Borosilicate Glass in Drug Delivery Systems
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Borosilicate Glass in Drug Delivery: Background and Objectives
Borosilicate glass has emerged as a pivotal material in the field of drug delivery systems, offering unique properties that make it particularly suitable for pharmaceutical applications. The development of this specialized glass can be traced back to the late 19th century when German glassmaker Otto Schott first produced it. Since then, borosilicate glass has undergone significant advancements, evolving to meet the stringent requirements of the pharmaceutical industry.
The primary objective of researching borosilicate glass in drug delivery systems is to enhance the efficacy, safety, and stability of pharmaceutical products. This glass type is renowned for its exceptional chemical resistance, low thermal expansion coefficient, and high durability, making it an ideal candidate for drug containment and delivery. These properties ensure that the glass does not interact with the drug formulations, maintaining their integrity over extended periods.
In recent years, the pharmaceutical industry has witnessed a growing demand for advanced drug delivery systems that can improve patient compliance and treatment outcomes. This trend has led to increased interest in exploring the potential of borosilicate glass in various drug delivery applications, including parenteral packaging, implantable devices, and controlled release systems.
The evolution of borosilicate glass technology in drug delivery has been driven by several factors. These include the need for materials that can withstand sterilization processes, protect sensitive drug formulations from light and moisture, and provide precise dosing capabilities. Additionally, the push towards personalized medicine and the development of complex biopharmaceuticals have further emphasized the importance of advanced packaging and delivery materials.
As research in this field progresses, scientists and engineers are focusing on optimizing the composition and surface properties of borosilicate glass to enhance its performance in drug delivery applications. This includes developing coatings that can improve drug stability, reduce protein adsorption, and control drug release kinetics. Furthermore, efforts are being made to integrate smart technologies into borosilicate glass-based drug delivery systems, enabling real-time monitoring and controlled release of medications.
The objectives of current research initiatives encompass several key areas. These include improving the biocompatibility of borosilicate glass for long-term implantable devices, developing novel surface modification techniques to enhance drug loading and release profiles, and exploring the potential of borosilicate glass in targeted drug delivery systems. Additionally, researchers are investigating ways to combine borosilicate glass with other materials to create hybrid systems that leverage the strengths of multiple components.
The primary objective of researching borosilicate glass in drug delivery systems is to enhance the efficacy, safety, and stability of pharmaceutical products. This glass type is renowned for its exceptional chemical resistance, low thermal expansion coefficient, and high durability, making it an ideal candidate for drug containment and delivery. These properties ensure that the glass does not interact with the drug formulations, maintaining their integrity over extended periods.
In recent years, the pharmaceutical industry has witnessed a growing demand for advanced drug delivery systems that can improve patient compliance and treatment outcomes. This trend has led to increased interest in exploring the potential of borosilicate glass in various drug delivery applications, including parenteral packaging, implantable devices, and controlled release systems.
The evolution of borosilicate glass technology in drug delivery has been driven by several factors. These include the need for materials that can withstand sterilization processes, protect sensitive drug formulations from light and moisture, and provide precise dosing capabilities. Additionally, the push towards personalized medicine and the development of complex biopharmaceuticals have further emphasized the importance of advanced packaging and delivery materials.
As research in this field progresses, scientists and engineers are focusing on optimizing the composition and surface properties of borosilicate glass to enhance its performance in drug delivery applications. This includes developing coatings that can improve drug stability, reduce protein adsorption, and control drug release kinetics. Furthermore, efforts are being made to integrate smart technologies into borosilicate glass-based drug delivery systems, enabling real-time monitoring and controlled release of medications.
The objectives of current research initiatives encompass several key areas. These include improving the biocompatibility of borosilicate glass for long-term implantable devices, developing novel surface modification techniques to enhance drug loading and release profiles, and exploring the potential of borosilicate glass in targeted drug delivery systems. Additionally, researchers are investigating ways to combine borosilicate glass with other materials to create hybrid systems that leverage the strengths of multiple components.
