Understanding Barium Hydroxide’s Functionality in Controlled Drug Release
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide in Drug Delivery: Background and Objectives
Barium hydroxide has emerged as a significant compound in the field of controlled drug release, marking a notable advancement in pharmaceutical technology. The evolution of drug delivery systems has been driven by the need for more efficient and targeted therapeutic approaches. In this context, barium hydroxide's unique properties have attracted considerable attention from researchers and pharmaceutical companies alike.
The primary objective of exploring barium hydroxide in drug delivery is to enhance the efficacy and safety of pharmaceutical formulations. By leveraging its chemical characteristics, scientists aim to develop innovative drug release mechanisms that can precisely control the timing and rate of medication dispersal within the body. This controlled release approach holds the potential to revolutionize treatment regimens for various medical conditions, particularly those requiring sustained drug concentrations over extended periods.
Historically, the pharmaceutical industry has faced challenges in maintaining consistent drug levels in patients' systems, often resulting in suboptimal therapeutic outcomes. The introduction of barium hydroxide into drug delivery systems represents a strategic response to these limitations. Its ability to form complexes with certain drug molecules and its pH-dependent solubility offer promising avenues for creating advanced drug formulations.
The technical goals associated with barium hydroxide in controlled drug release are multifaceted. Researchers are focusing on optimizing its integration into various drug delivery platforms, such as matrix tablets, microspheres, and transdermal patches. The aim is to achieve precise control over drug release kinetics, improve bioavailability, and minimize side effects associated with fluctuating drug concentrations in the bloodstream.
Furthermore, the exploration of barium hydroxide in this context aligns with the broader trend towards personalized medicine. By fine-tuning drug release profiles, healthcare providers can potentially tailor treatments to individual patient needs, considering factors such as metabolism, disease progression, and lifestyle.
As the field progresses, there is a growing emphasis on understanding the molecular interactions between barium hydroxide and different classes of pharmaceutical compounds. This fundamental research is crucial for expanding the range of drugs that can benefit from this technology and for developing predictive models to streamline formulation processes.
The trajectory of barium hydroxide in controlled drug release is closely tied to advancements in materials science and nanotechnology. These interdisciplinary connections are driving innovation in drug delivery systems, promising more sophisticated and effective therapeutic solutions for the future of healthcare.
The primary objective of exploring barium hydroxide in drug delivery is to enhance the efficacy and safety of pharmaceutical formulations. By leveraging its chemical characteristics, scientists aim to develop innovative drug release mechanisms that can precisely control the timing and rate of medication dispersal within the body. This controlled release approach holds the potential to revolutionize treatment regimens for various medical conditions, particularly those requiring sustained drug concentrations over extended periods.
Historically, the pharmaceutical industry has faced challenges in maintaining consistent drug levels in patients' systems, often resulting in suboptimal therapeutic outcomes. The introduction of barium hydroxide into drug delivery systems represents a strategic response to these limitations. Its ability to form complexes with certain drug molecules and its pH-dependent solubility offer promising avenues for creating advanced drug formulations.
The technical goals associated with barium hydroxide in controlled drug release are multifaceted. Researchers are focusing on optimizing its integration into various drug delivery platforms, such as matrix tablets, microspheres, and transdermal patches. The aim is to achieve precise control over drug release kinetics, improve bioavailability, and minimize side effects associated with fluctuating drug concentrations in the bloodstream.
Furthermore, the exploration of barium hydroxide in this context aligns with the broader trend towards personalized medicine. By fine-tuning drug release profiles, healthcare providers can potentially tailor treatments to individual patient needs, considering factors such as metabolism, disease progression, and lifestyle.
As the field progresses, there is a growing emphasis on understanding the molecular interactions between barium hydroxide and different classes of pharmaceutical compounds. This fundamental research is crucial for expanding the range of drugs that can benefit from this technology and for developing predictive models to streamline formulation processes.
The trajectory of barium hydroxide in controlled drug release is closely tied to advancements in materials science and nanotechnology. These interdisciplinary connections are driving innovation in drug delivery systems, promising more sophisticated and effective therapeutic solutions for the future of healthcare.
