Carbonyl Reaction Mechanisms in Advanced Drug Development
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Reaction Mechanisms in Drug Development
Carbonyl reactions play a pivotal role in advanced drug development, serving as key mechanisms for the synthesis and modification of pharmaceutical compounds. These reactions involve the transformation of carbonyl groups, which are ubiquitous in organic molecules and essential in many drug structures. The carbonyl group's unique reactivity stems from its polarized nature, with the carbon atom bearing a partial positive charge and the oxygen atom a partial negative charge.
In drug development, carbonyl reactions are utilized in various stages, from initial lead compound synthesis to late-stage functionalization. One of the most common carbonyl reactions in pharmaceutical research is nucleophilic addition, where nucleophiles attack the electrophilic carbonyl carbon. This reaction is crucial for creating new carbon-carbon bonds and introducing functional groups that can enhance drug efficacy or improve pharmacokinetic properties.
Another significant carbonyl reaction mechanism is the aldol condensation, which allows for the formation of β-hydroxy carbonyl compounds. This reaction is particularly valuable in creating complex molecular scaffolds and introducing stereochemistry, both of which are critical factors in drug design. The reversibility of aldol reactions also provides opportunities for dynamic combinatorial chemistry, a powerful tool in drug discovery.
Reductive amination, involving the reaction of carbonyl compounds with amines followed by reduction, is extensively used in the synthesis of amine-containing drugs. This reaction is prized for its versatility and efficiency in creating carbon-nitrogen bonds, which are prevalent in many pharmaceuticals. The ability to control the stereochemistry of the resulting amine products makes this reaction particularly valuable in the development of chiral drugs.
Carbonyl chemistry also encompasses oxidation and reduction reactions, which are essential for modifying the oxidation state of drug molecules. These transformations can significantly alter a compound's biological activity and are often employed in the optimization of lead compounds. For instance, the selective reduction of ketones to alcohols can improve a drug's solubility or binding affinity to target proteins.
In recent years, advances in catalysis have revolutionized carbonyl chemistry in drug development. Asymmetric catalysis, in particular, has enabled the synthesis of enantiomerically pure compounds, which is crucial for developing safer and more effective drugs. Organocatalysis and transition metal catalysis have expanded the toolkit of carbonyl reactions, allowing for milder reaction conditions, higher yields, and improved selectivity.
The understanding and manipulation of carbonyl reaction mechanisms continue to evolve, driven by the need for more efficient and sustainable drug synthesis processes. Researchers are exploring novel carbonyl transformations, such as C-H functionalization adjacent to carbonyl groups and cascade reactions involving multiple carbonyl transformations. These advancements promise to streamline drug development pipelines and facilitate the discovery of new chemical entities with enhanced therapeutic properties.
In drug development, carbonyl reactions are utilized in various stages, from initial lead compound synthesis to late-stage functionalization. One of the most common carbonyl reactions in pharmaceutical research is nucleophilic addition, where nucleophiles attack the electrophilic carbonyl carbon. This reaction is crucial for creating new carbon-carbon bonds and introducing functional groups that can enhance drug efficacy or improve pharmacokinetic properties.
Another significant carbonyl reaction mechanism is the aldol condensation, which allows for the formation of β-hydroxy carbonyl compounds. This reaction is particularly valuable in creating complex molecular scaffolds and introducing stereochemistry, both of which are critical factors in drug design. The reversibility of aldol reactions also provides opportunities for dynamic combinatorial chemistry, a powerful tool in drug discovery.
Reductive amination, involving the reaction of carbonyl compounds with amines followed by reduction, is extensively used in the synthesis of amine-containing drugs. This reaction is prized for its versatility and efficiency in creating carbon-nitrogen bonds, which are prevalent in many pharmaceuticals. The ability to control the stereochemistry of the resulting amine products makes this reaction particularly valuable in the development of chiral drugs.
Carbonyl chemistry also encompasses oxidation and reduction reactions, which are essential for modifying the oxidation state of drug molecules. These transformations can significantly alter a compound's biological activity and are often employed in the optimization of lead compounds. For instance, the selective reduction of ketones to alcohols can improve a drug's solubility or binding affinity to target proteins.
In recent years, advances in catalysis have revolutionized carbonyl chemistry in drug development. Asymmetric catalysis, in particular, has enabled the synthesis of enantiomerically pure compounds, which is crucial for developing safer and more effective drugs. Organocatalysis and transition metal catalysis have expanded the toolkit of carbonyl reactions, allowing for milder reaction conditions, higher yields, and improved selectivity.
