Impact of Barium Hydroxide on Environmental Protection Methods
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide and Environmental Protection Overview
Barium hydroxide, a chemical compound with the formula Ba(OH)2, plays a significant role in various environmental protection methods. This alkaline substance has garnered attention due to its unique properties and potential applications in addressing environmental challenges. As concerns about pollution and ecological degradation continue to grow, researchers and industry professionals are exploring innovative ways to leverage barium hydroxide's characteristics for sustainable solutions.
In the context of environmental protection, barium hydroxide has shown promise in several key areas. One of its primary applications is in flue gas desulfurization processes, where it effectively removes sulfur dioxide from industrial emissions. This capability is crucial in mitigating air pollution and reducing the environmental impact of fossil fuel combustion. Additionally, barium hydroxide has demonstrated potential in wastewater treatment, particularly in the removal of heavy metals and other contaminants from industrial effluents.
The compound's high alkalinity makes it an effective neutralizing agent for acidic waste streams, helping to maintain pH balance in aquatic ecosystems. Furthermore, barium hydroxide has been investigated for its role in carbon capture and storage technologies, offering a potential avenue for reducing greenhouse gas emissions and combating climate change.
However, the use of barium hydroxide in environmental protection methods is not without challenges. Its toxicity and potential environmental risks necessitate careful handling and disposal practices. Researchers are actively working on developing safer and more efficient ways to utilize barium hydroxide while minimizing its potential negative impacts on ecosystems and human health.
As environmental regulations become increasingly stringent worldwide, the demand for effective pollution control technologies continues to rise. This has led to a growing interest in exploring the full potential of barium hydroxide and similar compounds in environmental applications. Ongoing research focuses on optimizing its use in existing processes and discovering novel applications that could further enhance its role in environmental protection strategies.
The impact of barium hydroxide on environmental protection methods extends beyond its direct applications. Its use has spurred innovation in related fields, such as materials science and chemical engineering, leading to the development of new technologies and processes that complement or enhance its effectiveness. This ripple effect contributes to the overall advancement of environmental protection technologies and practices across various industries.
In the context of environmental protection, barium hydroxide has shown promise in several key areas. One of its primary applications is in flue gas desulfurization processes, where it effectively removes sulfur dioxide from industrial emissions. This capability is crucial in mitigating air pollution and reducing the environmental impact of fossil fuel combustion. Additionally, barium hydroxide has demonstrated potential in wastewater treatment, particularly in the removal of heavy metals and other contaminants from industrial effluents.
The compound's high alkalinity makes it an effective neutralizing agent for acidic waste streams, helping to maintain pH balance in aquatic ecosystems. Furthermore, barium hydroxide has been investigated for its role in carbon capture and storage technologies, offering a potential avenue for reducing greenhouse gas emissions and combating climate change.
However, the use of barium hydroxide in environmental protection methods is not without challenges. Its toxicity and potential environmental risks necessitate careful handling and disposal practices. Researchers are actively working on developing safer and more efficient ways to utilize barium hydroxide while minimizing its potential negative impacts on ecosystems and human health.
As environmental regulations become increasingly stringent worldwide, the demand for effective pollution control technologies continues to rise. This has led to a growing interest in exploring the full potential of barium hydroxide and similar compounds in environmental applications. Ongoing research focuses on optimizing its use in existing processes and discovering novel applications that could further enhance its role in environmental protection strategies.
The impact of barium hydroxide on environmental protection methods extends beyond its direct applications. Its use has spurred innovation in related fields, such as materials science and chemical engineering, leading to the development of new technologies and processes that complement or enhance its effectiveness. This ripple effect contributes to the overall advancement of environmental protection technologies and practices across various industries.
Market Analysis for Eco-friendly Barium Hydroxide Applications
The market for eco-friendly barium hydroxide applications has shown significant growth potential in recent years, driven by increasing environmental concerns and stringent regulations. Barium hydroxide, traditionally used in various industrial processes, is now being explored for its potential in environmental protection methods.
The global market for barium hydroxide in eco-friendly applications is expected to expand at a steady rate, with a particular focus on water treatment, air pollution control, and soil remediation. In the water treatment sector, barium hydroxide has demonstrated effectiveness in removing sulfates and heavy metals from industrial wastewater, addressing a critical environmental challenge faced by many industries.
