Investigating Barium Hydroxide in Controlled Crystallization Processes
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide Crystallization Background
Barium hydroxide crystallization has been a subject of significant interest in various industrial and scientific applications for decades. The controlled crystallization of barium hydroxide plays a crucial role in numerous processes, including water treatment, chemical manufacturing, and materials science. The study of this compound's crystallization behavior dates back to the early 20th century, with researchers exploring its unique properties and potential applications.
The evolution of barium hydroxide crystallization research has been marked by several key developments. Initially, the focus was primarily on understanding the basic principles of crystal formation and growth. As analytical techniques advanced, researchers gained deeper insights into the molecular structure and behavior of barium hydroxide during crystallization. This led to the discovery of various polymorphs and hydrates, each with distinct properties and potential applications.
In recent years, the emphasis has shifted towards controlling and manipulating the crystallization process to achieve specific outcomes. This includes tailoring crystal size, morphology, and purity for targeted applications. The advent of advanced characterization techniques, such as X-ray diffraction and electron microscopy, has greatly enhanced our ability to study and optimize crystallization parameters.
The technological goals in this field have evolved to address several key challenges. One primary objective is to develop precise control mechanisms for crystal nucleation and growth, allowing for the production of uniform, high-quality crystals. Another important goal is to enhance the efficiency and scalability of barium hydroxide crystallization processes, making them more suitable for industrial applications.
Researchers are also exploring the potential of barium hydroxide crystallization in emerging fields such as nanotechnology and advanced materials. This includes investigating its role in the synthesis of novel nanostructures and composite materials with unique properties. Additionally, there is growing interest in understanding and mitigating the environmental impacts of barium hydroxide crystallization, particularly in the context of industrial waste management and water treatment.
The current trajectory of research in this area points towards several promising directions. These include the development of innovative crystallization techniques, such as template-assisted growth and electrochemical crystallization, to achieve greater control over crystal properties. There is also a growing focus on integrating computational modeling and machine learning approaches to predict and optimize crystallization outcomes, potentially revolutionizing process design and control in industrial settings.
The evolution of barium hydroxide crystallization research has been marked by several key developments. Initially, the focus was primarily on understanding the basic principles of crystal formation and growth. As analytical techniques advanced, researchers gained deeper insights into the molecular structure and behavior of barium hydroxide during crystallization. This led to the discovery of various polymorphs and hydrates, each with distinct properties and potential applications.
In recent years, the emphasis has shifted towards controlling and manipulating the crystallization process to achieve specific outcomes. This includes tailoring crystal size, morphology, and purity for targeted applications. The advent of advanced characterization techniques, such as X-ray diffraction and electron microscopy, has greatly enhanced our ability to study and optimize crystallization parameters.
The technological goals in this field have evolved to address several key challenges. One primary objective is to develop precise control mechanisms for crystal nucleation and growth, allowing for the production of uniform, high-quality crystals. Another important goal is to enhance the efficiency and scalability of barium hydroxide crystallization processes, making them more suitable for industrial applications.
Researchers are also exploring the potential of barium hydroxide crystallization in emerging fields such as nanotechnology and advanced materials. This includes investigating its role in the synthesis of novel nanostructures and composite materials with unique properties. Additionally, there is growing interest in understanding and mitigating the environmental impacts of barium hydroxide crystallization, particularly in the context of industrial waste management and water treatment.
The current trajectory of research in this area points towards several promising directions. These include the development of innovative crystallization techniques, such as template-assisted growth and electrochemical crystallization, to achieve greater control over crystal properties. There is also a growing focus on integrating computational modeling and machine learning approaches to predict and optimize crystallization outcomes, potentially revolutionizing process design and control in industrial settings.
Market Analysis for Controlled Crystallization
The controlled crystallization market has witnessed significant growth in recent years, driven by increasing demand across various industries such as pharmaceuticals, food and beverages, and materials science. The global market for controlled crystallization processes is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years.
In the pharmaceutical sector, controlled crystallization plays a crucial role in drug development and manufacturing. The industry's focus on improving drug efficacy, bioavailability, and stability has led to a surge in demand for advanced crystallization techniques. This trend is further amplified by the growing emphasis on personalized medicine and the need for precise control over drug properties.
The food and beverage industry has also emerged as a significant contributor to the controlled crystallization market. With consumers increasingly demanding healthier and more natural products, manufacturers are turning to controlled crystallization to develop low-fat, low-sugar, and clean-label alternatives. This has opened up new opportunities for innovative crystallization technologies and solutions.