The understanding and manipulation of carbonyl reaction mechanisms continue to evolve, driven by the need for more efficient and sustainable drug synthesis processes. Researchers are exploring novel carbonyl transformations, such as C-H functionalization adjacent to carbonyl groups and cascade reactions involving multiple carbonyl transformations. These advancements promise to streamline drug development pipelines and facilitate the discovery of new chemical entities with enhanced therapeutic properties.
Market Demand for Advanced Drug Synthesis
The market demand for advanced drug synthesis techniques, particularly those involving carbonyl reaction mechanisms, has been steadily increasing in recent years. This growth is driven by the pharmaceutical industry's need for more efficient, cost-effective, and environmentally friendly methods to produce complex drug molecules. The carbonyl group, being a versatile functional group in organic chemistry, plays a crucial role in the synthesis of many pharmaceuticals, making it a focal point for research and development efforts.
The global pharmaceutical market, valued at over $1.4 trillion in 2021, is projected to grow at a compound annual growth rate (CAGR) of 5-6% through 2026. This expansion is fueling the demand for innovative drug synthesis methods, with a significant portion of this demand focused on carbonyl chemistry. The increasing prevalence of chronic diseases, coupled with the aging population in many developed countries, is driving the need for new and improved medications, further boosting the market for advanced synthesis techniques.
In the realm of drug discovery and development, there is a growing emphasis on green chemistry principles and sustainable practices. This trend has led to increased interest in catalytic methods for carbonyl reactions, which offer improved atom economy and reduced waste generation. The market for catalysts used in pharmaceutical synthesis is expected to grow substantially, with some estimates suggesting a CAGR of 6-7% over the next five years.
The demand for chiral drugs, which often involve asymmetric carbonyl reactions in their synthesis, is another significant driver of market growth. The global chiral technology market, closely tied to advanced drug synthesis, is projected to reach $10 billion by 2025. This growth is partly attributed to the increasing recognition of the importance of stereochemistry in drug efficacy and safety.
Furthermore, the rise of personalized medicine and biologics has created new challenges and opportunities in drug synthesis. While biologics themselves may not directly involve carbonyl chemistry, the production of small molecule drugs that complement or enhance biologic therapies often relies on advanced carbonyl reaction mechanisms. This synergy between small molecules and biologics is expected to drive further innovation in synthesis techniques.
The contract development and manufacturing organization (CDMO) sector, which heavily utilizes advanced synthesis methods, is experiencing rapid growth. This sector is projected to expand at a CAGR of 7-8% through 2026, indicating a strong market demand for outsourced drug synthesis capabilities, including those involving complex carbonyl chemistry.
In conclusion, the market demand for advanced drug synthesis, particularly involving carbonyl reaction mechanisms, is robust and multifaceted. It is driven by the overall growth of the pharmaceutical industry, the push for more sustainable practices, the increasing complexity of drug molecules, and the evolving landscape of personalized medicine. As the industry continues to evolve, research into carbonyl reaction mechanisms will remain a critical area for innovation and investment in drug development.
The global pharmaceutical market, valued at over $1.4 trillion in 2021, is projected to grow at a compound annual growth rate (CAGR) of 5-6% through 2026. This expansion is fueling the demand for innovative drug synthesis methods, with a significant portion of this demand focused on carbonyl chemistry. The increasing prevalence of chronic diseases, coupled with the aging population in many developed countries, is driving the need for new and improved medications, further boosting the market for advanced synthesis techniques.
In the realm of drug discovery and development, there is a growing emphasis on green chemistry principles and sustainable practices. This trend has led to increased interest in catalytic methods for carbonyl reactions, which offer improved atom economy and reduced waste generation. The market for catalysts used in pharmaceutical synthesis is expected to grow substantially, with some estimates suggesting a CAGR of 6-7% over the next five years.
The demand for chiral drugs, which often involve asymmetric carbonyl reactions in their synthesis, is another significant driver of market growth. The global chiral technology market, closely tied to advanced drug synthesis, is projected to reach $10 billion by 2025. This growth is partly attributed to the increasing recognition of the importance of stereochemistry in drug efficacy and safety.
Furthermore, the rise of personalized medicine and biologics has created new challenges and opportunities in drug synthesis. While biologics themselves may not directly involve carbonyl chemistry, the production of small molecule drugs that complement or enhance biologic therapies often relies on advanced carbonyl reaction mechanisms. This synergy between small molecules and biologics is expected to drive further innovation in synthesis techniques.
