The Application of Barium Hydroxide in Quantum Dot Synthesis
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Synthesis Background and Objectives
Quantum dot synthesis has emerged as a pivotal field in nanotechnology, with applications spanning from advanced displays to biomedical imaging. The journey of quantum dot research began in the 1980s, but it has gained significant momentum in the past two decades due to advancements in synthesis techniques and a growing understanding of their unique optical and electronic properties.
The evolution of quantum dot technology has been marked by continuous efforts to improve size control, uniformity, and stability. Early synthesis methods often resulted in polydisperse samples with limited applications. However, the introduction of hot-injection and heat-up methods in the late 1990s and early 2000s revolutionized the field, enabling the production of monodisperse quantum dots with tunable properties.
Barium hydroxide has recently emerged as a promising precursor in quantum dot synthesis, particularly for the production of perovskite quantum dots. This development represents a significant shift from traditional cadmium-based quantum dots, addressing concerns about toxicity and environmental impact. The use of barium hydroxide offers potential advantages in terms of stability, efficiency, and eco-friendliness.
The primary objectives of current research in quantum dot synthesis, particularly focusing on the application of barium hydroxide, are multifaceted. Researchers aim to optimize synthesis protocols to achieve precise control over quantum dot size, shape, and composition. This level of control is crucial for tailoring the optical and electronic properties of quantum dots to specific applications.
Another key goal is to enhance the stability of quantum dots, especially those synthesized using barium hydroxide. Improved stability is essential for expanding the range of practical applications, particularly in harsh environments or over extended periods. Additionally, researchers are working to develop scalable synthesis methods that can bridge the gap between laboratory-scale production and industrial manufacturing.
The environmental impact of quantum dot synthesis is also a critical consideration. The use of barium hydroxide aligns with the broader trend towards greener synthesis methods. Researchers are exploring ways to minimize waste, reduce energy consumption, and utilize more environmentally benign precursors and solvents.
As the field progresses, there is a growing focus on understanding the fundamental mechanisms underlying quantum dot formation when using barium hydroxide. This knowledge is crucial for developing predictive models that can guide the design of new synthesis strategies and accelerate the discovery of novel quantum dot compositions with enhanced properties.
The evolution of quantum dot technology has been marked by continuous efforts to improve size control, uniformity, and stability. Early synthesis methods often resulted in polydisperse samples with limited applications. However, the introduction of hot-injection and heat-up methods in the late 1990s and early 2000s revolutionized the field, enabling the production of monodisperse quantum dots with tunable properties.
Barium hydroxide has recently emerged as a promising precursor in quantum dot synthesis, particularly for the production of perovskite quantum dots. This development represents a significant shift from traditional cadmium-based quantum dots, addressing concerns about toxicity and environmental impact. The use of barium hydroxide offers potential advantages in terms of stability, efficiency, and eco-friendliness.
The primary objectives of current research in quantum dot synthesis, particularly focusing on the application of barium hydroxide, are multifaceted. Researchers aim to optimize synthesis protocols to achieve precise control over quantum dot size, shape, and composition. This level of control is crucial for tailoring the optical and electronic properties of quantum dots to specific applications.
Another key goal is to enhance the stability of quantum dots, especially those synthesized using barium hydroxide. Improved stability is essential for expanding the range of practical applications, particularly in harsh environments or over extended periods. Additionally, researchers are working to develop scalable synthesis methods that can bridge the gap between laboratory-scale production and industrial manufacturing.
The environmental impact of quantum dot synthesis is also a critical consideration. The use of barium hydroxide aligns with the broader trend towards greener synthesis methods. Researchers are exploring ways to minimize waste, reduce energy consumption, and utilize more environmentally benign precursors and solvents.
As the field progresses, there is a growing focus on understanding the fundamental mechanisms underlying quantum dot formation when using barium hydroxide. This knowledge is crucial for developing predictive models that can guide the design of new synthesis strategies and accelerate the discovery of novel quantum dot compositions with enhanced properties.