Market Analysis for Pharmaceutical Packaging
The pharmaceutical packaging market has witnessed significant growth in recent years, driven by the increasing demand for drug delivery systems that ensure product safety, efficacy, and patient compliance. Borosilicate glass, with its unique properties, has emerged as a crucial material in this sector, particularly for packaging sensitive drugs and biologics.
The global pharmaceutical packaging market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many other industries. This growth is attributed to several factors, including the rising prevalence of chronic diseases, the aging population, and the increasing focus on personalized medicine. These trends have led to a surge in demand for advanced drug delivery systems, where borosilicate glass plays a vital role.
Borosilicate glass offers numerous advantages in pharmaceutical packaging, making it a preferred choice for many applications. Its chemical inertness ensures minimal interaction with the packaged drugs, preserving their efficacy and extending shelf life. The material's excellent thermal resistance allows for sterilization processes without compromising the integrity of the packaging. Additionally, its transparency enables visual inspection of the contents, a critical feature for quality control in pharmaceutical manufacturing.
The market for borosilicate glass in drug delivery systems is segmented based on various factors, including product type, drug type, and region. Vials, ampoules, and prefilled syringes are among the most common borosilicate glass products used in pharmaceutical packaging. The demand for these products varies across different regions, with developed markets showing a higher adoption rate of advanced drug delivery systems.
Emerging markets present significant growth opportunities for borosilicate glass in pharmaceutical packaging. As healthcare infrastructure improves and regulatory standards become more stringent in these regions, the demand for high-quality packaging materials is expected to rise. This trend is further supported by the increasing focus on biosimilars and generic drugs in developing countries, which often require specialized packaging solutions.
However, the market faces challenges, including competition from alternative materials such as plastic and concerns about the environmental impact of glass production. Manufacturers are addressing these issues through innovations in lightweight glass formulations and improved recycling processes. The industry is also witnessing a shift towards smart packaging solutions, incorporating technologies like RFID and NFC for improved traceability and patient engagement.
In conclusion, the market analysis for pharmaceutical packaging, particularly focusing on borosilicate glass in drug delivery systems, reveals a sector with strong growth potential. The material's unique properties align well with the industry's needs for safety, efficacy, and quality. As the pharmaceutical landscape continues to evolve, borosilicate glass is expected to maintain its crucial role in drug delivery systems, adapting to new challenges and opportunities in the market.
The global pharmaceutical packaging market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many other industries. This growth is attributed to several factors, including the rising prevalence of chronic diseases, the aging population, and the increasing focus on personalized medicine. These trends have led to a surge in demand for advanced drug delivery systems, where borosilicate glass plays a vital role.
Borosilicate glass offers numerous advantages in pharmaceutical packaging, making it a preferred choice for many applications. Its chemical inertness ensures minimal interaction with the packaged drugs, preserving their efficacy and extending shelf life. The material's excellent thermal resistance allows for sterilization processes without compromising the integrity of the packaging. Additionally, its transparency enables visual inspection of the contents, a critical feature for quality control in pharmaceutical manufacturing.
The market for borosilicate glass in drug delivery systems is segmented based on various factors, including product type, drug type, and region. Vials, ampoules, and prefilled syringes are among the most common borosilicate glass products used in pharmaceutical packaging. The demand for these products varies across different regions, with developed markets showing a higher adoption rate of advanced drug delivery systems.
Emerging markets present significant growth opportunities for borosilicate glass in pharmaceutical packaging. As healthcare infrastructure improves and regulatory standards become more stringent in these regions, the demand for high-quality packaging materials is expected to rise. This trend is further supported by the increasing focus on biosimilars and generic drugs in developing countries, which often require specialized packaging solutions.