The contract development and manufacturing organization (CDMO) sector, which heavily utilizes advanced synthesis methods, is experiencing rapid growth. This sector is projected to expand at a CAGR of 7-8% through 2026, indicating a strong market demand for outsourced drug synthesis capabilities, including those involving complex carbonyl chemistry.
In conclusion, the market demand for advanced drug synthesis, particularly involving carbonyl reaction mechanisms, is robust and multifaceted. It is driven by the overall growth of the pharmaceutical industry, the push for more sustainable practices, the increasing complexity of drug molecules, and the evolving landscape of personalized medicine. As the industry continues to evolve, research into carbonyl reaction mechanisms will remain a critical area for innovation and investment in drug development.
Current Challenges in Carbonyl Chemistry
Carbonyl chemistry plays a pivotal role in advanced drug development, yet it faces several significant challenges that hinder progress in this field. One of the primary obstacles is the complexity of carbonyl reaction mechanisms, which often involve multiple steps and intermediates. This complexity makes it difficult to predict and control reaction outcomes, leading to reduced efficiency in drug synthesis processes.
The reactivity of carbonyl compounds poses another challenge. While their high reactivity is beneficial for many transformations, it can also lead to undesired side reactions and product instability. This is particularly problematic in the context of drug development, where precise control over molecular structure is crucial for achieving desired pharmacological properties.
Stereochemistry presents a further hurdle in carbonyl chemistry. Many drug molecules require specific stereochemical configurations to function effectively. However, controlling stereoselectivity in carbonyl reactions remains challenging, often resulting in mixtures of stereoisomers that require costly and time-consuming separation processes.
The sensitivity of carbonyl compounds to reaction conditions is another significant issue. Factors such as pH, temperature, and solvent can dramatically affect reaction outcomes, making it difficult to scale up processes from laboratory to industrial production. This sensitivity also complicates the development of robust and reproducible synthetic routes for drug candidates.
Environmental concerns add another layer of complexity to carbonyl chemistry in drug development. Traditional carbonyl reactions often rely on toxic or environmentally harmful reagents and solvents. There is a growing need for greener alternatives that maintain reaction efficiency while reducing environmental impact, aligning with the principles of sustainable chemistry.
The formation of stable carbon-carbon bonds through carbonyl chemistry is crucial in drug synthesis but remains challenging in many cases. Developing efficient methods for carbon-carbon bond formation, especially in complex molecular environments, is an ongoing area of research with significant implications for drug discovery and development.
Lastly, the integration of carbonyl chemistry with emerging technologies, such as flow chemistry and automated synthesis platforms, presents both opportunities and challenges. While these technologies offer the potential for increased efficiency and scalability, adapting traditional carbonyl reactions to these new paradigms requires overcoming significant technical hurdles.
The reactivity of carbonyl compounds poses another challenge. While their high reactivity is beneficial for many transformations, it can also lead to undesired side reactions and product instability. This is particularly problematic in the context of drug development, where precise control over molecular structure is crucial for achieving desired pharmacological properties.
Stereochemistry presents a further hurdle in carbonyl chemistry. Many drug molecules require specific stereochemical configurations to function effectively. However, controlling stereoselectivity in carbonyl reactions remains challenging, often resulting in mixtures of stereoisomers that require costly and time-consuming separation processes.
The sensitivity of carbonyl compounds to reaction conditions is another significant issue. Factors such as pH, temperature, and solvent can dramatically affect reaction outcomes, making it difficult to scale up processes from laboratory to industrial production. This sensitivity also complicates the development of robust and reproducible synthetic routes for drug candidates.
Environmental concerns add another layer of complexity to carbonyl chemistry in drug development. Traditional carbonyl reactions often rely on toxic or environmentally harmful reagents and solvents. There is a growing need for greener alternatives that maintain reaction efficiency while reducing environmental impact, aligning with the principles of sustainable chemistry.
The formation of stable carbon-carbon bonds through carbonyl chemistry is crucial in drug synthesis but remains challenging in many cases. Developing efficient methods for carbon-carbon bond formation, especially in complex molecular environments, is an ongoing area of research with significant implications for drug discovery and development.
Lastly, the integration of carbonyl chemistry with emerging technologies, such as flow chemistry and automated synthesis platforms, presents both opportunities and challenges. While these technologies offer the potential for increased efficiency and scalability, adapting traditional carbonyl reactions to these new paradigms requires overcoming significant technical hurdles.