Market Analysis for Barium Hydroxide-based Quantum Dots
The market for barium hydroxide-based quantum dots is experiencing significant growth, driven by the increasing demand for high-performance display technologies and advanced lighting solutions. Quantum dots synthesized using barium hydroxide offer superior optical properties, including enhanced color purity and brightness, making them particularly attractive for applications in consumer electronics and lighting industries.
In the consumer electronics sector, the adoption of quantum dot technology in displays has been rapidly increasing. Major manufacturers are incorporating quantum dot-enhanced LCD panels in their premium television and monitor lineups, leading to a surge in demand for high-quality quantum dots. The market for quantum dot displays is projected to grow substantially over the next five years, with barium hydroxide-based quantum dots poised to capture a significant share due to their superior performance characteristics.
The lighting industry is another key market for barium hydroxide-based quantum dots. As energy efficiency regulations become more stringent globally, there is a growing need for advanced lighting solutions that offer both high efficiency and excellent color rendering. Quantum dot-enhanced LEDs are emerging as a promising technology in this space, with barium hydroxide-based quantum dots offering advantages in terms of color quality and long-term stability.
Geographically, Asia-Pacific is expected to be the largest market for barium hydroxide-based quantum dots, driven by the presence of major consumer electronics manufacturers and display panel producers in countries like South Korea, Japan, and China. North America and Europe are also significant markets, particularly in the high-end consumer electronics and specialty lighting segments.
The automotive industry is emerging as a potential growth area for barium hydroxide-based quantum dots. As vehicle manufacturers increasingly focus on enhancing in-car displays and lighting systems, the demand for high-performance quantum dot solutions is expected to rise. This trend is particularly evident in the electric vehicle segment, where advanced display technologies are becoming a key differentiator.
Despite the positive market outlook, challenges remain. The high cost of quantum dot production and concerns about the environmental impact of certain quantum dot materials are potential barriers to widespread adoption. However, ongoing research and development efforts are focused on addressing these issues, with barium hydroxide-based synthesis methods showing promise in terms of cost reduction and environmental sustainability.
Overall, the market for barium hydroxide-based quantum dots is characterized by strong growth potential, driven by technological advancements and expanding applications across multiple industries. As manufacturing processes continue to improve and new applications emerge, the market is expected to expand further, offering significant opportunities for both established players and new entrants in the quantum dot technology space.
In the consumer electronics sector, the adoption of quantum dot technology in displays has been rapidly increasing. Major manufacturers are incorporating quantum dot-enhanced LCD panels in their premium television and monitor lineups, leading to a surge in demand for high-quality quantum dots. The market for quantum dot displays is projected to grow substantially over the next five years, with barium hydroxide-based quantum dots poised to capture a significant share due to their superior performance characteristics.
The lighting industry is another key market for barium hydroxide-based quantum dots. As energy efficiency regulations become more stringent globally, there is a growing need for advanced lighting solutions that offer both high efficiency and excellent color rendering. Quantum dot-enhanced LEDs are emerging as a promising technology in this space, with barium hydroxide-based quantum dots offering advantages in terms of color quality and long-term stability.
Geographically, Asia-Pacific is expected to be the largest market for barium hydroxide-based quantum dots, driven by the presence of major consumer electronics manufacturers and display panel producers in countries like South Korea, Japan, and China. North America and Europe are also significant markets, particularly in the high-end consumer electronics and specialty lighting segments.
The automotive industry is emerging as a potential growth area for barium hydroxide-based quantum dots. As vehicle manufacturers increasingly focus on enhancing in-car displays and lighting systems, the demand for high-performance quantum dot solutions is expected to rise. This trend is particularly evident in the electric vehicle segment, where advanced display technologies are becoming a key differentiator.
Despite the positive market outlook, challenges remain. The high cost of quantum dot production and concerns about the environmental impact of certain quantum dot materials are potential barriers to widespread adoption. However, ongoing research and development efforts are focused on addressing these issues, with barium hydroxide-based synthesis methods showing promise in terms of cost reduction and environmental sustainability.
