Borosilicate Glass Kinetics in Devitrification Processes
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Borosilicate Glass Devitrification Background
Borosilicate glass, renowned for its exceptional thermal and chemical resistance, has been a cornerstone material in various industries for over a century. Its development can be traced back to the late 19th century when German glassmaker Otto Schott pioneered the addition of boron to traditional glass compositions. This innovation led to the creation of a glass with significantly improved properties, particularly its ability to withstand thermal shock and chemical corrosion.
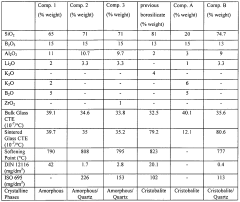
The unique properties of borosilicate glass stem from its composition, typically consisting of silica (70-80%), boron oxide (7-13%), sodium oxide, and aluminum oxide. This formulation results in a glass with a low coefficient of thermal expansion, high durability, and excellent resistance to chemical attack. These characteristics have made borosilicate glass indispensable in laboratory equipment, pharmaceutical packaging, and high-performance optical applications.
Despite its numerous advantages, borosilicate glass is not immune to the phenomenon of devitrification. Devitrification is the process by which the amorphous structure of glass transforms into a crystalline state, often triggered by prolonged exposure to elevated temperatures or specific environmental conditions. This process can significantly alter the glass's properties, potentially compromising its performance and integrity in critical applications.
The study of devitrification kinetics in borosilicate glass is crucial for several reasons. Firstly, it aids in understanding the long-term stability and performance of borosilicate glass products, particularly in high-temperature or chemically aggressive environments. Secondly, it provides insights into the fundamental mechanisms of crystal nucleation and growth in complex glass systems, contributing to the broader field of materials science.
Research on borosilicate glass devitrification has evolved significantly over the years, driven by both academic curiosity and industrial needs. Early studies focused primarily on observing and describing the phenomenon, while more recent investigations have delved into the molecular-level processes and kinetics involved. Advanced analytical techniques, such as X-ray diffraction, electron microscopy, and thermal analysis, have played a pivotal role in elucidating the intricate details of the devitrification process.
Understanding the kinetics of devitrification is not only crucial for predicting the long-term behavior of borosilicate glass but also for developing strategies to either prevent or control the process. This knowledge is particularly valuable in applications where the glass's performance is critical, such as in nuclear waste storage, high-temperature processing equipment, and precision optical systems.
The unique properties of borosilicate glass stem from its composition, typically consisting of silica (70-80%), boron oxide (7-13%), sodium oxide, and aluminum oxide. This formulation results in a glass with a low coefficient of thermal expansion, high durability, and excellent resistance to chemical attack. These characteristics have made borosilicate glass indispensable in laboratory equipment, pharmaceutical packaging, and high-performance optical applications.
Despite its numerous advantages, borosilicate glass is not immune to the phenomenon of devitrification. Devitrification is the process by which the amorphous structure of glass transforms into a crystalline state, often triggered by prolonged exposure to elevated temperatures or specific environmental conditions. This process can significantly alter the glass's properties, potentially compromising its performance and integrity in critical applications.
The study of devitrification kinetics in borosilicate glass is crucial for several reasons. Firstly, it aids in understanding the long-term stability and performance of borosilicate glass products, particularly in high-temperature or chemically aggressive environments. Secondly, it provides insights into the fundamental mechanisms of crystal nucleation and growth in complex glass systems, contributing to the broader field of materials science.
Research on borosilicate glass devitrification has evolved significantly over the years, driven by both academic curiosity and industrial needs. Early studies focused primarily on observing and describing the phenomenon, while more recent investigations have delved into the molecular-level processes and kinetics involved. Advanced analytical techniques, such as X-ray diffraction, electron microscopy, and thermal analysis, have played a pivotal role in elucidating the intricate details of the devitrification process.
Understanding the kinetics of devitrification is not only crucial for predicting the long-term behavior of borosilicate glass but also for developing strategies to either prevent or control the process. This knowledge is particularly valuable in applications where the glass's performance is critical, such as in nuclear waste storage, high-temperature processing equipment, and precision optical systems.