In the materials science sector, controlled crystallization is gaining traction in the development of advanced materials with specific properties. Industries such as electronics, aerospace, and energy storage are driving demand for materials with tailored crystalline structures, leading to increased investment in research and development of controlled crystallization processes.
The market for controlled crystallization equipment and services is highly fragmented, with a mix of large multinational corporations and specialized niche players. Key market players are focusing on technological advancements, such as the integration of artificial intelligence and machine learning in crystallization processes, to gain a competitive edge.
Geographically, North America and Europe currently dominate the controlled crystallization market, owing to their well-established pharmaceutical and food industries. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing R&D investments, and growing demand for high-quality products in emerging economies like China and India.
The use of barium hydroxide in controlled crystallization processes represents a niche but growing segment within the broader market. Its applications in areas such as wastewater treatment, specialty chemicals production, and certain pharmaceutical processes are gaining attention. As industries seek more efficient and environmentally friendly crystallization agents, barium hydroxide's unique properties position it as a potential alternative in specific applications, contributing to the diversification of the controlled crystallization market.
In the pharmaceutical sector, controlled crystallization plays a crucial role in drug development and manufacturing. The industry's focus on improving drug efficacy, bioavailability, and stability has led to a surge in demand for advanced crystallization techniques. This trend is further amplified by the growing emphasis on personalized medicine and the need for precise control over drug properties.
The food and beverage industry has also emerged as a significant contributor to the controlled crystallization market. With consumers increasingly demanding healthier and more natural products, manufacturers are turning to controlled crystallization to develop low-fat, low-sugar, and clean-label alternatives. This has opened up new opportunities for innovative crystallization technologies and solutions.
In the materials science sector, controlled crystallization is gaining traction in the development of advanced materials with specific properties. Industries such as electronics, aerospace, and energy storage are driving demand for materials with tailored crystalline structures, leading to increased investment in research and development of controlled crystallization processes.
The market for controlled crystallization equipment and services is highly fragmented, with a mix of large multinational corporations and specialized niche players. Key market players are focusing on technological advancements, such as the integration of artificial intelligence and machine learning in crystallization processes, to gain a competitive edge.
Geographically, North America and Europe currently dominate the controlled crystallization market, owing to their well-established pharmaceutical and food industries. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing R&D investments, and growing demand for high-quality products in emerging economies like China and India.
The use of barium hydroxide in controlled crystallization processes represents a niche but growing segment within the broader market. Its applications in areas such as wastewater treatment, specialty chemicals production, and certain pharmaceutical processes are gaining attention. As industries seek more efficient and environmentally friendly crystallization agents, barium hydroxide's unique properties position it as a potential alternative in specific applications, contributing to the diversification of the controlled crystallization market.
Current Challenges in Barium Hydroxide Use
The use of barium hydroxide in controlled crystallization processes presents several significant challenges that researchers and industry professionals must address. One of the primary issues is the high sensitivity of barium hydroxide to environmental conditions, particularly temperature and humidity. This sensitivity can lead to inconsistent crystallization outcomes, making it difficult to achieve reproducible results in industrial settings.
Another challenge lies in the control of crystal morphology and size distribution. Barium hydroxide's rapid crystallization kinetics can result in the formation of irregular crystal shapes and a wide range of particle sizes. This variability can negatively impact the final product's properties and performance, especially in applications where uniform crystal characteristics are crucial.
The solubility of barium hydroxide also poses difficulties in maintaining precise supersaturation levels during the crystallization process. Fluctuations in supersaturation can lead to uncontrolled nucleation and growth, resulting in product inconsistencies and reduced yield. Additionally, the presence of impurities in barium hydroxide solutions can significantly affect crystallization behavior, requiring stringent purification processes to ensure consistent results.
Safety concerns associated with barium hydroxide handling and disposal present another set of challenges. As a corrosive substance, it requires specialized equipment and safety protocols, which can increase operational costs and complexity. Furthermore, the potential environmental impact of barium hydroxide waste necessitates careful management and treatment procedures.
Scale-up issues are particularly prominent when transitioning from laboratory-scale experiments to industrial production. The heat transfer and mixing dynamics in larger reactors can differ substantially from small-scale setups, leading to unexpected crystallization behaviors and product variations. This scaling challenge often requires extensive process optimization and redesign of crystallization equipment.