Market Analysis of Controlled Release Drug Formulations
The controlled release drug formulation market has experienced significant growth in recent years, driven by the increasing demand for more efficient and patient-friendly drug delivery systems. This market segment is characterized by its ability to provide sustained and targeted drug release, improving therapeutic outcomes and patient compliance. The global controlled release drug delivery market was valued at approximately $29.8 billion in 2020 and is projected to reach $43.2 billion by 2026, growing at a CAGR of 7.2% during the forecast period.
Several factors contribute to the expanding market for controlled release drug formulations. The rising prevalence of chronic diseases, such as diabetes, cardiovascular disorders, and cancer, has increased the need for long-acting medications that can maintain therapeutic levels over extended periods. Additionally, the growing geriatric population, who often require multiple medications, benefits from controlled release formulations that reduce dosing frequency and minimize side effects.
The pharmaceutical industry's focus on developing innovative drug delivery technologies has also fueled market growth. Companies are investing heavily in research and development to create novel controlled release platforms, including polymer-based systems, liposomes, and nanoparticles. These advancements aim to enhance drug bioavailability, reduce dosing frequency, and minimize adverse effects, ultimately improving patient outcomes and quality of life.
Geographically, North America dominates the controlled release drug formulation market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure, high healthcare expenditure, and strong presence of pharmaceutical companies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by improving healthcare access and increasing investments in pharmaceutical research and development.
Key players in the controlled release drug formulation market include Johnson & Johnson, Pfizer, Merck & Co., Novartis, and AstraZeneca. These companies are actively developing and commercializing controlled release products across various therapeutic areas. Moreover, collaborations between pharmaceutical companies and drug delivery technology providers are becoming increasingly common, fostering innovation and market expansion.
The market for controlled release drug formulations faces some challenges, including the high cost of development and manufacturing, regulatory hurdles, and potential safety concerns. However, the benefits of improved patient compliance, reduced side effects, and enhanced therapeutic efficacy continue to drive market growth and innovation in this field.
Several factors contribute to the expanding market for controlled release drug formulations. The rising prevalence of chronic diseases, such as diabetes, cardiovascular disorders, and cancer, has increased the need for long-acting medications that can maintain therapeutic levels over extended periods. Additionally, the growing geriatric population, who often require multiple medications, benefits from controlled release formulations that reduce dosing frequency and minimize side effects.
The pharmaceutical industry's focus on developing innovative drug delivery technologies has also fueled market growth. Companies are investing heavily in research and development to create novel controlled release platforms, including polymer-based systems, liposomes, and nanoparticles. These advancements aim to enhance drug bioavailability, reduce dosing frequency, and minimize adverse effects, ultimately improving patient outcomes and quality of life.
Geographically, North America dominates the controlled release drug formulation market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure, high healthcare expenditure, and strong presence of pharmaceutical companies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by improving healthcare access and increasing investments in pharmaceutical research and development.
Key players in the controlled release drug formulation market include Johnson & Johnson, Pfizer, Merck & Co., Novartis, and AstraZeneca. These companies are actively developing and commercializing controlled release products across various therapeutic areas. Moreover, collaborations between pharmaceutical companies and drug delivery technology providers are becoming increasingly common, fostering innovation and market expansion.
The market for controlled release drug formulations faces some challenges, including the high cost of development and manufacturing, regulatory hurdles, and potential safety concerns. However, the benefits of improved patient compliance, reduced side effects, and enhanced therapeutic efficacy continue to drive market growth and innovation in this field.
Current Challenges in Barium Hydroxide-Based Drug Release Systems
Despite the promising potential of barium hydroxide in controlled drug release systems, several challenges currently hinder its widespread adoption and optimal performance. One of the primary issues is the limited understanding of barium hydroxide's interaction mechanisms with various drug compounds. This knowledge gap makes it difficult to predict and control release kinetics accurately, potentially leading to suboptimal therapeutic outcomes.
Another significant challenge lies in the pH-dependent solubility of barium hydroxide. While this property can be advantageous in certain applications, it also presents difficulties in maintaining consistent release rates across different physiological environments. Fluctuations in local pH levels within the body can lead to unpredictable drug release profiles, compromising the effectiveness of the delivery system.
The potential toxicity of barium ions remains a concern, particularly in long-term or high-dose applications. Although barium hydroxide is generally considered less toxic than other barium compounds, there is still a need for comprehensive safety studies to establish acceptable limits and potential side effects in various therapeutic contexts.