Market Analysis for Borosilicate Glass Applications
The market for borosilicate glass applications has been experiencing steady growth, driven by its unique properties and versatility across various industries. Borosilicate glass, known for its high resistance to thermal shock, chemical corrosion, and mechanical stress, finds extensive use in laboratory equipment, pharmaceutical packaging, cookware, and industrial applications.
In the laboratory and scientific research sector, borosilicate glass remains the material of choice for beakers, test tubes, and other precision glassware. The global laboratory glassware market, largely dominated by borosilicate products, is projected to expand significantly in the coming years, fueled by increased research and development activities in pharmaceuticals, biotechnology, and academic institutions.
The pharmaceutical industry represents another major market for borosilicate glass, particularly in packaging applications. With the growing emphasis on drug stability and shelf life, borosilicate glass vials and ampoules are preferred for their chemical inertness and ability to protect sensitive medications. The pharmaceutical glass packaging market is expected to witness substantial growth, driven by the increasing demand for biologics and injectable drugs.
In the consumer goods sector, borosilicate glass has gained popularity in kitchenware and cookware due to its heat resistance and durability. The trend towards eco-friendly and sustainable products has further boosted the demand for borosilicate glass containers and storage solutions as alternatives to plastic.
The automotive and aerospace industries are emerging markets for borosilicate glass applications. Its use in high-performance lighting, sensors, and display systems is increasing, driven by advancements in vehicle technology and the growing electric vehicle market.
Industrial applications of borosilicate glass, including its use in solar panels, optical fibers, and specialty lighting, are also contributing to market growth. The renewable energy sector, in particular, is expected to drive demand for borosilicate glass in solar thermal collectors and photovoltaic modules.
Geographically, North America and Europe currently lead the borosilicate glass market, owing to their well-established pharmaceutical and research sectors. However, the Asia-Pacific region is anticipated to witness the fastest growth, propelled by rapid industrialization, increasing healthcare expenditure, and growing research activities in countries like China and India.
Despite the positive outlook, the market faces challenges such as the high production costs of borosilicate glass compared to conventional soda-lime glass and the availability of alternative materials in certain applications. However, ongoing research into improving production efficiency and expanding the glass's properties is expected to address these challenges and open up new application areas.
In the laboratory and scientific research sector, borosilicate glass remains the material of choice for beakers, test tubes, and other precision glassware. The global laboratory glassware market, largely dominated by borosilicate products, is projected to expand significantly in the coming years, fueled by increased research and development activities in pharmaceuticals, biotechnology, and academic institutions.
The pharmaceutical industry represents another major market for borosilicate glass, particularly in packaging applications. With the growing emphasis on drug stability and shelf life, borosilicate glass vials and ampoules are preferred for their chemical inertness and ability to protect sensitive medications. The pharmaceutical glass packaging market is expected to witness substantial growth, driven by the increasing demand for biologics and injectable drugs.
In the consumer goods sector, borosilicate glass has gained popularity in kitchenware and cookware due to its heat resistance and durability. The trend towards eco-friendly and sustainable products has further boosted the demand for borosilicate glass containers and storage solutions as alternatives to plastic.
The automotive and aerospace industries are emerging markets for borosilicate glass applications. Its use in high-performance lighting, sensors, and display systems is increasing, driven by advancements in vehicle technology and the growing electric vehicle market.
Industrial applications of borosilicate glass, including its use in solar panels, optical fibers, and specialty lighting, are also contributing to market growth. The renewable energy sector, in particular, is expected to drive demand for borosilicate glass in solar thermal collectors and photovoltaic modules.
Geographically, North America and Europe currently lead the borosilicate glass market, owing to their well-established pharmaceutical and research sectors. However, the Asia-Pacific region is anticipated to witness the fastest growth, propelled by rapid industrialization, increasing healthcare expenditure, and growing research activities in countries like China and India.
Despite the positive outlook, the market faces challenges such as the high production costs of borosilicate glass compared to conventional soda-lime glass and the availability of alternative materials in certain applications. However, ongoing research into improving production efficiency and expanding the glass's properties is expected to address these challenges and open up new application areas.
Current Challenges in Devitrification Control
Devitrification control in borosilicate glass remains a significant challenge in the field of glass science and technology. The process of devitrification, where the amorphous structure of glass crystallizes, can significantly alter the properties and performance of borosilicate glass products. This phenomenon is particularly problematic in high-temperature applications and long-term storage scenarios.