The integration of barium hydroxide-based crystallization processes into existing manufacturing lines can also be problematic. Compatibility issues with current equipment materials and the need for specialized handling systems may necessitate significant infrastructure investments. Moreover, the potential for cross-contamination in multi-product facilities requires careful consideration and implementation of robust cleaning and changeover procedures.
Lastly, the development of in-situ monitoring and control systems for barium hydroxide crystallization processes remains a significant challenge. Real-time measurement of critical parameters such as supersaturation, crystal size, and morphology is essential for process optimization but is often difficult to achieve reliably in industrial settings. This limitation hinders the implementation of advanced control strategies that could improve product quality and process efficiency.
Another challenge lies in the control of crystal morphology and size distribution. Barium hydroxide's rapid crystallization kinetics can result in the formation of irregular crystal shapes and a wide range of particle sizes. This variability can negatively impact the final product's properties and performance, especially in applications where uniform crystal characteristics are crucial.
The solubility of barium hydroxide also poses difficulties in maintaining precise supersaturation levels during the crystallization process. Fluctuations in supersaturation can lead to uncontrolled nucleation and growth, resulting in product inconsistencies and reduced yield. Additionally, the presence of impurities in barium hydroxide solutions can significantly affect crystallization behavior, requiring stringent purification processes to ensure consistent results.
Safety concerns associated with barium hydroxide handling and disposal present another set of challenges. As a corrosive substance, it requires specialized equipment and safety protocols, which can increase operational costs and complexity. Furthermore, the potential environmental impact of barium hydroxide waste necessitates careful management and treatment procedures.
Scale-up issues are particularly prominent when transitioning from laboratory-scale experiments to industrial production. The heat transfer and mixing dynamics in larger reactors can differ substantially from small-scale setups, leading to unexpected crystallization behaviors and product variations. This scaling challenge often requires extensive process optimization and redesign of crystallization equipment.
The integration of barium hydroxide-based crystallization processes into existing manufacturing lines can also be problematic. Compatibility issues with current equipment materials and the need for specialized handling systems may necessitate significant infrastructure investments. Moreover, the potential for cross-contamination in multi-product facilities requires careful consideration and implementation of robust cleaning and changeover procedures.
Lastly, the development of in-situ monitoring and control systems for barium hydroxide crystallization processes remains a significant challenge. Real-time measurement of critical parameters such as supersaturation, crystal size, and morphology is essential for process optimization but is often difficult to achieve reliably in industrial settings. This limitation hinders the implementation of advanced control strategies that could improve product quality and process efficiency.
Existing Barium Hydroxide Crystallization Methods
01 Crystallization process for barium hydroxide
The crystallization of barium hydroxide involves specific techniques and conditions to produce high-quality crystals. This process may include controlled cooling, seeding, and agitation to promote crystal growth and purity. The method can be optimized for industrial-scale production of barium hydroxide crystals.- Crystallization process for barium hydroxide: Various methods and apparatus for crystallizing barium hydroxide from aqueous solutions. This includes controlled cooling, seeding, and agitation techniques to promote crystal formation and growth. The process often involves precise temperature control and may use specialized crystallization vessels or equipment.
- Purification of barium hydroxide through crystallization: Techniques for purifying barium hydroxide by selective crystallization. This may involve removing impurities through fractional crystallization, washing of crystals, or recrystallization steps. The process aims to produce high-purity barium hydroxide crystals suitable for industrial or laboratory use.
- Equipment design for barium hydroxide crystallization: Specialized equipment and apparatus designs for efficient barium hydroxide crystallization. This includes crystallizers, heat exchangers, filtration systems, and other process equipment optimized for handling barium hydroxide solutions and crystals. The designs focus on improving yield, purity, and process efficiency.
- Control systems for barium hydroxide crystallization: Advanced control systems and methods for managing the barium hydroxide crystallization process. This may include automated temperature control, concentration monitoring, and crystal size distribution analysis. The systems aim to optimize crystal quality, yield, and consistency in industrial-scale production.
- Recovery and recycling in barium hydroxide crystallization: Techniques for recovering and recycling barium hydroxide from process streams or waste products. This may involve reprocessing mother liquors, recovering barium compounds from other industrial processes, or integrating crystallization with other separation techniques to maximize resource efficiency.