However, the market faces challenges, including competition from alternative materials such as plastic and concerns about the environmental impact of glass production. Manufacturers are addressing these issues through innovations in lightweight glass formulations and improved recycling processes. The industry is also witnessing a shift towards smart packaging solutions, incorporating technologies like RFID and NFC for improved traceability and patient engagement.
In conclusion, the market analysis for pharmaceutical packaging, particularly focusing on borosilicate glass in drug delivery systems, reveals a sector with strong growth potential. The material's unique properties align well with the industry's needs for safety, efficacy, and quality. As the pharmaceutical landscape continues to evolve, borosilicate glass is expected to maintain its crucial role in drug delivery systems, adapting to new challenges and opportunities in the market.
Current Challenges in Borosilicate Glass for Drug Delivery
Despite the widespread use of borosilicate glass in drug delivery systems, several challenges persist that hinder its optimal performance and limit its applications. One of the primary concerns is the potential for glass delamination, particularly in high-pH formulations. This phenomenon can lead to the formation of glass flakes or lamellae, which may compromise drug stability and patient safety. The risk of delamination increases with prolonged storage times and exposure to certain drug formulations, necessitating careful consideration in packaging design and stability testing protocols.
Another significant challenge is the interaction between glass surfaces and protein-based drugs. Protein adsorption onto glass surfaces can result in loss of drug potency and potential immunogenicity issues. This is particularly problematic for biopharmaceuticals, which are increasingly prevalent in modern drug development. The need for surface treatments or coatings to mitigate protein adsorption while maintaining the integrity of the glass container presents a complex engineering challenge.
The variability in glass composition and surface properties across different manufacturers and batches poses challenges for standardization and quality control. Inconsistencies in the glass surface can lead to differences in drug-container interactions, potentially affecting drug stability and efficacy. This variability necessitates rigorous testing and characterization of glass containers, adding complexity and cost to the drug development and manufacturing processes.
Extractables and leachables from borosilicate glass containers remain a concern, particularly for sensitive drug formulations. While borosilicate glass is generally considered inert, trace amounts of elements such as silicon, boron, and sodium can leach into the drug product over time. This can potentially alter the drug's pH, stability, or even its pharmacological properties. Developing methods to minimize and accurately predict leachables over the shelf life of a drug product is an ongoing challenge for the industry.
The mechanical strength of borosilicate glass, while generally good, can be compromised during manufacturing, transportation, or handling. Microscopic flaws or stress points in the glass can lead to breakage, posing risks to both manufacturing personnel and end-users. Improving the resilience of glass containers without compromising their chemical inertness or transparency is an area of active research and development.
Lastly, the environmental impact of glass production and disposal is becoming increasingly important in the context of sustainability goals. While glass is recyclable, the specialized nature of pharmaceutical glass often precludes its inclusion in standard recycling streams. Developing more sustainable production methods and end-of-life solutions for borosilicate glass used in drug delivery systems is a growing challenge that intersects with broader environmental concerns in the pharmaceutical industry.
Another significant challenge is the interaction between glass surfaces and protein-based drugs. Protein adsorption onto glass surfaces can result in loss of drug potency and potential immunogenicity issues. This is particularly problematic for biopharmaceuticals, which are increasingly prevalent in modern drug development. The need for surface treatments or coatings to mitigate protein adsorption while maintaining the integrity of the glass container presents a complex engineering challenge.
The variability in glass composition and surface properties across different manufacturers and batches poses challenges for standardization and quality control. Inconsistencies in the glass surface can lead to differences in drug-container interactions, potentially affecting drug stability and efficacy. This variability necessitates rigorous testing and characterization of glass containers, adding complexity and cost to the drug development and manufacturing processes.
Extractables and leachables from borosilicate glass containers remain a concern, particularly for sensitive drug formulations. While borosilicate glass is generally considered inert, trace amounts of elements such as silicon, boron, and sodium can leach into the drug product over time. This can potentially alter the drug's pH, stability, or even its pharmacological properties. Developing methods to minimize and accurately predict leachables over the shelf life of a drug product is an ongoing challenge for the industry.