One of the primary challenges in controlling devitrification is the complex interplay between glass composition, thermal history, and environmental factors. Borosilicate glasses, while known for their thermal and chemical resistance, can still undergo devitrification under certain conditions. The presence of nucleating agents, either intentionally added or as impurities, can accelerate this process, making it difficult to predict and control.
The kinetics of devitrification in borosilicate glasses presents another significant hurdle. The rate and extent of crystallization can vary widely depending on factors such as temperature, time, and the presence of stress or surface defects. This variability makes it challenging to develop universally applicable models for predicting devitrification behavior across different borosilicate glass compositions and processing conditions.
Surface crystallization is a particular concern in devitrification control. The glass surface often serves as a preferential site for crystal nucleation and growth, leading to the formation of a crystalline layer that can compromise the glass's optical, mechanical, and chemical properties. Developing effective strategies to inhibit surface crystallization without altering the bulk properties of the glass remains a significant challenge.
The development of in-situ monitoring techniques for devitrification processes is another area of ongoing research. Current methods often rely on post-process analysis, which limits the ability to intervene and control devitrification in real-time. Advanced sensing technologies capable of detecting early stages of crystallization during glass formation or subsequent heat treatments are needed to enable more proactive control strategies.
Furthermore, the long-term stability of borosilicate glasses under various environmental conditions poses challenges for predicting and preventing devitrification. This is particularly crucial in applications such as nuclear waste storage, where glass stability must be ensured over thousands of years. Developing accelerated testing methods that can accurately simulate long-term devitrification behavior remains a significant challenge in the field.
Lastly, the optimization of glass compositions to enhance devitrification resistance while maintaining desired properties is an ongoing challenge. Balancing the need for thermal stability with other critical characteristics such as chemical durability, optical transparency, and mechanical strength requires a deep understanding of structure-property relationships in borosilicate glasses and innovative approaches to glass formulation.
One of the primary challenges in controlling devitrification is the complex interplay between glass composition, thermal history, and environmental factors. Borosilicate glasses, while known for their thermal and chemical resistance, can still undergo devitrification under certain conditions. The presence of nucleating agents, either intentionally added or as impurities, can accelerate this process, making it difficult to predict and control.
The kinetics of devitrification in borosilicate glasses presents another significant hurdle. The rate and extent of crystallization can vary widely depending on factors such as temperature, time, and the presence of stress or surface defects. This variability makes it challenging to develop universally applicable models for predicting devitrification behavior across different borosilicate glass compositions and processing conditions.
Surface crystallization is a particular concern in devitrification control. The glass surface often serves as a preferential site for crystal nucleation and growth, leading to the formation of a crystalline layer that can compromise the glass's optical, mechanical, and chemical properties. Developing effective strategies to inhibit surface crystallization without altering the bulk properties of the glass remains a significant challenge.
The development of in-situ monitoring techniques for devitrification processes is another area of ongoing research. Current methods often rely on post-process analysis, which limits the ability to intervene and control devitrification in real-time. Advanced sensing technologies capable of detecting early stages of crystallization during glass formation or subsequent heat treatments are needed to enable more proactive control strategies.
Furthermore, the long-term stability of borosilicate glasses under various environmental conditions poses challenges for predicting and preventing devitrification. This is particularly crucial in applications such as nuclear waste storage, where glass stability must be ensured over thousands of years. Developing accelerated testing methods that can accurately simulate long-term devitrification behavior remains a significant challenge in the field.
Lastly, the optimization of glass compositions to enhance devitrification resistance while maintaining desired properties is an ongoing challenge. Balancing the need for thermal stability with other critical characteristics such as chemical durability, optical transparency, and mechanical strength requires a deep understanding of structure-property relationships in borosilicate glasses and innovative approaches to glass formulation.
Existing Devitrification Prevention Methods
01 Composition and properties of borosilicate glass
Borosilicate glass is known for its unique composition and properties, including high thermal shock resistance and low thermal expansion. The kinetics of borosilicate glass formation and its behavior under various conditions are influenced by its chemical composition, which typically includes silica, boron oxide, and other elements.- Composition and properties of borosilicate glass: Borosilicate glass is known for its unique composition and properties, including high thermal shock resistance and low thermal expansion. The kinetics of borosilicate glass formation and its behavior under various conditions are influenced by its chemical composition, which typically includes silica, boron oxide, and other elements.