02 Equipment for barium hydroxide crystallization
Specialized equipment is used in the crystallization of barium hydroxide, including crystallizers, heat exchangers, and filtration systems. These devices are designed to control temperature, supersaturation, and crystal size distribution during the crystallization process, ensuring efficient and consistent production of barium hydroxide crystals.Expand Specific Solutions03 Purification methods for barium hydroxide crystals
Various purification techniques are employed to enhance the quality of barium hydroxide crystals. These may include recrystallization, washing processes, and the use of additives to remove impurities. The purification steps are crucial for obtaining high-purity barium hydroxide suitable for industrial applications.Expand Specific Solutions04 Control of crystal size and morphology
Methods for controlling the size and shape of barium hydroxide crystals are essential for specific applications. This involves manipulating crystallization parameters such as temperature, supersaturation, and additives to achieve desired crystal characteristics. The control of crystal size and morphology can significantly impact the properties and performance of the final product.Expand Specific Solutions05 Recovery and recycling of barium hydroxide
Processes for recovering and recycling barium hydroxide from industrial waste streams or byproducts are developed to improve efficiency and reduce environmental impact. These methods may involve precipitation, filtration, and recrystallization techniques to reclaim and purify barium hydroxide for reuse in various applications.Expand Specific Solutions
Key Industry Players and Competitors
The investigation of barium hydroxide in controlled crystallization processes is currently in an emerging phase, with growing interest from both academic institutions and industrial players. The market size for this technology is relatively small but expanding, driven by applications in materials science, chemical engineering, and pharmaceutical industries. The technology's maturity is still developing, with key players like Sakai Chemical Industry Co., Ltd. and Ecolab USA, Inc. leading industrial applications. Academic research is spearheaded by institutions such as the National Cheng Kung University and Dalian University of Technology, focusing on fundamental aspects and potential new applications. Companies like Venator Germany GmbH and Anhui Hengwei Bismuth Industry Co., Ltd. are exploring niche applications, indicating a diversifying competitive landscape as the technology matures.
Sakai Chemical Industry Co., Ltd.
Technical Solution: Sakai Chemical Industry Co., Ltd. has developed an innovative approach to controlled crystallization processes using barium hydroxide. Their method involves precise control of temperature, pH, and supersaturation levels to manipulate crystal growth and morphology[1]. The company has implemented a continuous flow crystallization system that allows for real-time adjustments of process parameters, resulting in highly uniform barium hydroxide crystals with specific size distributions[3]. Additionally, they have incorporated advanced in-situ monitoring techniques, such as focused beam reflectance measurement (FBRM) and particle vision microscopy (PVM), to provide real-time feedback on crystal properties during the crystallization process[5].
Strengths: High level of control over crystal properties, real-time process optimization, and consistent product quality. Weaknesses: Potentially higher equipment costs and complexity in process control systems.
Ecolab USA, Inc.
Technical Solution: Ecolab USA, Inc. has developed a proprietary crystallization process for barium hydroxide that focuses on environmental sustainability and water conservation. Their approach utilizes a closed-loop system that recycles process water and minimizes waste generation[2]. The company has implemented advanced membrane separation technologies to purify and concentrate barium hydroxide solutions before crystallization, reducing energy consumption and improving yield[4]. Ecolab's process also incorporates intelligent control systems that optimize crystallization conditions based on real-time data analytics, ensuring consistent crystal quality while minimizing resource usage[6].
Strengths: Environmentally friendly process, reduced water and energy consumption, and high efficiency. Weaknesses: Potential limitations in scaling up the process for very large production volumes.
Core Innovations in Controlled Crystallization
Crystallisation of barium hydroxide
PatentInactiveGB980813A
Innovation
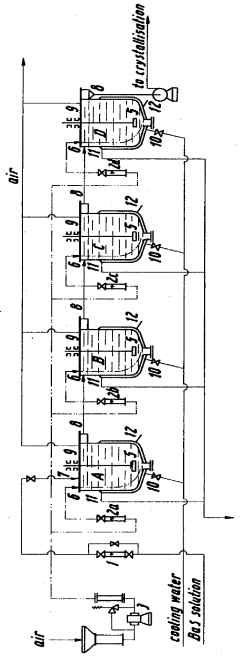
- A process involving cooling an aqueous barium sulphide solution to form barium hydroxide crystals, which are then passed through a washing zone with a counter-current stream of water or aqueous barium hydroxide solution to reduce impurity content, using a single column under isothermal conditions, with adjustable washing ratios and flow rates to maximize purification.