Existing Carbonyl Reaction Strategies
01 Nucleophilic addition to carbonyl compounds
This mechanism involves the addition of nucleophiles to the electrophilic carbon of carbonyl groups. The process typically results in the formation of new carbon-carbon or carbon-heteroatom bonds. Various nucleophiles, such as organometallic reagents or hydride donors, can participate in these reactions, leading to the formation of alcohols or other functionalized products.- Nucleophilic addition to carbonyl compounds: This mechanism involves the addition of nucleophiles to the electrophilic carbon of carbonyl groups. The process typically results in the formation of new carbon-carbon or carbon-heteroatom bonds. Various nucleophiles can participate in this reaction, including organometallic compounds, amines, and alcohols. The reaction often proceeds through a tetrahedral intermediate before forming the final product.
- Oxidation and reduction of carbonyl compounds: Carbonyl compounds can undergo oxidation or reduction reactions, leading to the formation of various functional groups. Oxidation can convert aldehydes to carboxylic acids, while reduction can transform ketones into alcohols. These reactions often involve the use of specific reagents and catalysts to control the selectivity and yield of the desired products.
- Carbonyl condensation reactions: Condensation reactions involving carbonyl compounds are essential in organic synthesis. These reactions typically involve the combination of two carbonyl compounds or a carbonyl compound with another nucleophile, often resulting in the formation of carbon-carbon bonds. Examples include aldol condensation, Claisen condensation, and Knoevenagel condensation. These reactions often require basic or acidic catalysts to proceed efficiently.
- Carbonyl rearrangement reactions: Various rearrangement reactions can occur with carbonyl compounds, leading to the formation of new structural isomers. These reactions often involve the migration of atoms or groups within the molecule. Examples include the Beckmann rearrangement, Favorskii rearrangement, and benzilic acid rearrangement. These transformations are valuable in the synthesis of complex organic molecules and pharmaceutical intermediates.
- Carbonyl protection and deprotection strategies: In multi-step organic syntheses, it is often necessary to protect carbonyl groups to prevent unwanted side reactions. Various protecting groups can be used, such as acetals, ketals, and enol ethers. These protecting groups can be selectively removed under specific conditions to regenerate the carbonyl functionality when needed. The choice of protecting group depends on the reaction conditions and the overall synthetic strategy.
02 Carbonyl condensation reactions
Condensation reactions involving carbonyl compounds are essential in organic synthesis. These reactions often involve the formation of carbon-carbon bonds between two carbonyl-containing molecules or a carbonyl compound and another nucleophile. Examples include aldol condensations, Claisen condensations, and related processes that result in the formation of larger, more complex molecules.Expand Specific Solutions03 Oxidation and reduction of carbonyl compounds
Carbonyl groups can undergo various oxidation and reduction reactions. Oxidation can lead to the formation of carboxylic acids or other higher oxidation state compounds, while reduction can result in the formation of alcohols or hydrocarbons. These transformations often involve the use of specific reagents or catalysts and play crucial roles in the interconversion of functional groups.Expand Specific Solutions04 Carbonyl-mediated rearrangement reactions
Certain carbonyl compounds can undergo intramolecular rearrangements or participate in intermolecular rearrangement reactions. These processes often involve the migration of atoms or groups within a molecule, leading to structural reorganization. Examples include pinacol rearrangement, Beckmann rearrangement, and related transformations that result in the formation of new molecular frameworks.Expand Specific Solutions05 Catalytic transformations of carbonyl compounds
Various catalytic processes can be applied to carbonyl compounds to facilitate their transformation into other valuable products. These may include metal-catalyzed reactions, organocatalytic processes, or enzymatic transformations. Such catalytic methods often provide efficient and selective routes for the modification of carbonyl-containing molecules, enabling the synthesis of complex organic compounds.Expand Specific Solutions
Key Players in Pharmaceutical Research
The research on carbonyl reaction mechanisms in advanced drug development is currently in a mature stage, with significant market potential and ongoing innovation. The field is characterized by a competitive landscape involving both academic institutions and pharmaceutical companies. Key players like Novo Nordisk, Bayer AG, and Merck Sharp & Dohme are driving commercial applications, while research institutions such as the Shanghai Institute of Pharmaceutical Industry and Centre National de la Recherche Scientifique are contributing to fundamental advancements. The market size is substantial, reflecting the critical role of carbonyl chemistry in drug discovery and development. Technological maturity is evident, with established methodologies coexisting with emerging techniques, indicating a balance between proven approaches and cutting-edge innovations in the field.