- Thermal behavior and phase transitions: The kinetics of borosilicate glass are significantly affected by temperature changes. This includes studying phase transitions, crystallization processes, and the glass transition temperature. Understanding these thermal behaviors is crucial for optimizing the glass production process and tailoring its properties for specific applications.
- Surface reactions and chemical durability: The surface kinetics of borosilicate glass, including reactions with various chemicals and environmental factors, play a crucial role in its durability and performance. This involves studying corrosion resistance, ion exchange processes, and surface modifications to enhance the glass's chemical stability and longevity.
- Diffusion processes and ion mobility: Understanding the diffusion kinetics of various ions within the borosilicate glass structure is essential for predicting and controlling its properties. This includes studying the mobility of alkali ions, the formation of diffusion barriers, and the impact of these processes on the glass's electrical and optical characteristics.
- Manufacturing processes and kinetics optimization: The kinetics of borosilicate glass formation during manufacturing processes, such as melting, forming, and annealing, significantly impact the final product quality. Optimizing these kinetics through careful control of process parameters helps improve glass homogeneity, reduce defects, and enhance overall performance.
02 Thermal behavior and phase transitions
The kinetics of borosilicate glass are significantly affected by temperature changes. This includes studying phase transitions, crystallization processes, and the glass transition temperature. Understanding these thermal behaviors is crucial for optimizing the glass production process and tailoring its properties for specific applications.Expand Specific Solutions03 Surface reactions and chemical durability
The surface kinetics of borosilicate glass, including reactions with various chemicals and environmental factors, play a crucial role in its durability and performance. This involves studying corrosion resistance, ion exchange processes, and surface modifications that can enhance or alter the glass properties.Expand Specific Solutions04 Kinetics of glass formation and melting
The kinetics of borosilicate glass formation during the melting and cooling processes are essential for controlling the final product quality. This includes studying nucleation and growth rates, viscosity changes, and the effects of various additives on the glass-forming process.Expand Specific Solutions05 Mechanical properties and stress relaxation
The mechanical behavior of borosilicate glass, including stress relaxation and fracture mechanics, is governed by complex kinetic processes. Understanding these kinetics is crucial for predicting and improving the glass's strength, toughness, and long-term performance under various loading conditions.Expand Specific Solutions
Key Players in Borosilicate Glass Industry
The research on borosilicate glass kinetics in devitrification processes is currently in a mature stage of development, with a significant market presence and ongoing technological advancements. The global market for borosilicate glass is substantial, driven by its wide-ranging applications in pharmaceuticals, laboratory equipment, and consumer goods. Key players like SCHOTT AG, Corning, Inc., and Nippon Electric Glass Co., Ltd. are at the forefront of innovation, leveraging their extensive experience and research capabilities. These companies, along with others such as AGC, Inc. and Saint-Gobain, are continuously improving the material's properties and manufacturing processes, focusing on enhancing thermal resistance, chemical durability, and optical clarity.
SCHOTT AG
Technical Solution: SCHOTT AG has developed advanced techniques for studying borosilicate glass kinetics during devitrification. They utilize high-temperature X-ray diffraction (HT-XRD) to monitor crystallization processes in real-time[1]. Their research focuses on tailoring glass compositions to control devitrification rates, crucial for applications in pharmaceutical packaging and optical systems. SCHOTT has also implemented machine learning algorithms to predict devitrification behavior based on glass composition and thermal history[2]. This approach allows for rapid optimization of glass formulations for specific applications, reducing development time and costs.
Strengths: Extensive experience in borosilicate glass manufacturing; cutting-edge research facilities. Weaknesses: High research and development costs; potential for intellectual property conflicts with competitors.
Corning, Inc.
Technical Solution: Corning's research on borosilicate glass kinetics in devitrification processes involves advanced in-situ characterization techniques. They employ synchrotron-based X-ray scattering and spectroscopy to study the atomic-scale structural changes during devitrification[3]. Corning has developed proprietary models to predict nucleation and crystal growth rates in borosilicate glasses under various thermal conditions. Their approach combines experimental data with molecular dynamics simulations to provide a comprehensive understanding of devitrification mechanisms[4]. This research has led to the development of highly stable borosilicate glasses for use in extreme environments, such as high-temperature industrial processes and aerospace applications.