The air pollution control market segment has also shown promise for barium hydroxide applications. Its ability to neutralize acidic gases in flue emissions has attracted attention from power plants and manufacturing facilities seeking to comply with tightening air quality standards. This application is particularly relevant in regions with high industrial activity and stringent emission regulations.
Soil remediation represents another growing market for eco-friendly barium hydroxide applications. Its use in stabilizing contaminated soils and reducing the mobility of heavy metals has gained traction in brownfield redevelopment projects and environmental restoration initiatives.
Geographically, North America and Europe lead in adopting eco-friendly barium hydroxide solutions, primarily due to their advanced environmental regulations and well-established industrial sectors. However, emerging economies in Asia-Pacific, particularly China and India, are expected to become significant markets as they intensify efforts to combat industrial pollution and improve environmental quality.
The market is characterized by a mix of established chemical companies and innovative environmental technology firms. Key players are investing in research and development to enhance the efficiency and cost-effectiveness of barium hydroxide-based environmental solutions. Collaborations between industry and academic institutions are also driving innovation in this field.
Despite the positive outlook, challenges remain. The high cost of implementing barium hydroxide-based solutions compared to traditional methods poses a barrier to widespread adoption, especially in price-sensitive markets. Additionally, concerns about the potential environmental impact of barium itself need to be addressed through comprehensive life cycle assessments and responsible waste management practices.
Looking ahead, the market for eco-friendly barium hydroxide applications is poised for growth, driven by increasing environmental awareness, regulatory pressures, and technological advancements. As industries seek sustainable solutions to meet environmental targets, the demand for innovative applications of barium hydroxide in environmental protection methods is expected to rise, creating opportunities for market expansion and technological innovation.
The global market for barium hydroxide in eco-friendly applications is expected to expand at a steady rate, with a particular focus on water treatment, air pollution control, and soil remediation. In the water treatment sector, barium hydroxide has demonstrated effectiveness in removing sulfates and heavy metals from industrial wastewater, addressing a critical environmental challenge faced by many industries.
The air pollution control market segment has also shown promise for barium hydroxide applications. Its ability to neutralize acidic gases in flue emissions has attracted attention from power plants and manufacturing facilities seeking to comply with tightening air quality standards. This application is particularly relevant in regions with high industrial activity and stringent emission regulations.
Soil remediation represents another growing market for eco-friendly barium hydroxide applications. Its use in stabilizing contaminated soils and reducing the mobility of heavy metals has gained traction in brownfield redevelopment projects and environmental restoration initiatives.
Geographically, North America and Europe lead in adopting eco-friendly barium hydroxide solutions, primarily due to their advanced environmental regulations and well-established industrial sectors. However, emerging economies in Asia-Pacific, particularly China and India, are expected to become significant markets as they intensify efforts to combat industrial pollution and improve environmental quality.
The market is characterized by a mix of established chemical companies and innovative environmental technology firms. Key players are investing in research and development to enhance the efficiency and cost-effectiveness of barium hydroxide-based environmental solutions. Collaborations between industry and academic institutions are also driving innovation in this field.
Despite the positive outlook, challenges remain. The high cost of implementing barium hydroxide-based solutions compared to traditional methods poses a barrier to widespread adoption, especially in price-sensitive markets. Additionally, concerns about the potential environmental impact of barium itself need to be addressed through comprehensive life cycle assessments and responsible waste management practices.
Looking ahead, the market for eco-friendly barium hydroxide applications is poised for growth, driven by increasing environmental awareness, regulatory pressures, and technological advancements. As industries seek sustainable solutions to meet environmental targets, the demand for innovative applications of barium hydroxide in environmental protection methods is expected to rise, creating opportunities for market expansion and technological innovation.
Current Challenges in Barium Hydroxide Environmental Management
The management of barium hydroxide in environmental contexts presents several significant challenges that require immediate attention and innovative solutions. One of the primary concerns is the high solubility of barium hydroxide in water, which can lead to its rapid dispersion in aquatic ecosystems. This property makes it difficult to contain and control barium hydroxide once it enters water bodies, potentially causing widespread contamination.
Another critical challenge is the toxicity of barium compounds to various organisms, including humans. Exposure to high levels of barium can result in serious health issues, such as hypertension, cardiac arrhythmias, and gastrointestinal problems. The environmental persistence of barium compounds further exacerbates this issue, as they can accumulate in soil and sediments over time, creating long-term ecological risks.