The mechanical strength of borosilicate glass, while generally good, can be compromised during manufacturing, transportation, or handling. Microscopic flaws or stress points in the glass can lead to breakage, posing risks to both manufacturing personnel and end-users. Improving the resilience of glass containers without compromising their chemical inertness or transparency is an area of active research and development.
Lastly, the environmental impact of glass production and disposal is becoming increasingly important in the context of sustainability goals. While glass is recyclable, the specialized nature of pharmaceutical glass often precludes its inclusion in standard recycling streams. Developing more sustainable production methods and end-of-life solutions for borosilicate glass used in drug delivery systems is a growing challenge that intersects with broader environmental concerns in the pharmaceutical industry.
Existing Solutions for Drug-Glass Interactions
01 Composition and properties of borosilicate glass
Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. It is known for its low thermal expansion coefficient, high chemical resistance, and excellent thermal shock resistance. These properties make it suitable for various applications in laboratory equipment, cookware, and industrial uses.- Composition and properties of borosilicate glass: Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. It is known for its low thermal expansion coefficient, high chemical resistance, and excellent thermal shock resistance. These properties make it suitable for various applications in laboratory equipment, cookware, and industrial uses.
- Manufacturing processes for borosilicate glass: Various manufacturing processes are employed to produce borosilicate glass, including melting, forming, and annealing. Advanced techniques such as float glass production and precision molding are used to create different forms of borosilicate glass products. The manufacturing process often involves careful control of temperature and composition to achieve desired properties.
- Applications of borosilicate glass in laboratory and industrial settings: Borosilicate glass is widely used in laboratory glassware, such as beakers, test tubes, and pipettes, due to its chemical resistance and thermal stability. It is also employed in industrial applications, including sight glasses, process vessels, and heat exchangers. The material's durability and transparency make it ideal for these demanding environments.
- Borosilicate glass in consumer products: Borosilicate glass is increasingly used in consumer products, particularly in kitchenware and home appliances. Its heat resistance and durability make it suitable for bakeware, storage containers, and coffee makers. The material's clarity and ability to withstand temperature changes also make it popular in lighting fixtures and decorative items.
- Innovations and modifications in borosilicate glass: Ongoing research and development in borosilicate glass focus on improving its properties and expanding its applications. This includes developing new compositions with enhanced characteristics, such as increased strength or improved optical properties. Innovations also involve surface treatments, coatings, and the incorporation of additional materials to create specialized borosilicate glass products for specific industries or applications.
02 Manufacturing processes for borosilicate glass
Various manufacturing processes are employed to produce borosilicate glass, including melting, forming, and annealing. Advanced techniques such as float glass production and precision molding are used to create different forms of borosilicate glass products. The manufacturing process often involves careful control of temperature and composition to achieve desired properties.Expand Specific Solutions03 Applications of borosilicate glass in laboratory and industrial settings
Borosilicate glass is widely used in laboratory glassware, such as beakers, test tubes, and pipettes, due to its chemical resistance and thermal stability. It is also employed in industrial applications, including sight glasses, process vessels, and heat exchangers. The material's durability and transparency make it ideal for these demanding environments.Expand Specific Solutions04 Borosilicate glass in consumer products
Borosilicate glass is increasingly used in consumer products, particularly in kitchenware and household items. Its heat resistance and durability make it suitable for bakeware, storage containers, and coffee makers. The material's clarity and eco-friendly nature also make it popular for reusable water bottles and food storage solutions.Expand Specific Solutions05 Innovations and modifications in borosilicate glass
Ongoing research and development in borosilicate glass focus on improving its properties and expanding its applications. This includes developing new compositions with enhanced characteristics, such as increased strength or improved optical properties. Innovations also involve surface treatments, coatings, and the incorporation of additional elements to tailor the glass for specific uses.Expand Specific Solutions
Key Players in Borosilicate Glass Manufacturing
The research on borosilicate glass in drug delivery systems is in a growth phase, with increasing market size and technological advancements. The global pharmaceutical glass packaging market, which includes borosilicate glass, is projected to expand significantly in the coming years. Key players like SCHOTT AG, Nippon Electric Glass, and SiO2 Medical Products are driving innovation in this field. These companies are developing specialized borosilicate glass formulations and coatings to enhance drug stability, reduce interactions, and improve overall performance in drug delivery systems. The technology is maturing, with a focus on improving chemical durability, thermal resistance, and compatibility with various pharmaceutical formulations. Emerging players such as Hunan Kibing Pharmaceutical Material Technology and Gansu Xukang Material Technology are also contributing to the competitive landscape, indicating a growing interest in this specialized market segment.