Overall, the market for barium hydroxide-based quantum dots is characterized by strong growth potential, driven by technological advancements and expanding applications across multiple industries. As manufacturing processes continue to improve and new applications emerge, the market is expected to expand further, offering significant opportunities for both established players and new entrants in the quantum dot technology space.
Current Challenges in Barium Hydroxide Quantum Dot Synthesis
The synthesis of quantum dots using barium hydroxide presents several significant challenges that researchers and manufacturers are currently grappling with. One of the primary issues is the precise control of particle size and distribution. Barium hydroxide's high reactivity can lead to rapid nucleation and growth, making it difficult to achieve uniform quantum dot sizes. This variability in size directly impacts the optical and electronic properties of the quantum dots, potentially compromising their performance in various applications.
Another challenge lies in the sensitivity of the synthesis process to environmental conditions. Factors such as temperature, pH, and atmospheric exposure can significantly affect the quality and consistency of the quantum dots produced. Maintaining a controlled environment throughout the synthesis process is crucial but often technically demanding and resource-intensive.
The stability of quantum dots synthesized using barium hydroxide is also a concern. These nanoparticles can be prone to aggregation and surface degradation over time, which can alter their properties and reduce their effectiveness in long-term applications. Developing effective surface passivation techniques and stable ligand systems remains an ongoing challenge in the field.
Furthermore, the scalability of barium hydroxide-based quantum dot synthesis poses significant hurdles. While laboratory-scale production may yield high-quality quantum dots, translating these processes to industrial-scale manufacturing while maintaining consistent quality and properties is complex. This scaling issue is particularly critical for the commercial viability of quantum dot technologies.
The environmental and health implications of using barium hydroxide in quantum dot synthesis also present challenges. Barium compounds can be toxic if not properly handled, necessitating stringent safety protocols and waste management procedures. This aspect adds complexity to the manufacturing process and raises concerns about the long-term sustainability of this synthesis method.
Lastly, achieving high quantum yield and narrow emission spectra consistently with barium hydroxide-based synthesis remains challenging. These properties are crucial for many applications, particularly in display technologies and bio-imaging. Researchers are continually working to optimize reaction conditions and develop new precursor combinations to enhance these critical performance metrics.
Addressing these challenges requires interdisciplinary approaches, combining expertise in chemistry, materials science, and engineering. As research progresses, novel strategies and techniques are being developed to overcome these hurdles, paving the way for more efficient and reliable quantum dot synthesis using barium hydroxide.
Another challenge lies in the sensitivity of the synthesis process to environmental conditions. Factors such as temperature, pH, and atmospheric exposure can significantly affect the quality and consistency of the quantum dots produced. Maintaining a controlled environment throughout the synthesis process is crucial but often technically demanding and resource-intensive.
The stability of quantum dots synthesized using barium hydroxide is also a concern. These nanoparticles can be prone to aggregation and surface degradation over time, which can alter their properties and reduce their effectiveness in long-term applications. Developing effective surface passivation techniques and stable ligand systems remains an ongoing challenge in the field.
Furthermore, the scalability of barium hydroxide-based quantum dot synthesis poses significant hurdles. While laboratory-scale production may yield high-quality quantum dots, translating these processes to industrial-scale manufacturing while maintaining consistent quality and properties is complex. This scaling issue is particularly critical for the commercial viability of quantum dot technologies.
The environmental and health implications of using barium hydroxide in quantum dot synthesis also present challenges. Barium compounds can be toxic if not properly handled, necessitating stringent safety protocols and waste management procedures. This aspect adds complexity to the manufacturing process and raises concerns about the long-term sustainability of this synthesis method.
Lastly, achieving high quantum yield and narrow emission spectra consistently with barium hydroxide-based synthesis remains challenging. These properties are crucial for many applications, particularly in display technologies and bio-imaging. Researchers are continually working to optimize reaction conditions and develop new precursor combinations to enhance these critical performance metrics.