Formulation stability poses another challenge, as barium hydroxide can be sensitive to environmental factors such as temperature and humidity. This sensitivity can lead to changes in the physical and chemical properties of the drug delivery system during storage or administration, potentially affecting its performance and shelf life.
The scalability of barium hydroxide-based drug release systems presents technical hurdles in manufacturing processes. Ensuring uniform distribution of barium hydroxide within the delivery matrix and maintaining consistent quality across large-scale production batches remain areas of concern for pharmaceutical companies.
Additionally, there is a lack of standardized methods for characterizing and evaluating barium hydroxide-based drug release systems. This absence of established protocols makes it challenging to compare different formulations and assess their relative efficacy, hindering the development and optimization of these systems.
Regulatory considerations also pose challenges, as the use of barium compounds in pharmaceutical applications may require extensive safety data and approval processes. The regulatory landscape for novel excipients like barium hydroxide can be complex and time-consuming, potentially slowing down the development and commercialization of new drug delivery technologies.
Another significant challenge lies in the pH-dependent solubility of barium hydroxide. While this property can be advantageous in certain applications, it also presents difficulties in maintaining consistent release rates across different physiological environments. Fluctuations in local pH levels within the body can lead to unpredictable drug release profiles, compromising the effectiveness of the delivery system.
The potential toxicity of barium ions remains a concern, particularly in long-term or high-dose applications. Although barium hydroxide is generally considered less toxic than other barium compounds, there is still a need for comprehensive safety studies to establish acceptable limits and potential side effects in various therapeutic contexts.
Formulation stability poses another challenge, as barium hydroxide can be sensitive to environmental factors such as temperature and humidity. This sensitivity can lead to changes in the physical and chemical properties of the drug delivery system during storage or administration, potentially affecting its performance and shelf life.
The scalability of barium hydroxide-based drug release systems presents technical hurdles in manufacturing processes. Ensuring uniform distribution of barium hydroxide within the delivery matrix and maintaining consistent quality across large-scale production batches remain areas of concern for pharmaceutical companies.