Method for the production of barium hydroxide
PatentInactiveUS3652217A
Innovation
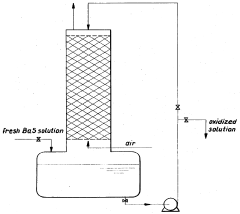
- The oxidation process is carried out in multiple stages with continuous cooling and efficient stirring, using oxygen-containing gases and flocculation agents to manage foam, allowing for the continuous production of barium hydroxide with high purity by crystallization and separation.
Environmental Impact Assessment
The use of barium hydroxide in controlled crystallization processes necessitates a thorough environmental impact assessment to ensure sustainable and responsible implementation. The primary environmental concerns associated with this process revolve around the potential release of barium compounds into ecosystems and water systems.
Barium, in its ionic form, can be toxic to aquatic life and pose risks to human health if ingested in high concentrations. Therefore, stringent waste management protocols must be established to prevent the discharge of barium-containing effluents into natural water bodies. This includes implementing advanced filtration and treatment systems to remove barium ions from wastewater before release.
Air quality is another critical factor to consider. While barium hydroxide itself is not volatile, the crystallization process may generate fine particulate matter that could become airborne. Proper ventilation systems and air filtration mechanisms should be installed to mitigate the risk of atmospheric contamination and protect workers and surrounding communities from potential respiratory hazards.
The production and transportation of barium hydroxide also warrant attention in the environmental impact assessment. Mining and processing of barium ores can lead to habitat disruption and soil contamination if not managed properly. Sustainable sourcing practices and rehabilitation plans for mining sites should be integral to the overall environmental strategy.
Energy consumption is a significant aspect of the environmental footprint of controlled crystallization processes. The use of barium hydroxide may require specific temperature and pressure conditions, potentially leading to increased energy demands. Implementing energy-efficient technologies and exploring renewable energy sources can help minimize the carbon footprint associated with these processes.
Waste reduction and recycling opportunities should be thoroughly explored. Developing closed-loop systems that allow for the recovery and reuse of barium compounds can significantly reduce the environmental impact and improve the overall sustainability of the process. This may include techniques such as ion exchange or electrodialysis to reclaim barium from waste streams.
Long-term ecological monitoring programs should be established to assess the cumulative effects of barium hydroxide use on local ecosystems. This includes regular soil and water testing, as well as biodiversity assessments to detect any potential bioaccumulation of barium in food chains.
In conclusion, while the use of barium hydroxide in controlled crystallization processes offers technological benefits, it is crucial to implement comprehensive environmental safeguards. By addressing waste management, air quality, sustainable sourcing, energy efficiency, and long-term ecological impacts, industries can minimize environmental risks and ensure responsible use of this chemical in their processes.
Barium, in its ionic form, can be toxic to aquatic life and pose risks to human health if ingested in high concentrations. Therefore, stringent waste management protocols must be established to prevent the discharge of barium-containing effluents into natural water bodies. This includes implementing advanced filtration and treatment systems to remove barium ions from wastewater before release.
Air quality is another critical factor to consider. While barium hydroxide itself is not volatile, the crystallization process may generate fine particulate matter that could become airborne. Proper ventilation systems and air filtration mechanisms should be installed to mitigate the risk of atmospheric contamination and protect workers and surrounding communities from potential respiratory hazards.
The production and transportation of barium hydroxide also warrant attention in the environmental impact assessment. Mining and processing of barium ores can lead to habitat disruption and soil contamination if not managed properly. Sustainable sourcing practices and rehabilitation plans for mining sites should be integral to the overall environmental strategy.
Energy consumption is a significant aspect of the environmental footprint of controlled crystallization processes. The use of barium hydroxide may require specific temperature and pressure conditions, potentially leading to increased energy demands. Implementing energy-efficient technologies and exploring renewable energy sources can help minimize the carbon footprint associated with these processes.
Waste reduction and recycling opportunities should be thoroughly explored. Developing closed-loop systems that allow for the recovery and reuse of barium compounds can significantly reduce the environmental impact and improve the overall sustainability of the process. This may include techniques such as ion exchange or electrodialysis to reclaim barium from waste streams.
Long-term ecological monitoring programs should be established to assess the cumulative effects of barium hydroxide use on local ecosystems. This includes regular soil and water testing, as well as biodiversity assessments to detect any potential bioaccumulation of barium in food chains.