Nippon Electric Glass Co., Ltd.
Technical Solution: Nippon Electric Glass (NEG) has developed advanced borosilicate glass solutions for pharmaceutical packaging, focusing on their NEOPHARMA® series. This glass is designed to meet the stringent requirements of drug delivery systems, offering excellent chemical durability and thermal shock resistance. NEG's technology includes a proprietary melting process that ensures high purity and homogeneity in the glass composition[9]. They have also introduced innovative surface treatments to enhance the glass's hydrolytic resistance and reduce alkali elution, crucial for maintaining drug stability. NEG's recent developments include ultra-thin glass tubes for pre-fillable syringes, allowing for more precise dosing and reduced material usage[10].
Strengths: High chemical durability, thermal shock resistance, advanced surface treatments. Weaknesses: Limited global presence compared to some competitors, may face challenges in market penetration outside Asia.
SCHOTT AG
Technical Solution: SCHOTT AG has developed advanced borosilicate glass solutions for drug delivery systems, focusing on their SCHOTT FIOLAX® platform. This high-quality borosilicate glass is specifically designed for pharmaceutical packaging, offering superior chemical stability and low extractables and leachables. SCHOTT's technology includes a unique perfeXion® process, which uses big data and artificial intelligence for 100% quality control of each glass tube[1]. They have also introduced SCHOTT EVERIC® pure vials, which feature an improved surface chemistry to minimize drug-container interactions[2]. Additionally, SCHOTT has developed adaptiQ® ready-to-use vials, pre-sterilized and nested in tubs, facilitating aseptic fill-and-finish processes for pharmaceutical companies[3].
Strengths: Superior chemical stability, low extractables/leachables, advanced quality control, and ready-to-use solutions. Weaknesses: Potentially higher cost compared to traditional glass packaging, may require specialized handling and filling equipment.
Innovations in Borosilicate Glass Composition
Borosilicate glass for medicament container
PatentActiveJP2018193297A
Innovation
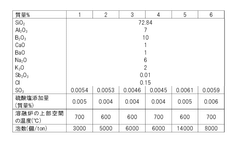
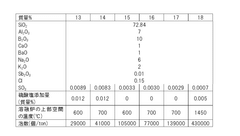
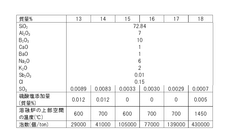
- A BaO-free borosilicate glass composition with specific ratios of SiO2, Al2O3, B2O3, Na2O, K2O, Li2O, and controlled ratios of MgO, CaO, and SrO to achieve low working temperatures without compromising chemical durability and hydrolysis resistance, using a glass composition that limits BaO content to 0.05% or less.
Borosilicate glass for pharmaceutical drug container, glass tube for pharmaceutical drug container, and method for producing borosilicate glass for pharmaceutical drug container
PatentWO2023089917A1
Innovation
- A borosilicate glass composition with specific ranges of SiO2, B2O3, SO3, Al2O3, CaO, BaO, Na2O, and K2O, along with controlled sulfate addition during the melting process, to minimize bubble formation and maintain clarity, using sulfate as a fining agent in controlled amounts to prevent reboiling.