Additionally, there is a lack of standardized methods for characterizing and evaluating barium hydroxide-based drug release systems. This absence of established protocols makes it challenging to compare different formulations and assess their relative efficacy, hindering the development and optimization of these systems.
Regulatory considerations also pose challenges, as the use of barium compounds in pharmaceutical applications may require extensive safety data and approval processes. The regulatory landscape for novel excipients like barium hydroxide can be complex and time-consuming, potentially slowing down the development and commercialization of new drug delivery technologies.
Existing Barium Hydroxide Formulation Strategies
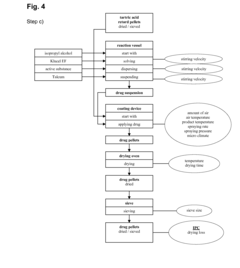
01 Use of barium hydroxide in controlled drug release formulations
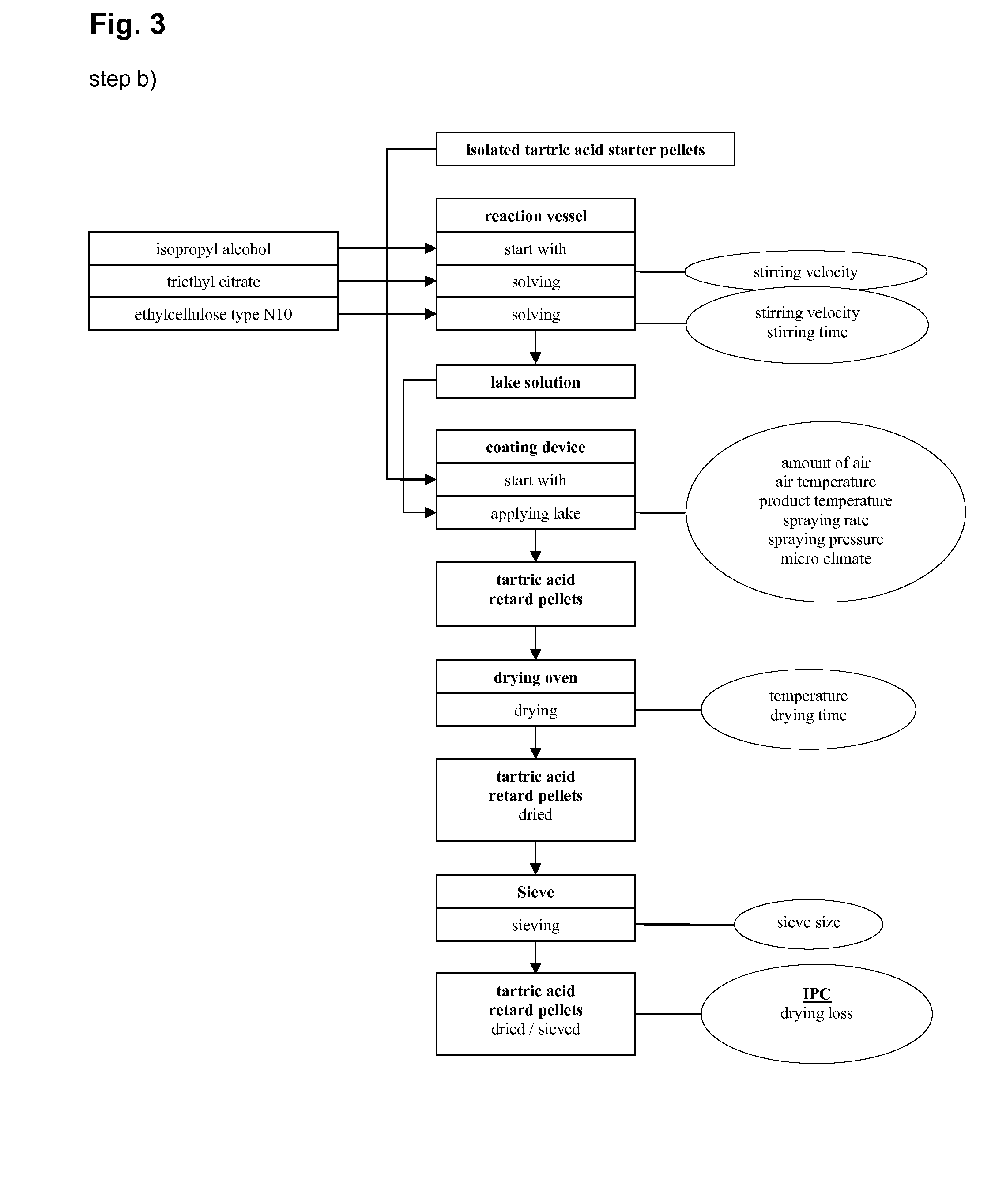
Barium hydroxide can be utilized in pharmaceutical formulations to control the release rate of drugs. Its alkaline properties can influence the dissolution of certain drug compounds, potentially allowing for sustained or delayed release profiles. This approach may be particularly useful for drugs that are pH-sensitive or require specific release kinetics.- Use of barium hydroxide in controlled drug release systems: Barium hydroxide can be utilized in controlled drug release formulations to modify the release rate of active pharmaceutical ingredients. Its alkaline nature can affect the solubility and dissolution of certain drugs, potentially allowing for sustained or delayed release profiles.
- Barium hydroxide as a pH modifier in pharmaceutical compositions: Barium hydroxide can serve as a pH modifier in drug formulations, helping to create an alkaline environment that may enhance the stability or solubility of certain drugs. This property can be exploited to optimize drug release kinetics in various dosage forms.
- Incorporation of barium hydroxide in matrix systems for drug delivery: Barium hydroxide can be incorporated into matrix systems, such as hydrogels or polymer-based formulations, to modulate drug release. Its interaction with the matrix material can influence the swelling, erosion, or degradation properties, thereby affecting the release profile of the encapsulated drug.
- Barium hydroxide in nanoparticle-based drug delivery systems: Barium hydroxide can be used in the synthesis or modification of nanoparticles for drug delivery applications. Its presence can affect the surface properties, charge, or porosity of nanoparticles, potentially influencing drug loading capacity and release characteristics.
- Barium hydroxide in transdermal drug delivery systems: Barium hydroxide may be employed in transdermal drug delivery formulations to enhance skin permeation or modify the release of drugs from patch or gel-based systems. Its alkaline nature could potentially affect the ionization state of certain drugs, influencing their transdermal absorption.