The industrial use of barium hydroxide, particularly in the production of lubricants, plastics, and rubber, poses significant waste management challenges. Improper disposal of barium-containing waste can lead to soil and groundwater contamination. Developing effective treatment and disposal methods for barium-rich industrial effluents is crucial but technically challenging due to the compound's chemical properties.
Monitoring and detecting barium hydroxide in the environment is another area of concern. Current analytical methods may not be sufficiently sensitive or cost-effective for widespread implementation, especially in resource-limited settings. This gap in detection capabilities hampers efforts to assess the full extent of barium contamination and implement timely remediation measures.
The regulatory framework surrounding barium hydroxide management is often inadequate or inconsistent across different regions. This lack of standardized guidelines and enforcement mechanisms complicates efforts to implement effective environmental protection strategies on a global scale. Harmonizing regulations and establishing clear, science-based standards for barium hydroxide handling and disposal is a complex but necessary task.
Climate change and its associated environmental shifts present additional challenges in managing barium hydroxide. Increased frequency of extreme weather events, such as floods, can mobilize barium compounds from contaminated sites, potentially spreading pollution to previously unaffected areas. Adapting environmental management strategies to account for these changing conditions requires ongoing research and flexible policy approaches.
Lastly, the economic implications of stringent barium hydroxide management practices pose a challenge for industries reliant on this compound. Balancing environmental protection with economic considerations is a delicate task that requires innovative technological solutions and supportive policy frameworks to encourage sustainable practices without unduly burdening businesses.
Another critical challenge is the toxicity of barium compounds to various organisms, including humans. Exposure to high levels of barium can result in serious health issues, such as hypertension, cardiac arrhythmias, and gastrointestinal problems. The environmental persistence of barium compounds further exacerbates this issue, as they can accumulate in soil and sediments over time, creating long-term ecological risks.
The industrial use of barium hydroxide, particularly in the production of lubricants, plastics, and rubber, poses significant waste management challenges. Improper disposal of barium-containing waste can lead to soil and groundwater contamination. Developing effective treatment and disposal methods for barium-rich industrial effluents is crucial but technically challenging due to the compound's chemical properties.
Monitoring and detecting barium hydroxide in the environment is another area of concern. Current analytical methods may not be sufficiently sensitive or cost-effective for widespread implementation, especially in resource-limited settings. This gap in detection capabilities hampers efforts to assess the full extent of barium contamination and implement timely remediation measures.
The regulatory framework surrounding barium hydroxide management is often inadequate or inconsistent across different regions. This lack of standardized guidelines and enforcement mechanisms complicates efforts to implement effective environmental protection strategies on a global scale. Harmonizing regulations and establishing clear, science-based standards for barium hydroxide handling and disposal is a complex but necessary task.
Climate change and its associated environmental shifts present additional challenges in managing barium hydroxide. Increased frequency of extreme weather events, such as floods, can mobilize barium compounds from contaminated sites, potentially spreading pollution to previously unaffected areas. Adapting environmental management strategies to account for these changing conditions requires ongoing research and flexible policy approaches.
Lastly, the economic implications of stringent barium hydroxide management practices pose a challenge for industries reliant on this compound. Balancing environmental protection with economic considerations is a delicate task that requires innovative technological solutions and supportive policy frameworks to encourage sustainable practices without unduly burdening businesses.
Existing Environmental Protection Methods for Barium Hydroxide
01 Production and purification of barium hydroxide
Various methods for producing and purifying barium hydroxide are described. These processes involve different raw materials, reaction conditions, and purification steps to obtain high-quality barium hydroxide for industrial applications.- Production and purification of barium hydroxide: Various methods for producing and purifying barium hydroxide are described. These processes involve different raw materials, reaction conditions, and purification steps to obtain high-quality barium hydroxide for industrial applications.
- Use of barium hydroxide in chemical reactions: Barium hydroxide is utilized as a reagent or catalyst in various chemical reactions. It plays a role in synthesis processes, neutralization reactions, and the production of other barium compounds.
- Applications in water treatment and environmental processes: Barium hydroxide finds applications in water treatment processes, including the removal of sulfates and other contaminants. It is also used in environmental remediation and waste treatment applications.
- Industrial uses of barium hydroxide: Barium hydroxide has various industrial applications, including its use in the production of lubricants, ceramics, and glass. It is also employed in the manufacturing of other barium compounds and as a component in specialized industrial processes.