Strengths: Strong R&D capabilities; diverse application portfolio. Weaknesses: High capital investment required for research; potential market saturation in some sectors.
Core Innovations in Devitrification Kinetics
Borosilicate glass compositions and uses thereof
PatentWO2004050575A1
Innovation
- A borosilicate glass composition with silicon dioxide, boric oxide, aluminum oxide, and at least one alkali oxide, which resists devitrification without the addition of inhibitor oxides, maintaining a suitable coefficient of thermal expansion and mechanical strength.
Borosilicate glass compositions and uses thereof
PatentInactiveUS7596968B2
Innovation
- A borosilicate glass composition with specific ranges of silicon dioxide, boric oxide, aluminum oxide, and alkali oxides is formulated to resist devitrification and maintain thermal expansion within a desired range without the need for inhibitor oxides, enhancing mechanical strength and chemical durability.
Environmental Impact of Borosilicate Glass Production
The production of borosilicate glass, while essential for various industries, carries significant environmental implications that warrant careful consideration. The manufacturing process involves high-temperature melting of raw materials, including silica, boron oxide, and other additives, which consumes substantial energy and contributes to greenhouse gas emissions. The primary environmental concerns stem from the energy-intensive nature of glass production and the potential release of harmful substances during the manufacturing process.
Energy consumption in borosilicate glass production is a major contributor to its environmental footprint. The melting process typically requires temperatures exceeding 1500°C, maintained for extended periods to ensure proper fusion and homogenization of the glass components. This high energy demand often relies on fossil fuel combustion, leading to considerable CO2 emissions. However, advancements in furnace technology and the integration of renewable energy sources have shown promise in reducing the carbon footprint of glass production.
Air emissions from borosilicate glass manufacturing pose another environmental challenge. The process can release particulate matter, nitrogen oxides, sulfur oxides, and volatile organic compounds. These emissions contribute to air pollution and may have adverse effects on local air quality and human health. Modern production facilities employ various pollution control technologies, such as electrostatic precipitators and scrubbers, to mitigate these emissions and comply with increasingly stringent environmental regulations.
Water usage and wastewater management are additional environmental concerns in borosilicate glass production. The manufacturing process requires water for cooling and cleaning purposes, potentially leading to thermal pollution and the discharge of contaminated wastewater. Implementing closed-loop water systems and advanced treatment technologies can significantly reduce water consumption and minimize the environmental impact of wastewater discharge.
Raw material extraction for borosilicate glass production also has environmental implications. Mining activities for silica and boron compounds can lead to habitat disruption, soil erosion, and potential groundwater contamination. Sustainable sourcing practices and the use of recycled glass (cullet) as a raw material can help mitigate these impacts and promote a more circular economy approach in glass production.
The disposal and recycling of borosilicate glass products present both challenges and opportunities from an environmental perspective. While borosilicate glass is recyclable, its specialized composition often requires separate processing from common soda-lime glass, potentially complicating recycling efforts. Developing efficient recycling systems and promoting the use of recycled borosilicate glass in new products can significantly reduce the environmental burden associated with raw material extraction and energy-intensive primary production.
Energy consumption in borosilicate glass production is a major contributor to its environmental footprint. The melting process typically requires temperatures exceeding 1500°C, maintained for extended periods to ensure proper fusion and homogenization of the glass components. This high energy demand often relies on fossil fuel combustion, leading to considerable CO2 emissions. However, advancements in furnace technology and the integration of renewable energy sources have shown promise in reducing the carbon footprint of glass production.
Air emissions from borosilicate glass manufacturing pose another environmental challenge. The process can release particulate matter, nitrogen oxides, sulfur oxides, and volatile organic compounds. These emissions contribute to air pollution and may have adverse effects on local air quality and human health. Modern production facilities employ various pollution control technologies, such as electrostatic precipitators and scrubbers, to mitigate these emissions and comply with increasingly stringent environmental regulations.
Water usage and wastewater management are additional environmental concerns in borosilicate glass production. The manufacturing process requires water for cooling and cleaning purposes, potentially leading to thermal pollution and the discharge of contaminated wastewater. Implementing closed-loop water systems and advanced treatment technologies can significantly reduce water consumption and minimize the environmental impact of wastewater discharge.
