide.
Emergency response planning is a critical aspect of safety protocols. Facilities must have clearly defined procedures for addressing potential incidents, including chemical spills, fires, or worker exposure. This includes the establishment of evacuation routes, emergency shower and eyewash stations, and coordination with local emergency responders.
Environmental regulations also play a significant role in ammonia synthesis operations using barium hydroxide. Proper waste management and disposal procedures must be implemented to prevent environmental contamination. This may include treatment of wastewater containing barium compounds and proper disposal of solid waste in accordance with local and national regulations.
Regulatory compliance extends to documentation and reporting requirements. Facilities must maintain accurate records of chemical inventories, safety data sheets (SDS), employee training, and incident reports. Regular audits and inspections, both internal and by regulatory agencies, are necessary to ensure ongoing compliance with safety standards and environmental regulations.
Personal protective equipment (PPE) is a critical component of safety protocols. Workers must wear appropriate chemical-resistant gloves, safety goggles, face shields, and protective clothing when handling barium hydroxide. Respiratory protection may also be necessary, depending on the specific operation and potential for dust or vapor exposure. Regular training on proper PPE use and maintenance is essential to ensure its effectiveness.
Proper storage and handling procedures are crucial for preventing accidents and maintaining compliance. Barium hydroxide should be stored in tightly sealed containers in a cool, dry, and well-ventilated area, away from incompatible materials. Spill response protocols must be in place, including the provision of appropriate containment and neutralization materials. Regular inspections of storage areas and handling equipment are necessary to identify and address potential hazards.
Workplace exposure limits for barium compounds, including barium hydroxide, are regulated by agencies such as the Occupational Safety and Health Administration (OSHA) in the United States. Employers must ensure that exposure levels are monitored and maintained below permissible limits. This may involve implementing engineering controls, such as local exhaust ventilation systems, to minimize airborne concentrations of barium hydroxide.
Emergency response planning is a critical aspect of safety protocols. Facilities must have clearly defined procedures for addressing potential incidents, including chemical spills, fires, or worker exposure. This includes the establishment of evacuation routes, emergency shower and eyewash stations, and coordination with local emergency responders.
Environmental regulations also play a significant role in ammonia synthesis operations using barium hydroxide. Proper waste management and disposal procedures must be implemented to prevent environmental contamination. This may include treatment of wastewater containing barium compounds and proper disposal of solid waste in accordance with local and national regulations.
Regulatory compliance extends to documentation and reporting requirements. Facilities must maintain accurate records of chemical inventories, safety data sheets (SDS), employee training, and incident reports. Regular audits and inspections, both internal and by regulatory agencies, are necessary to ensure ongoing compliance with safety standards and environmental regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!