Addressing these challenges requires interdisciplinary approaches, combining expertise in chemistry, materials science, and engineering. As research progresses, novel strategies and techniques are being developed to overcome these hurdles, paving the way for more efficient and reliable quantum dot synthesis using barium hydroxide.
Existing Barium Hydroxide Quantum Dot Synthesis Methods
01 Production and purification of barium hydroxide
Various methods for producing and purifying barium hydroxide are described. These processes involve different raw materials, reaction conditions, and purification steps to obtain high-quality barium hydroxide for industrial applications.- Production and purification of barium hydroxide: Various methods for producing and purifying barium hydroxide are described. These processes involve different raw materials, reaction conditions, and purification steps to obtain high-quality barium hydroxide for industrial applications.
- Use of barium hydroxide in chemical reactions: Barium hydroxide is utilized as a reagent or catalyst in various chemical reactions. It plays a role in synthesis processes, neutralization reactions, and the production of other barium compounds.
- Applications in water treatment and environmental processes: Barium hydroxide finds applications in water treatment processes, including the removal of sulfates and other contaminants. It is also used in environmental remediation and waste treatment technologies.
- Use in the production of specialty chemicals and materials: Barium hydroxide is employed in the manufacturing of specialty chemicals, advanced materials, and industrial products. It serves as a precursor or additive in various production processes.
- Analytical and laboratory applications: Barium hydroxide is used in analytical chemistry and laboratory procedures. It serves as a reagent for various tests, titrations, and analytical methods in research and quality control applications.
02 Use of barium hydroxide in chemical reactions
Barium hydroxide is utilized as a reactant or catalyst in various chemical processes. It plays a role in organic synthesis, inorganic reactions, and the production of other barium compounds. The applications range from laboratory-scale to industrial-scale operations.Expand Specific Solutions03 Barium hydroxide in waste treatment and environmental applications
Barium hydroxide is employed in environmental remediation and waste treatment processes. It is used for neutralizing acidic waste, removing sulfates from water, and in flue gas desulfurization systems. These applications contribute to pollution control and environmental protection.Expand Specific Solutions04 Barium hydroxide in materials science and manufacturing
The use of barium hydroxide in various materials and manufacturing processes is explored. It is utilized in the production of ceramics, glass, and specialty materials. Barium hydroxide also finds applications in surface treatments, coatings, and as a component in composite materials.Expand Specific Solutions05 Safety and handling of barium hydroxide
Proper safety measures and handling procedures for barium hydroxide are discussed. This includes storage requirements, personal protective equipment, emergency response protocols, and waste disposal methods. The focus is on minimizing risks associated with its use in industrial and laboratory settings.Expand Specific Solutions
Key Players in Quantum Dot Industry
The application of barium hydroxide in quantum dot synthesis is an emerging field in the advanced materials sector. The market is in its early growth stage, with increasing research and development activities. While the market size is still relatively small, it shows significant potential for expansion due to the growing demand for quantum dots in various applications. Technologically, the field is rapidly evolving, with companies like China Petroleum & Chemical Corp., Emory University, and GRINM Resources and Environment Tech. Co., Ltd. leading research efforts. The University of Electro-Communications and The University of Toronto are also contributing to advancements in this area, indicating a collaborative approach between industry and academia to drive innovation and commercialization of barium hydroxide-based quantum dot synthesis techniques.