- Handling and safety considerations for barium hydroxide: Due to its alkaline nature and potential health hazards, specific handling and safety measures are required when working with barium hydroxide. This includes proper storage, protective equipment, and disposal procedures to ensure safe usage in industrial and laboratory settings.
02 Use of barium hydroxide in chemical reactions
Barium hydroxide is utilized in various chemical reactions as a reagent or catalyst. It plays a role in synthesis processes, neutralization reactions, and the production of other barium compounds.Expand Specific Solutions03 Applications in water treatment and environmental processes
Barium hydroxide finds applications in water treatment and environmental processes. It is used for removing impurities, adjusting pH levels, and in the treatment of industrial effluents and waste streams.Expand Specific Solutions04 Use in the production of specialty chemicals and materials
Barium hydroxide is employed in the production of specialty chemicals and materials. It serves as a precursor or intermediate in the synthesis of various compounds, including lubricants, plastics, and ceramics.Expand Specific Solutions05 Safety and handling considerations for barium hydroxide
Due to its alkaline nature and potential health hazards, specific safety measures and handling procedures are required when working with barium hydroxide. This includes proper storage, personal protective equipment, and disposal methods.Expand Specific Solutions
Key Players in Barium Hydroxide and Environmental Solutions
The impact of barium hydroxide on environmental protection methods is in an early development stage, with a growing market driven by increasing environmental concerns. The technology's maturity is still evolving, as evidenced by the diverse range of companies involved. Veolia Water Solutions & Technologies Support SAS and Siemens AG are leveraging their expertise in water treatment and industrial processes to explore applications. Smaller firms like Binzhou Kunbao Chemical Co., Ltd. are focusing on production, while academic institutions such as Beijing University of Technology and Southeast University are conducting research to advance the technology. The involvement of both established corporations and specialized chemical companies indicates a competitive landscape with potential for significant growth and innovation in environmental protection applications.
Veolia Water Solutions & Technologies Support SAS
Technical Solution: Veolia has developed an innovative water treatment process utilizing barium hydroxide for environmental protection. Their method involves using barium hydroxide as a precipitating agent to remove sulfates and other contaminants from industrial wastewater [1]. The process includes a two-stage treatment: first, barium hydroxide is added to precipitate sulfates, then the resulting barium sulfate is removed through settling or filtration. This technology has shown particular effectiveness in treating mining effluents and other high-sulfate wastewaters [2]. Veolia's approach also incorporates a barium recovery system, where the precipitated barium sulfate is thermally reduced to regenerate barium hydroxide, making the process more sustainable and cost-effective [3].
Strengths: Highly effective for sulfate removal, applicable to various industrial wastewaters, and includes a barium recovery system for sustainability. Weaknesses: Potential environmental risks if barium is not properly managed, and relatively high initial implementation costs.
Siemens AG
Technical Solution: Siemens has developed an advanced flue gas desulfurization (FGD) system that incorporates barium hydroxide for enhanced environmental protection. Their technology uses a wet scrubbing process where flue gas is treated with a slurry containing barium hydroxide [4]. This method not only removes sulfur dioxide but also captures other acidic gases and particulates. The barium hydroxide reacts with sulfur dioxide to form barium sulfite, which is then oxidized to barium sulfate. Siemens' system includes a proprietary oxidation catalyst that enhances this conversion, improving overall efficiency [5]. Additionally, they have implemented a closed-loop process that recycles and regenerates the barium compounds, minimizing waste and reducing operational costs [6].
Strengths: High sulfur dioxide removal efficiency, capability to remove multiple pollutants, and closed-loop design for sustainability. Weaknesses: Complex system requiring skilled operation and maintenance, and potential for barium contamination if not properly managed.
Innovative Approaches to Barium Hydroxide Waste Management
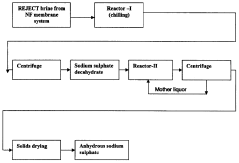
A process for the recovery of sodium sulphate from the brine resulting from nanofiltration membrane system in chloralkali process
PatentInactiveIN748CHE2007A
Innovation
- A process involving cooling the depleted brine to -20 to +2°C to form sodium sulphate decahydrate, followed by centrifugation, dissolution in a saturated sodium sulphate solution, and heating to convert it to anhydrous sodium sulphate, which is then centrifuged and dried, eliminating the need for toxic chemicals and sludge formation.