02 Barium hydroxide as a pH modifier in drug delivery systems
The incorporation of barium hydroxide in drug delivery systems can serve as a pH modifier. By adjusting the local pH environment, it can affect drug solubility, stability, and absorption. This property can be exploited to design targeted drug release mechanisms or to enhance the bioavailability of certain pharmaceutical compounds.Expand Specific Solutions03 Barium hydroxide in matrix formulations for sustained drug release
Barium hydroxide can be used in the development of matrix formulations for sustained drug release. When incorporated into polymer matrices, it can influence the swelling and erosion properties of the matrix, thereby modulating the release of the active pharmaceutical ingredient over an extended period.Expand Specific Solutions04 Interaction of barium hydroxide with other excipients for drug release
The interaction between barium hydroxide and other pharmaceutical excipients can be leveraged to create novel drug release mechanisms. These interactions may alter the physical or chemical properties of the formulation, leading to unique release profiles or improved stability of the drug product.Expand Specific Solutions05 Barium hydroxide in nanoparticle-based drug delivery systems
Barium hydroxide can play a role in the development of nanoparticle-based drug delivery systems. It may be used in the synthesis process of nanoparticles or as a component to modify their surface properties, potentially enhancing drug loading capacity, targeting ability, or controlled release characteristics.Expand Specific Solutions
Key Players in Pharmaceutical Excipient Industry
The controlled drug release market utilizing barium hydroxide is in a growth phase, with increasing demand for advanced drug delivery systems. The market size is expanding due to rising interest in targeted and sustained-release medications. Technologically, the field is progressing rapidly, with companies like Sunshine Lake Pharma, Lupin Ltd, and DURECT Corp. leading innovation. These firms are developing sophisticated formulations and delivery mechanisms, leveraging barium hydroxide's properties. Academic institutions such as Trinity College Dublin and the University of California are contributing significant research, bridging the gap between theoretical understanding and practical applications. The competitive landscape is diverse, with pharmaceutical giants like Boehringer Ingelheim and emerging players collaborating to advance this technology.
Boehringer Ingelheim International GmbH
Technical Solution: Boehringer Ingelheim has developed a novel controlled release formulation utilizing barium hydroxide as a pH-modifying agent. Their approach involves incorporating barium hydroxide into a matrix tablet containing the active pharmaceutical ingredient (API). As the tablet dissolves, barium hydroxide creates a localized alkaline microenvironment, which can modulate the solubility and release rate of pH-sensitive drugs[1]. This technology has shown particular promise for weakly basic drugs, where the alkaline conditions promote dissolution and absorption in the intestinal tract[2]. The company has also explored combining this approach with other release-controlling polymers to achieve tailored release profiles for different therapeutic needs[3].
Strengths: Enhances bioavailability of weakly basic drugs, allows for customizable release profiles. Weaknesses: May not be suitable for all types of drugs, potential for barium toxicity needs careful consideration.
DURECT Corp.
Technical Solution: DURECT Corporation has pioneered the SABER (Sucrose Acetate Isobutyrate Extended Release) technology, which incorporates barium hydroxide as a key component. In this system, barium hydroxide acts as a stabilizing agent and pH modifier within a biodegradable matrix. The SABER technology creates a depot formulation that, when injected subcutaneously, forms a viscous implant releasing the drug over an extended period[4]. Barium hydroxide helps maintain the stability of the formulation and can modulate the release rate of certain APIs. DURECT has successfully applied this technology to develop long-acting injectables for various therapeutic areas, including pain management and central nervous system disorders[5].
Strengths: Enables long-acting injectable formulations, reduces dosing frequency. Weaknesses: Limited to parenteral administration, may require specialized injection techniques.