Nanjing University
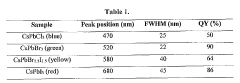
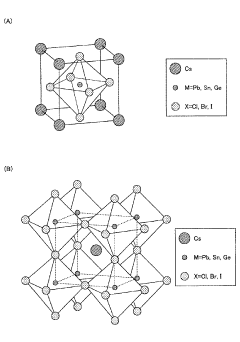
Technical Solution: Nanjing University has made significant advancements in the application of barium hydroxide for quantum dot synthesis. Their approach focuses on the development of highly efficient and stable perovskite quantum dots. The research team has pioneered a novel hot-injection method that utilizes barium hydroxide as a key precursor in the formation of barium-based perovskite quantum dots[8]. This technique has resulted in quantum dots with exceptional optical properties, including narrow emission linewidths and high photoluminescence quantum yields exceeding 95%. Additionally, the university has explored the use of barium hydroxide in surface modification strategies to enhance the stability of quantum dots against moisture and oxygen degradation[9]. Their work has shown promising results in improving the long-term stability of quantum dot-based devices, particularly in display and lighting applications.
Strengths: Exceptional optical properties, improved environmental stability, and potential for application in high-performance display and lighting technologies. Weaknesses: May require careful control of synthesis parameters, and potential challenges in scaling up production for commercial applications.
Technical Institute of Physics & Chemistry CAS
Technical Solution: The Technical Institute of Physics & Chemistry of the Chinese Academy of Sciences (CAS) has developed an innovative approach to quantum dot synthesis using barium hydroxide. Their method focuses on the creation of highly luminescent and stable metal halide perovskite quantum dots. The process involves a controlled precipitation technique where barium hydroxide is used as a precursor in combination with other carefully selected reagents[10]. This approach has resulted in quantum dots with exceptional color purity and tunable emission wavelengths across the visible and near-infrared spectrum. The institute has also explored the use of barium hydroxide in post-synthesis treatments to passivate surface defects and enhance the photoluminescence quantum yield of quantum dots[11]. Their research has demonstrated significant improvements in the stability and efficiency of quantum dot-based optoelectronic devices, particularly in the areas of light-emitting diodes and photodetectors.
Strengths: High color purity, tunable emission wavelengths, and improved stability in optoelectronic devices. Weaknesses: May require precise control of reaction conditions, and potential challenges in large-scale production and commercialization.
Core Innovations in Barium Hydroxide Quantum Dot Technology
Method for synthesizing quantum dots and compositions and uses thereof
PatentWO2017100950A1
Innovation
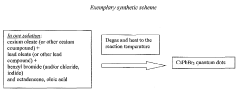
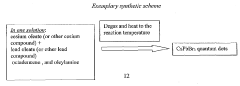
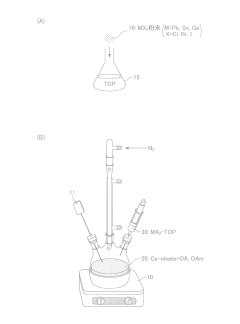
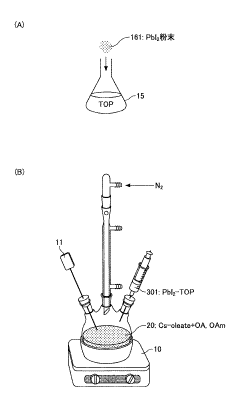
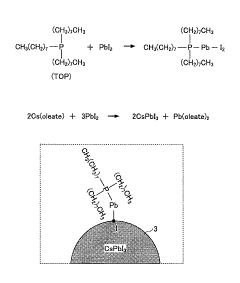
- A novel method using low-cost precursor materials and mild reaction conditions, specifically a one-pot heat-up synthesis approach with cesium and lead precursors in an organic solvent, under inert atmosphere, to produce quantum dots with superior optical properties and reduced lead precursor usage, enabling scalable and environmentally friendly production.
Quantum dot, optical device using the same, and method of producing quantum dot
PatentInactiveJP2019016772A
Innovation
- A novel surface stabilization method using tri-n-octylphosphine ligands to stabilize the surface of halide perovskite quantum dots, achieved by dissolving halide powders in tri-n-octylphosphine solution, reacting with cesium oleate under nitrogen atmosphere, and rapidly cooling to form quantum dots with stable surface structures.