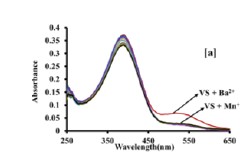
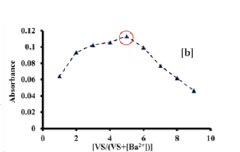
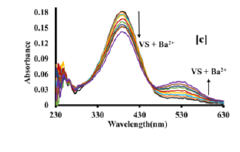
Colorimetric sensor for the detection of barium metal ion in water
PatentPendingIN202211066531A
Innovation
- A portable colorimetric sensor system utilizing synthesized substituted benzothiazolinic spiropyran salts that change color upon barium ion detection, compatible with smartphones for real-time monitoring and capable of detecting barium ions in the presence of other metal ions without pretreatment.
Regulatory Framework for Barium Hydroxide Use and Disposal
The regulatory framework for barium hydroxide use and disposal is a critical aspect of environmental protection, given the potential hazards associated with this chemical compound. Governments and environmental agencies worldwide have established comprehensive guidelines to manage its lifecycle, from production to disposal.
In the United States, the Environmental Protection Agency (EPA) plays a pivotal role in regulating barium hydroxide. Under the Resource Conservation and Recovery Act (RCRA), barium compounds are classified as hazardous waste when they exceed specific concentration thresholds. This classification mandates strict handling, storage, and disposal procedures for industries utilizing barium hydroxide.
The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for barium compounds in the workplace, including barium hydroxide. These regulations require employers to implement safety measures, provide personal protective equipment, and conduct regular air quality monitoring to ensure worker safety.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of barium hydroxide. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The Classification, Labelling and Packaging (CLP) Regulation further ensures that the hazards of barium hydroxide are clearly communicated to workers and consumers.
Many countries have adopted the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), which provides a standardized approach to hazard communication. Under GHS, barium hydroxide is typically classified as harmful if swallowed and as an irritant to skin and eyes, necessitating specific labeling and safety data sheet requirements.
Disposal regulations for barium hydroxide often require treatment to reduce its solubility before landfill disposal. Some jurisdictions mandate the conversion of barium hydroxide to less soluble forms, such as barium sulfate, prior to disposal. Wastewater containing barium hydroxide must undergo treatment to remove barium ions before release into the environment, often through precipitation or ion exchange processes.
Transportation of barium hydroxide is regulated under various international agreements, such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging, labeling, and documentation requirements to ensure safe transport and emergency response preparedness.
As environmental concerns grow, many jurisdictions are tightening regulations on barium hydroxide and other potentially harmful chemicals. This includes stricter emission controls, more frequent environmental impact assessments, and increased reporting requirements for industries using or producing barium hydroxide.
In the United States, the Environmental Protection Agency (EPA) plays a pivotal role in regulating barium hydroxide. Under the Resource Conservation and Recovery Act (RCRA), barium compounds are classified as hazardous waste when they exceed specific concentration thresholds. This classification mandates strict handling, storage, and disposal procedures for industries utilizing barium hydroxide.
The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for barium compounds in the workplace, including barium hydroxide. These regulations require employers to implement safety measures, provide personal protective equipment, and conduct regular air quality monitoring to ensure worker safety.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of barium hydroxide. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The Classification, Labelling and Packaging (CLP) Regulation further ensures that the hazards of barium hydroxide are clearly communicated to workers and consumers.
Many countries have adopted the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), which provides a standardized approach to hazard communication. Under GHS, barium hydroxide is typically classified as harmful if swallowed and as an irritant to skin and eyes, necessitating specific labeling and safety data sheet requirements.
Disposal regulations for barium hydroxide often require treatment to reduce its solubility before landfill disposal. Some jurisdictions mandate the conversion of barium hydroxide to less soluble forms, such as barium sulfate, prior to disposal. Wastewater containing barium hydroxide must undergo treatment to remove barium ions before release into the environment, often through precipitation or ion exchange processes.
Transportation of barium hydroxide is regulated under various international agreements, such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging, labeling, and documentation requirements to ensure safe transport and emergency response preparedness.
As environmental concerns grow, many jurisdictions are tightening regulations on barium hydroxide and other potentially harmful chemicals. This includes stricter emission controls, more frequent environmental impact assessments, and increased reporting requirements for industries using or producing barium hydroxide.