Innovative Approaches in Barium Hydroxide Drug Release Mechanisms
Controlled release system and method for manufacturing the same
PatentWO2008022932A2
Innovation
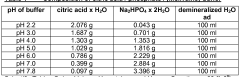
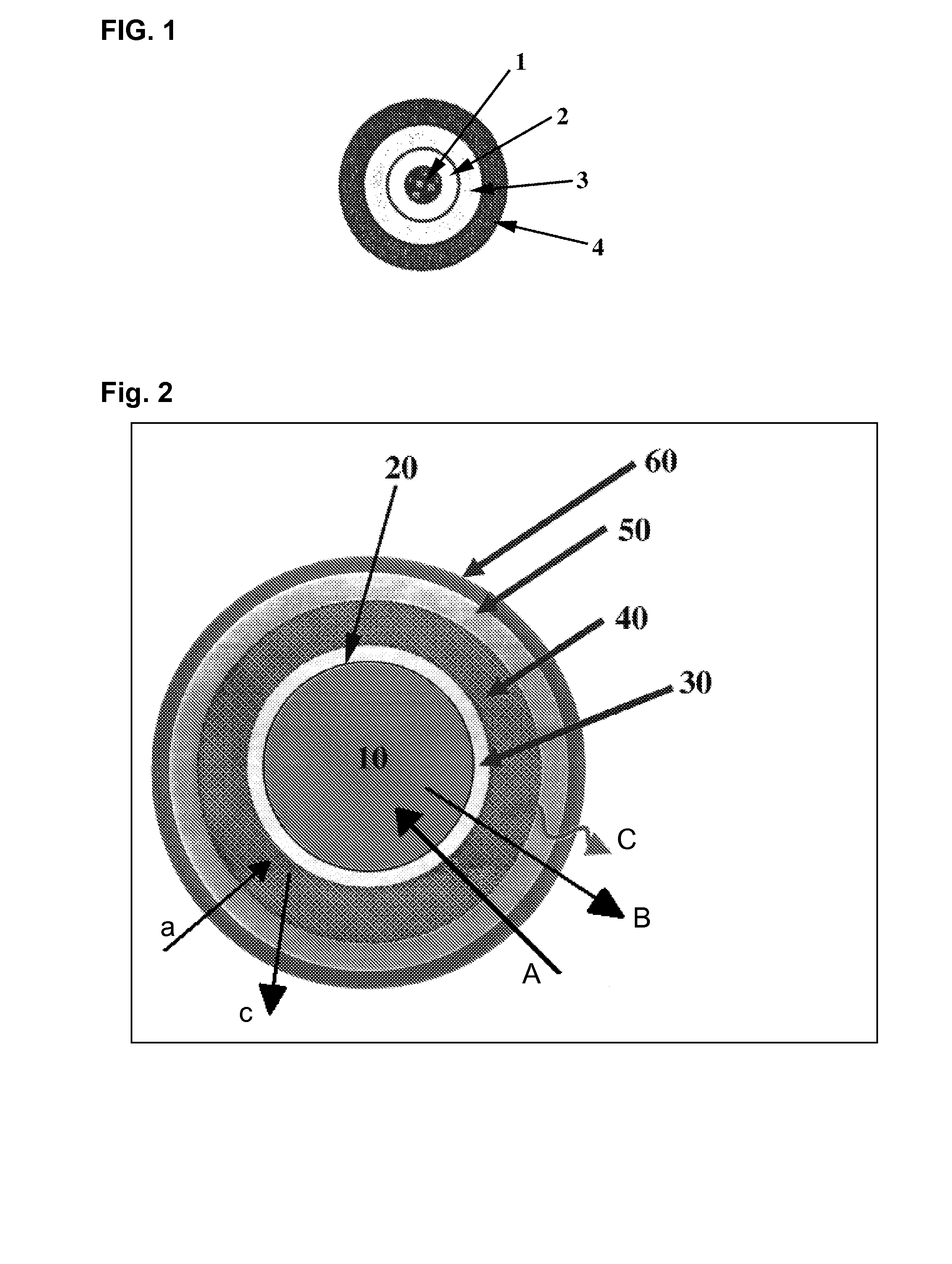
- A controlled release system comprising a core material with pH modifiers, an insulating layer, a first layer of water-insoluble polymers, a second layer with pH-dependent active substances, and an optional third layer of anionic polymers, which allows for pH-independent release profiles by spatially separating the pH modifier from the active substance, preventing undesirable interactions and stabilizing the formulation.
Controlled release system and method for manufacturing the same
PatentActiveUS20080069873A1
Innovation
- A controlled release system comprising a core material with pH modifiers, an insulating layer, a first layer of water-insoluble polymers, a second layer with pH-dependent active substances, and a third layer of anionic or non-ionic polymers, which allows for pH-independent release profiles by spatially separating the pH modifier from the active substance until administration.