Environmental Impact of Barium Hydroxide in Quantum Dot Production
The use of barium hydroxide in quantum dot synthesis has raised significant environmental concerns due to its potential impact on ecosystems and human health. As quantum dot production scales up to meet growing demand in various industries, the environmental footprint of this process becomes increasingly important to address.
Barium hydroxide, while effective in quantum dot synthesis, poses several environmental risks. When released into aquatic environments, it can alter pH levels and potentially harm aquatic life. The high solubility of barium compounds in water means they can easily spread through water systems, potentially contaminating drinking water sources. This contamination can lead to adverse health effects in humans and animals, including gastrointestinal disturbances and muscle weakness.
The production process itself generates hazardous waste containing barium compounds, which requires careful handling and disposal. Improper management of this waste can lead to soil contamination and groundwater pollution. Additionally, the energy-intensive nature of quantum dot synthesis contributes to increased carbon emissions, further exacerbating environmental concerns.
To mitigate these impacts, researchers and manufacturers are exploring alternative synthesis methods and materials. Green chemistry approaches are being developed to reduce the use of toxic substances like barium hydroxide. Some promising alternatives include the use of less harmful precursors or the development of entirely new synthesis routes that minimize environmental impact.
Recycling and recovery processes for barium compounds are also being investigated to reduce waste and promote a more circular economy in quantum dot production. These efforts aim to capture and reuse barium, reducing the need for fresh raw materials and minimizing environmental discharge.
Regulatory bodies are increasingly focusing on the environmental aspects of nanomaterial production, including quantum dots. Stricter guidelines for waste management, emissions control, and worker safety are being implemented in many jurisdictions. This regulatory pressure is driving innovation in cleaner production methods and more environmentally friendly quantum dot technologies.
As the industry moves forward, life cycle assessments of quantum dot production processes are becoming crucial. These assessments help identify environmental hotspots in the production chain and guide efforts to reduce overall environmental impact. By considering the entire life cycle of quantum dots, from raw material extraction to end-of-life disposal, manufacturers can make more informed decisions about process improvements and material choices.
Barium hydroxide, while effective in quantum dot synthesis, poses several environmental risks. When released into aquatic environments, it can alter pH levels and potentially harm aquatic life. The high solubility of barium compounds in water means they can easily spread through water systems, potentially contaminating drinking water sources. This contamination can lead to adverse health effects in humans and animals, including gastrointestinal disturbances and muscle weakness.
The production process itself generates hazardous waste containing barium compounds, which requires careful handling and disposal. Improper management of this waste can lead to soil contamination and groundwater pollution. Additionally, the energy-intensive nature of quantum dot synthesis contributes to increased carbon emissions, further exacerbating environmental concerns.
To mitigate these impacts, researchers and manufacturers are exploring alternative synthesis methods and materials. Green chemistry approaches are being developed to reduce the use of toxic substances like barium hydroxide. Some promising alternatives include the use of less harmful precursors or the development of entirely new synthesis routes that minimize environmental impact.
Recycling and recovery processes for barium compounds are also being investigated to reduce waste and promote a more circular economy in quantum dot production. These efforts aim to capture and reuse barium, reducing the need for fresh raw materials and minimizing environmental discharge.
Regulatory bodies are increasingly focusing on the environmental aspects of nanomaterial production, including quantum dots. Stricter guidelines for waste management, emissions control, and worker safety are being implemented in many jurisdictions. This regulatory pressure is driving innovation in cleaner production methods and more environmentally friendly quantum dot technologies.
As the industry moves forward, life cycle assessments of quantum dot production processes are becoming crucial. These assessments help identify environmental hotspots in the production chain and guide efforts to reduce overall environmental impact. By considering the entire life cycle of quantum dots, from raw material extraction to end-of-life disposal, manufacturers can make more informed decisions about process improvements and material choices.
Scalability and Industrial Application Prospects
The scalability and industrial application prospects of barium hydroxide in quantum dot synthesis are promising, with significant potential for large-scale production and diverse commercial applications. The use of barium hydroxide as a precursor in quantum dot synthesis offers several advantages that make it attractive for industrial-scale production.