Raw material extraction for borosilicate glass production also has environmental implications. Mining activities for silica and boron compounds can lead to habitat disruption, soil erosion, and potential groundwater contamination. Sustainable sourcing practices and the use of recycled glass (cullet) as a raw material can help mitigate these impacts and promote a more circular economy approach in glass production.
The disposal and recycling of borosilicate glass products present both challenges and opportunities from an environmental perspective. While borosilicate glass is recyclable, its specialized composition often requires separate processing from common soda-lime glass, potentially complicating recycling efforts. Developing efficient recycling systems and promoting the use of recycled borosilicate glass in new products can significantly reduce the environmental burden associated with raw material extraction and energy-intensive primary production.
Regulatory Framework for Glass Manufacturing
The regulatory framework for glass manufacturing, particularly in the context of borosilicate glass production and devitrification processes, is a complex and multifaceted system designed to ensure product safety, environmental protection, and quality standards. At the international level, organizations such as the International Organization for Standardization (ISO) and the World Health Organization (WHO) provide guidelines that influence national regulations.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating borosilicate glass used in pharmaceutical and food packaging. The FDA's Code of Federal Regulations Title 21, Part 177 specifically addresses the requirements for glass and ceramic articles intended for food contact. Additionally, the Environmental Protection Agency (EPA) oversees environmental aspects of glass manufacturing, including emissions control and waste management.
The European Union has established comprehensive regulations through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) framework, which impacts the production and use of borosilicate glass. The EU's Directive 2011/65/EU on the Restriction of Hazardous Substances (RoHS) also applies to certain glass products, limiting the use of specific hazardous materials.
In terms of workplace safety, regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) set standards for protecting workers in glass manufacturing facilities. These regulations cover aspects such as exposure limits to silica dust, heat stress management, and personal protective equipment requirements.
Specific to devitrification processes, regulatory frameworks often focus on the end-use applications of the glass products. For instance, borosilicate glass used in laboratory equipment must meet stringent chemical resistance and thermal shock resistance standards, as outlined in ASTM International's specifications.
Emerging regulations are increasingly addressing the environmental impact of glass manufacturing. Many countries are implementing stricter energy efficiency standards and promoting the use of recycled glass in production processes. The circular economy concept is gaining traction, with regulations encouraging the development of more sustainable glass manufacturing practices and improved recycling technologies.
As research on borosilicate glass kinetics in devitrification processes advances, regulatory bodies are likely to update their frameworks to incorporate new scientific findings. This may lead to more precise specifications for heat treatment processes, cooling rates, and compositional controls to prevent unwanted devitrification in final products.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating borosilicate glass used in pharmaceutical and food packaging. The FDA's Code of Federal Regulations Title 21, Part 177 specifically addresses the requirements for glass and ceramic articles intended for food contact. Additionally, the Environmental Protection Agency (EPA) oversees environmental aspects of glass manufacturing, including emissions control and waste management.
The European Union has established comprehensive regulations through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) framework, which impacts the production and use of borosilicate glass. The EU's Directive 2011/65/EU on the Restriction of Hazardous Substances (RoHS) also applies to certain glass products, limiting the use of specific hazardous materials.
In terms of workplace safety, regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) set standards for protecting workers in glass manufacturing facilities. These regulations cover aspects such as exposure limits to silica dust, heat stress management, and personal protective equipment requirements.
Specific to devitrification processes, regulatory frameworks often focus on the end-use applications of the glass products. For instance, borosilicate glass used in laboratory equipment must meet stringent chemical resistance and thermal shock resistance standards, as outlined in ASTM International's specifications.
Emerging regulations are increasingly addressing the environmental impact of glass manufacturing. Many countries are implementing stricter energy efficiency standards and promoting the use of recycled glass in production processes. The circular economy concept is gaining traction, with regulations encouraging the development of more sustainable glass manufacturing practices and improved recycling technologies.
As research on borosilicate glass kinetics in devitrification processes advances, regulatory bodies are likely to update their frameworks to incorporate new scientific findings. This may lead to more precise specifications for heat treatment processes, cooling rates, and compositional controls to prevent unwanted devitrification in final products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!