In conclusion, while the use of barium hydroxide in controlled crystallization processes offers technological benefits, it is crucial to implement comprehensive environmental safeguards. By addressing waste management, air quality, sustainable sourcing, energy efficiency, and long-term ecological impacts, industries can minimize environmental risks and ensure responsible use of this chemical in their processes.
Scalability and Industrial Applications
The scalability and industrial applications of barium hydroxide in controlled crystallization processes present significant opportunities for various sectors. The unique properties of barium hydroxide, particularly its high solubility and ability to form supersaturated solutions, make it an attractive candidate for large-scale crystallization operations.
In industrial settings, barium hydroxide's controlled crystallization can be leveraged in water treatment processes, where it effectively removes sulfates from wastewater streams. This application is particularly valuable in industries such as mining, oil and gas, and power generation, where sulfate-rich effluents are common. The scalability of this process allows for the treatment of large volumes of water, making it economically viable for industrial-scale operations.
The pharmaceutical industry also stands to benefit from the scalability of barium hydroxide crystallization. In drug manufacturing, precise control over crystal size, shape, and purity is crucial for ensuring product quality and efficacy. By employing barium hydroxide in controlled crystallization processes, pharmaceutical companies can potentially achieve more consistent and higher-quality drug formulations at scale.
Another promising industrial application lies in the production of advanced materials. Barium hydroxide's controlled crystallization can be utilized to synthesize specialized barium compounds with specific crystal structures and properties. These materials find applications in electronics, optics, and energy storage technologies. The ability to scale up these processes enables the mass production of high-performance materials for emerging technologies.
In the field of chemical manufacturing, barium hydroxide's controlled crystallization offers opportunities for process intensification. By optimizing crystallization conditions, manufacturers can potentially reduce energy consumption, improve product yield, and enhance overall process efficiency. This scalability aspect is particularly attractive for bulk chemical production, where even small improvements in efficiency can translate to significant cost savings.
However, scaling up barium hydroxide crystallization processes also presents challenges. Maintaining precise control over crystallization parameters such as temperature, supersaturation, and mixing conditions becomes more complex at larger scales. Additionally, handling and disposing of barium compounds safely and environmentally responsibly require careful consideration and robust engineering solutions.
To address these challenges and fully realize the potential of barium hydroxide in industrial crystallization, ongoing research and development efforts are crucial. These include the development of advanced process control systems, innovative reactor designs, and improved separation and purification techniques. As these technologies mature, the industrial applications of barium hydroxide in controlled crystallization are likely to expand, offering new possibilities for process optimization and product innovation across multiple sectors.
In industrial settings, barium hydroxide's controlled crystallization can be leveraged in water treatment processes, where it effectively removes sulfates from wastewater streams. This application is particularly valuable in industries such as mining, oil and gas, and power generation, where sulfate-rich effluents are common. The scalability of this process allows for the treatment of large volumes of water, making it economically viable for industrial-scale operations.
The pharmaceutical industry also stands to benefit from the scalability of barium hydroxide crystallization. In drug manufacturing, precise control over crystal size, shape, and purity is crucial for ensuring product quality and efficacy. By employing barium hydroxide in controlled crystallization processes, pharmaceutical companies can potentially achieve more consistent and higher-quality drug formulations at scale.
Another promising industrial application lies in the production of advanced materials. Barium hydroxide's controlled crystallization can be utilized to synthesize specialized barium compounds with specific crystal structures and properties. These materials find applications in electronics, optics, and energy storage technologies. The ability to scale up these processes enables the mass production of high-performance materials for emerging technologies.
In the field of chemical manufacturing, barium hydroxide's controlled crystallization offers opportunities for process intensification. By optimizing crystallization conditions, manufacturers can potentially reduce energy consumption, improve product yield, and enhance overall process efficiency. This scalability aspect is particularly attractive for bulk chemical production, where even small improvements in efficiency can translate to significant cost savings.
However, scaling up barium hydroxide crystallization processes also presents challenges. Maintaining precise control over crystallization parameters such as temperature, supersaturation, and mixing conditions becomes more complex at larger scales. Additionally, handling and disposing of barium compounds safely and environmentally responsibly require careful consideration and robust engineering solutions.
To address these challenges and fully realize the potential of barium hydroxide in industrial crystallization, ongoing research and development efforts are crucial. These include the development of advanced process control systems, innovative reactor designs, and improved separation and purification techniques. As these technologies mature, the industrial applications of barium hydroxide in controlled crystallization are likely to expand, offering new possibilities for process optimization and product innovation across multiple sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!