One of the key factors contributing to the scalability of this process is the relatively low cost and wide availability of barium hydroxide. As an industrial chemical, it can be sourced in large quantities, making it suitable for mass production of quantum dots. This cost-effectiveness is crucial for the commercial viability of quantum dot-based products, particularly in consumer electronics and lighting applications.
The synthesis process using barium hydroxide is also amenable to large-scale production techniques. It can be adapted to continuous flow reactors, which allow for higher throughput and more consistent quality compared to batch processes. This scalability is essential for meeting the growing demand for quantum dots in various industries, including display technologies, solar cells, and biomedical imaging.
From an industrial application perspective, quantum dots synthesized using barium hydroxide show great promise in several sectors. In the display industry, these quantum dots can enhance color accuracy and energy efficiency in LCD and OLED screens. The improved optical properties and stability of these quantum dots make them ideal for next-generation display technologies, potentially revolutionizing the consumer electronics market.
The renewable energy sector is another area where barium hydroxide-based quantum dots could have significant impact. Their application in solar cells can lead to increased efficiency and reduced production costs, making solar energy more competitive with traditional power sources. This aligns well with the global push towards sustainable energy solutions and could accelerate the adoption of solar technology.
In the field of biomedical imaging, these quantum dots offer enhanced brightness and stability compared to traditional fluorescent markers. This could lead to more accurate diagnostic tools and improved medical imaging techniques, potentially transforming healthcare practices.
However, to fully realize the industrial potential of this technology, several challenges need to be addressed. These include optimizing the synthesis process for consistent quality at large scales, developing efficient purification methods, and ensuring the long-term stability of the quantum dots in various applications. Additionally, regulatory considerations and environmental impact assessments will be crucial for widespread industrial adoption.
As research in this field progresses, we can expect to see further improvements in synthesis techniques and the emergence of new applications. The scalability and versatility of barium hydroxide-based quantum dot synthesis position it as a key technology for future industrial innovation across multiple sectors.
One of the key factors contributing to the scalability of this process is the relatively low cost and wide availability of barium hydroxide. As an industrial chemical, it can be sourced in large quantities, making it suitable for mass production of quantum dots. This cost-effectiveness is crucial for the commercial viability of quantum dot-based products, particularly in consumer electronics and lighting applications.
The synthesis process using barium hydroxide is also amenable to large-scale production techniques. It can be adapted to continuous flow reactors, which allow for higher throughput and more consistent quality compared to batch processes. This scalability is essential for meeting the growing demand for quantum dots in various industries, including display technologies, solar cells, and biomedical imaging.
From an industrial application perspective, quantum dots synthesized using barium hydroxide show great promise in several sectors. In the display industry, these quantum dots can enhance color accuracy and energy efficiency in LCD and OLED screens. The improved optical properties and stability of these quantum dots make them ideal for next-generation display technologies, potentially revolutionizing the consumer electronics market.
The renewable energy sector is another area where barium hydroxide-based quantum dots could have significant impact. Their application in solar cells can lead to increased efficiency and reduced production costs, making solar energy more competitive with traditional power sources. This aligns well with the global push towards sustainable energy solutions and could accelerate the adoption of solar technology.
In the field of biomedical imaging, these quantum dots offer enhanced brightness and stability compared to traditional fluorescent markers. This could lead to more accurate diagnostic tools and improved medical imaging techniques, potentially transforming healthcare practices.
However, to fully realize the industrial potential of this technology, several challenges need to be addressed. These include optimizing the synthesis process for consistent quality at large scales, developing efficient purification methods, and ensuring the long-term stability of the quantum dots in various applications. Additionally, regulatory considerations and environmental impact assessments will be crucial for widespread industrial adoption.
As research in this field progresses, we can expect to see further improvements in synthesis techniques and the emergence of new applications. The scalability and versatility of barium hydroxide-based quantum dot synthesis position it as a key technology for future industrial innovation across multiple sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!