Regulatory Framework for Pharmaceutical Packaging Materials
The regulatory framework for pharmaceutical packaging materials, including borosilicate glass used in drug delivery systems, is a complex and critical aspect of the pharmaceutical industry. Regulatory bodies worldwide have established stringent guidelines to ensure the safety, efficacy, and quality of pharmaceutical products throughout their lifecycle.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating pharmaceutical packaging materials. The FDA's guidance documents, such as the "Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics," provide comprehensive requirements for packaging components, including glass containers. These guidelines emphasize the importance of chemical and physical compatibility between the packaging material and the drug product.
The European Medicines Agency (EMA) oversees regulations in the European Union, working in conjunction with national regulatory authorities. The EMA's guidelines, particularly those related to quality of medicines, address the requirements for packaging materials. The European Pharmacopoeia also provides specific monographs for glass containers used in pharmaceutical applications, detailing the quality standards and testing methods.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates pharmaceutical packaging materials. The Japanese Pharmacopoeia includes standards for glass containers, aligning with international best practices while addressing specific regional requirements.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has developed harmonized guidelines that are widely adopted. These guidelines, particularly ICH Q8, Q9, and Q10, emphasize a quality-by-design approach that includes considerations for packaging materials from the early stages of drug development.
Regulatory frameworks typically require manufacturers to demonstrate the suitability of packaging materials through extensive testing. This includes evaluations of chemical resistance, durability, and potential leachables or extractables that could interact with the drug product. For borosilicate glass, specific tests such as hydrolytic resistance and thermal shock resistance are often mandated.
Compliance with Good Manufacturing Practices (GMP) is another crucial aspect of the regulatory framework. Manufacturers of pharmaceutical packaging materials, including borosilicate glass, must adhere to GMP guidelines to ensure consistent quality and traceability of their products.
As the pharmaceutical industry evolves, regulatory frameworks are continuously updated to address emerging challenges and technologies. Recent trends include increased focus on sustainability and the environmental impact of packaging materials, as well as considerations for novel drug delivery systems and personalized medicine approaches.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating pharmaceutical packaging materials. The FDA's guidance documents, such as the "Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics," provide comprehensive requirements for packaging components, including glass containers. These guidelines emphasize the importance of chemical and physical compatibility between the packaging material and the drug product.
The European Medicines Agency (EMA) oversees regulations in the European Union, working in conjunction with national regulatory authorities. The EMA's guidelines, particularly those related to quality of medicines, address the requirements for packaging materials. The European Pharmacopoeia also provides specific monographs for glass containers used in pharmaceutical applications, detailing the quality standards and testing methods.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates pharmaceutical packaging materials. The Japanese Pharmacopoeia includes standards for glass containers, aligning with international best practices while addressing specific regional requirements.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has developed harmonized guidelines that are widely adopted. These guidelines, particularly ICH Q8, Q9, and Q10, emphasize a quality-by-design approach that includes considerations for packaging materials from the early stages of drug development.
Regulatory frameworks typically require manufacturers to demonstrate the suitability of packaging materials through extensive testing. This includes evaluations of chemical resistance, durability, and potential leachables or extractables that could interact with the drug product. For borosilicate glass, specific tests such as hydrolytic resistance and thermal shock resistance are often mandated.
Compliance with Good Manufacturing Practices (GMP) is another crucial aspect of the regulatory framework. Manufacturers of pharmaceutical packaging materials, including borosilicate glass, must adhere to GMP guidelines to ensure consistent quality and traceability of their products.
As the pharmaceutical industry evolves, regulatory frameworks are continuously updated to address emerging challenges and technologies. Recent trends include increased focus on sustainability and the environmental impact of packaging materials, as well as considerations for novel drug delivery systems and personalized medicine approaches.