Life Cycle Assessment of Barium Hydroxide in Industry
Life Cycle Assessment (LCA) of barium hydroxide in industry provides crucial insights into its environmental impact throughout its production, use, and disposal phases. The assessment begins with the extraction of raw materials, primarily barite ore, which is mined and processed to obtain barium sulfide. This initial stage involves significant energy consumption and potential land disturbance.
The production phase of barium hydroxide involves the reaction of barium sulfide with water, resulting in the formation of barium hydroxide and hydrogen sulfide. This process requires careful management of toxic hydrogen sulfide gas, necessitating robust safety measures and emission control systems. The energy requirements for this reaction and subsequent purification steps contribute to the overall environmental footprint.
During its use phase, barium hydroxide finds applications in various industries, including water treatment, lubricant production, and as a precursor in the synthesis of other barium compounds. Its role in water treatment, particularly in removing sulfates and radium from contaminated water, highlights its positive environmental contribution. However, the potential release of barium ions into the environment during these applications must be carefully monitored and controlled.
The disposal and end-of-life stage of barium hydroxide presents significant environmental challenges. Improper disposal can lead to soil and water contamination due to the high solubility and potential toxicity of barium compounds. Recycling and recovery processes for barium hydroxide are limited, often resulting in the need for specialized waste treatment methods to prevent environmental contamination.
Transportation throughout the life cycle stages adds to the overall environmental impact, with considerations for fuel consumption and emissions associated with moving raw materials, finished products, and waste. The global nature of the barium hydroxide industry means that these transportation impacts can be substantial.
Water usage is another critical aspect of the LCA, particularly in the production and use phases. The process of converting barium sulfide to barium hydroxide requires significant amounts of water, and its applications in water treatment further increase the water footprint of this compound.
Energy consumption across the life cycle, particularly in mining, processing, and manufacturing stages, contributes significantly to greenhouse gas emissions and overall environmental impact. The source of energy used in these processes (renewable vs. non-renewable) plays a crucial role in determining the extent of this impact.
In conclusion, the LCA of barium hydroxide in industry reveals a complex interplay of environmental benefits and challenges. While its applications in environmental protection, particularly water treatment, offer positive impacts, the environmental costs associated with its production, transportation, and disposal necessitate careful management and ongoing efforts to improve sustainability across its life cycle.
The production phase of barium hydroxide involves the reaction of barium sulfide with water, resulting in the formation of barium hydroxide and hydrogen sulfide. This process requires careful management of toxic hydrogen sulfide gas, necessitating robust safety measures and emission control systems. The energy requirements for this reaction and subsequent purification steps contribute to the overall environmental footprint.
During its use phase, barium hydroxide finds applications in various industries, including water treatment, lubricant production, and as a precursor in the synthesis of other barium compounds. Its role in water treatment, particularly in removing sulfates and radium from contaminated water, highlights its positive environmental contribution. However, the potential release of barium ions into the environment during these applications must be carefully monitored and controlled.
The disposal and end-of-life stage of barium hydroxide presents significant environmental challenges. Improper disposal can lead to soil and water contamination due to the high solubility and potential toxicity of barium compounds. Recycling and recovery processes for barium hydroxide are limited, often resulting in the need for specialized waste treatment methods to prevent environmental contamination.
Transportation throughout the life cycle stages adds to the overall environmental impact, with considerations for fuel consumption and emissions associated with moving raw materials, finished products, and waste. The global nature of the barium hydroxide industry means that these transportation impacts can be substantial.
Water usage is another critical aspect of the LCA, particularly in the production and use phases. The process of converting barium sulfide to barium hydroxide requires significant amounts of water, and its applications in water treatment further increase the water footprint of this compound.
Energy consumption across the life cycle, particularly in mining, processing, and manufacturing stages, contributes significantly to greenhouse gas emissions and overall environmental impact. The source of energy used in these processes (renewable vs. non-renewable) plays a crucial role in determining the extent of this impact.
In conclusion, the LCA of barium hydroxide in industry reveals a complex interplay of environmental benefits and challenges. While its applications in environmental protection, particularly water treatment, offer positive impacts, the environmental costs associated with its production, transportation, and disposal necessitate careful management and ongoing efforts to improve sustainability across its life cycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!