Regulatory Considerations for Barium-Based Pharmaceutical Excipients
The regulatory landscape for barium-based pharmaceutical excipients is complex and multifaceted, requiring careful consideration by drug manufacturers and regulatory bodies alike. The use of barium hydroxide in controlled drug release formulations necessitates a thorough understanding of the applicable regulations and guidelines across different jurisdictions.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the safety and efficacy of pharmaceutical excipients. Barium-based excipients, including barium hydroxide, fall under the scrutiny of the FDA's Center for Drug Evaluation and Research (CDER). Manufacturers must demonstrate compliance with Good Manufacturing Practices (GMP) and provide comprehensive safety data to support the use of barium hydroxide in drug formulations.
The European Medicines Agency (EMA) has its own set of regulations governing the use of excipients in pharmaceutical products. The EMA's guidelines on excipient safety assessment and the European Pharmacopoeia monographs provide essential frameworks for evaluating the suitability of barium-based excipients. Manufacturers targeting the European market must align their development processes with these regulatory requirements.
Internationally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) offers guidelines that aim to harmonize regulatory approaches across different regions. The ICH Q3D guideline on elemental impurities is particularly relevant for barium-based excipients, as it sets limits for elemental impurities in drug products.
Regulatory bodies often require extensive toxicological studies to assess the safety profile of barium hydroxide when used as an excipient. These studies must address potential risks associated with long-term exposure, bioaccumulation, and interactions with other components of the drug formulation. The results of these studies are critical in determining acceptable limits for barium content in pharmaceutical products.
Environmental regulations also come into play when considering the use of barium-based excipients. Manufacturers must address concerns related to the disposal of barium-containing waste and potential environmental impacts. Compliance with local and international environmental standards is essential for obtaining regulatory approval.
As the pharmaceutical industry continues to innovate in controlled drug release technologies, regulatory bodies may need to adapt their guidelines to address emerging concerns and applications of barium-based excipients. This dynamic regulatory environment requires manufacturers to maintain ongoing dialogue with regulatory agencies and stay abreast of evolving requirements.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the safety and efficacy of pharmaceutical excipients. Barium-based excipients, including barium hydroxide, fall under the scrutiny of the FDA's Center for Drug Evaluation and Research (CDER). Manufacturers must demonstrate compliance with Good Manufacturing Practices (GMP) and provide comprehensive safety data to support the use of barium hydroxide in drug formulations.
The European Medicines Agency (EMA) has its own set of regulations governing the use of excipients in pharmaceutical products. The EMA's guidelines on excipient safety assessment and the European Pharmacopoeia monographs provide essential frameworks for evaluating the suitability of barium-based excipients. Manufacturers targeting the European market must align their development processes with these regulatory requirements.
Internationally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) offers guidelines that aim to harmonize regulatory approaches across different regions. The ICH Q3D guideline on elemental impurities is particularly relevant for barium-based excipients, as it sets limits for elemental impurities in drug products.
Regulatory bodies often require extensive toxicological studies to assess the safety profile of barium hydroxide when used as an excipient. These studies must address potential risks associated with long-term exposure, bioaccumulation, and interactions with other components of the drug formulation. The results of these studies are critical in determining acceptable limits for barium content in pharmaceutical products.
Environmental regulations also come into play when considering the use of barium-based excipients. Manufacturers must address concerns related to the disposal of barium-containing waste and potential environmental impacts. Compliance with local and international environmental standards is essential for obtaining regulatory approval.
As the pharmaceutical industry continues to innovate in controlled drug release technologies, regulatory bodies may need to adapt their guidelines to address emerging concerns and applications of barium-based excipients. This dynamic regulatory environment requires manufacturers to maintain ongoing dialogue with regulatory agencies and stay abreast of evolving requirements.
Safety and Toxicology Profile of Barium Hydroxide in Drug Formulations
The safety and toxicology profile of barium hydroxide in drug formulations is a critical aspect to consider when evaluating its potential use in controlled drug release systems. Barium hydroxide, while offering unique properties for drug delivery, presents several safety concerns that must be thoroughly assessed before its implementation in pharmaceutical applications.
Acute toxicity studies have shown that barium hydroxide can cause severe irritation and corrosion to the gastrointestinal tract if ingested in significant quantities. The alkaline nature of the compound can lead to chemical burns and tissue damage. Inhalation of barium hydroxide dust or aerosols may cause respiratory irritation and, in severe cases, pulmonary edema. Dermal exposure can result in skin irritation and burns, particularly with prolonged contact.