Environmental Impact of Borosilicate Glass in Healthcare
The environmental impact of borosilicate glass in healthcare, particularly in drug delivery systems, is a multifaceted issue that warrants careful consideration. Borosilicate glass has long been a preferred material in pharmaceutical packaging and medical devices due to its exceptional chemical resistance, thermal stability, and inertness. These properties ensure the integrity and safety of drug formulations, contributing to extended shelf life and reduced risk of contamination.
However, the production of borosilicate glass is an energy-intensive process, requiring high temperatures and specialized manufacturing techniques. This energy consumption contributes to greenhouse gas emissions and overall carbon footprint. The raw materials used in borosilicate glass production, including silica sand, boric oxide, and other additives, also have environmental implications related to mining and extraction processes.
On the positive side, borosilicate glass is highly recyclable, which can significantly mitigate its environmental impact when proper recycling systems are in place. The durability of borosilicate glass also means that products made from this material have a longer lifespan, potentially reducing the need for frequent replacements and thereby decreasing overall waste generation in healthcare settings.
In the context of drug delivery systems, the use of borosilicate glass can lead to reduced packaging waste compared to some alternative materials. Its ability to withstand sterilization processes without degradation allows for the reuse of certain glass components in medical devices, further minimizing waste production.
The disposal of borosilicate glass in healthcare settings presents both challenges and opportunities. While it is non-biodegradable, its inert nature means it does not leach harmful chemicals into the environment when disposed of in landfills. However, the increasing focus on sustainable healthcare practices has led to growing efforts to implement comprehensive recycling programs for medical glass waste.
Recent innovations in borosilicate glass manufacturing have aimed to reduce its environmental footprint. These include the development of more energy-efficient production methods, the use of recycled glass in manufacturing, and the exploration of alternative raw materials to reduce the reliance on mined resources.
As the healthcare industry continues to prioritize sustainability, the role of borosilicate glass in drug delivery systems is likely to evolve. Future research may focus on further improving the recyclability of borosilicate glass products, developing closed-loop systems for glass waste in healthcare facilities, and exploring bio-based alternatives that can match the performance of borosilicate glass while offering enhanced environmental benefits.
However, the production of borosilicate glass is an energy-intensive process, requiring high temperatures and specialized manufacturing techniques. This energy consumption contributes to greenhouse gas emissions and overall carbon footprint. The raw materials used in borosilicate glass production, including silica sand, boric oxide, and other additives, also have environmental implications related to mining and extraction processes.
On the positive side, borosilicate glass is highly recyclable, which can significantly mitigate its environmental impact when proper recycling systems are in place. The durability of borosilicate glass also means that products made from this material have a longer lifespan, potentially reducing the need for frequent replacements and thereby decreasing overall waste generation in healthcare settings.
In the context of drug delivery systems, the use of borosilicate glass can lead to reduced packaging waste compared to some alternative materials. Its ability to withstand sterilization processes without degradation allows for the reuse of certain glass components in medical devices, further minimizing waste production.
The disposal of borosilicate glass in healthcare settings presents both challenges and opportunities. While it is non-biodegradable, its inert nature means it does not leach harmful chemicals into the environment when disposed of in landfills. However, the increasing focus on sustainable healthcare practices has led to growing efforts to implement comprehensive recycling programs for medical glass waste.
Recent innovations in borosilicate glass manufacturing have aimed to reduce its environmental footprint. These include the development of more energy-efficient production methods, the use of recycled glass in manufacturing, and the exploration of alternative raw materials to reduce the reliance on mined resources.
As the healthcare industry continues to prioritize sustainability, the role of borosilicate glass in drug delivery systems is likely to evolve. Future research may focus on further improving the recyclability of borosilicate glass products, developing closed-loop systems for glass waste in healthcare facilities, and exploring bio-based alternatives that can match the performance of borosilicate glass while offering enhanced environmental benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!