Chronic exposure to barium compounds, including barium hydroxide, has been associated with various health effects. Long-term ingestion of barium can lead to hypokalemia, which may cause cardiac arrhythmias and muscle weakness. Studies have also suggested potential effects on the nervous system, with some evidence of neurotoxicity in animal models. However, the specific long-term effects of barium hydroxide in controlled release formulations require further investigation.
The pharmacokinetics of barium hydroxide in drug formulations is an important consideration. While the compound itself is not intended for therapeutic use, its potential for systemic absorption and distribution must be evaluated. Research has shown that barium can be absorbed through the gastrointestinal tract, with subsequent distribution primarily to bone and muscle tissues. The elimination of barium is primarily through fecal excretion, with a smaller portion eliminated via urine.
Regulatory bodies, including the FDA and EMA, have established guidelines for the use of barium compounds in pharmaceutical products. These guidelines typically set strict limits on barium content and require thorough safety assessments. Manufacturers must demonstrate that the use of barium hydroxide in controlled release formulations does not pose unacceptable risks to patients. This often involves extensive toxicological studies and risk-benefit analyses.
To mitigate safety concerns, several strategies can be employed in the development of barium hydroxide-containing drug formulations. These may include encapsulation techniques to minimize direct contact between barium hydroxide and biological tissues, careful dosage control to ensure barium levels remain below toxic thresholds, and the use of protective coatings or matrices to modulate barium release. Additionally, the development of sensitive analytical methods for detecting and quantifying barium in biological samples is crucial for monitoring potential exposure and accumulation.
In conclusion, while barium hydroxide offers promising functionality in controlled drug release systems, its safety and toxicology profile necessitates careful consideration and rigorous testing. Balancing the potential benefits of improved drug delivery against the inherent risks of barium exposure remains a key challenge in the development of such formulations. Ongoing research and regulatory oversight will continue to shape the use of barium hydroxide in pharmaceutical applications, with a focus on ensuring patient safety while harnessing its unique properties for drug delivery innovation.
Acute toxicity studies have shown that barium hydroxide can cause severe irritation and corrosion to the gastrointestinal tract if ingested in significant quantities. The alkaline nature of the compound can lead to chemical burns and tissue damage. Inhalation of barium hydroxide dust or aerosols may cause respiratory irritation and, in severe cases, pulmonary edema. Dermal exposure can result in skin irritation and burns, particularly with prolonged contact.
Chronic exposure to barium compounds, including barium hydroxide, has been associated with various health effects. Long-term ingestion of barium can lead to hypokalemia, which may cause cardiac arrhythmias and muscle weakness. Studies have also suggested potential effects on the nervous system, with some evidence of neurotoxicity in animal models. However, the specific long-term effects of barium hydroxide in controlled release formulations require further investigation.
The pharmacokinetics of barium hydroxide in drug formulations is an important consideration. While the compound itself is not intended for therapeutic use, its potential for systemic absorption and distribution must be evaluated. Research has shown that barium can be absorbed through the gastrointestinal tract, with subsequent distribution primarily to bone and muscle tissues. The elimination of barium is primarily through fecal excretion, with a smaller portion eliminated via urine.
Regulatory bodies, including the FDA and EMA, have established guidelines for the use of barium compounds in pharmaceutical products. These guidelines typically set strict limits on barium content and require thorough safety assessments. Manufacturers must demonstrate that the use of barium hydroxide in controlled release formulations does not pose unacceptable risks to patients. This often involves extensive toxicological studies and risk-benefit analyses.
To mitigate safety concerns, several strategies can be employed in the development of barium hydroxide-containing drug formulations. These may include encapsulation techniques to minimize direct contact between barium hydroxide and biological tissues, careful dosage control to ensure barium levels remain below toxic thresholds, and the use of protective coatings or matrices to modulate barium release. Additionally, the development of sensitive analytical methods for detecting and quantifying barium in biological samples is crucial for monitoring potential exposure and accumulation.
In conclusion, while barium hydroxide offers promising functionality in controlled drug release systems, its safety and toxicology profile necessitates careful consideration and rigorous testing. Balancing the potential benefits of improved drug delivery against the inherent risks of barium exposure remains a key challenge in the development of such formulations. Ongoing research and regulatory oversight will continue to shape the use of barium hydroxide in pharmaceutical applications, with a focus on ensuring patient safety while harnessing its unique properties for drug delivery innovation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!