Novo Nordisk A/S
Technical Solution: Novo Nordisk A/S has made notable advancements in carbonyl reaction mechanisms research for drug development, particularly in the field of peptide and protein-based therapeutics. They have developed innovative approaches to selective modification of carbonyl groups in peptides, enabling the creation of novel drug conjugates with improved pharmacokinetic properties[12]. Novo Nordisk has also pioneered the use of enzymatic catalysis for carbonyl transformations in sensitive biomolecules, offering milder reaction conditions and higher selectivity compared to traditional chemical methods[13]. Their research includes the development of chemoselective ligation techniques for the synthesis of complex peptide therapeutics, utilizing carbonyl chemistry to achieve site-specific modifications[14]. Additionally, they have invested in computational tools for predicting carbonyl reactivity in large biomolecules, facilitating the design of more effective biopharmaceuticals[15].
Strengths: Expertise in peptide and protein chemistry, innovative enzymatic approaches, and advanced computational modeling capabilities. Weaknesses: Potential limitations in applying their research to small molecule drug development and challenges in scaling up biocatalytic processes.
Bayer AG
Technical Solution: Bayer AG has developed innovative approaches to carbonyl reaction mechanisms in advanced drug development. They utilize a combination of computational chemistry and experimental techniques to elucidate reaction pathways. Their research focuses on the application of transition metal catalysts to facilitate carbonyl transformations, particularly in the synthesis of complex pharmaceutical intermediates[1]. Bayer has also pioneered the use of flow chemistry systems for continuous carbonyl reactions, allowing for better control and scalability in drug manufacturing processes[2]. Additionally, they have made significant progress in asymmetric carbonyl reactions, developing novel chiral catalysts for the synthesis of enantiomerically pure drug candidates[3].
Strengths: Strong integration of computational and experimental methods, expertise in transition metal catalysis, and advanced flow chemistry capabilities. Weaknesses: High research costs and potential limitations in applying academic findings to industrial-scale processes.
Innovative Approaches in Carbonyl Chemistry
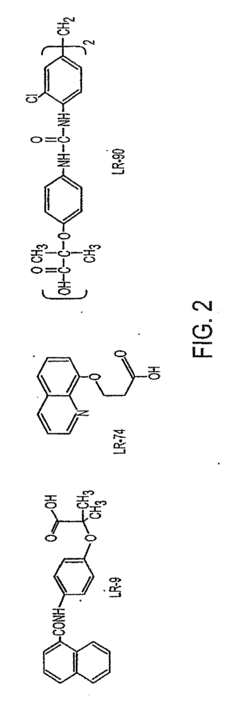
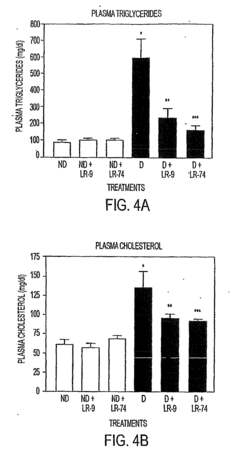
LR-9, LR-74 and LR-90 for use in treating complications resulting from diabetes
PatentInactiveEP2269601A1
Innovation
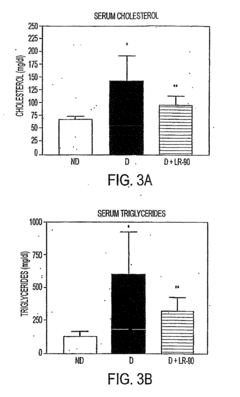
- Administration of compounds such as 4-(2-napthylcarboxamido) phenoxyisobutyric acid, 2-(8-quinolinoxy) propionic acid, and methylene bis (4,4=-(2-chlorophenylureidophenoxyisobutyric acid) to inhibit AGE and ALE formation, thereby reducing lipid levels and preventing diabetic complications.
Methods of suppression of rage gene expression and rage triggered inflammatory genes by selected age-inhibitors
PatentActiveUS20070117819A1
Innovation
- The compound LR-90, a novel aromatic derivative, is administered to suppress RAGE expression and inhibit AGE accumulation, thereby reducing pro-inflammatory signals and lipid concentrations, effectively addressing diabetic nephropathy and atherosclerosis.
Regulatory Considerations for Novel Drug Synthesis
In the realm of advanced drug development, regulatory considerations for novel drug synthesis play a crucial role in ensuring the safety, efficacy, and quality of new pharmaceutical products. The carbonyl reaction mechanisms, which are central to many drug synthesis processes, are subject to stringent regulatory oversight.
Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established comprehensive guidelines for the development and manufacturing of novel drugs. These guidelines encompass various aspects of the synthesis process, including the use of carbonyl reactions. Manufacturers must demonstrate a thorough understanding of the reaction mechanisms and their potential impact on drug safety and efficacy.
One key regulatory consideration is the control of impurities arising from carbonyl reactions. Regulatory agencies require detailed characterization and quantification of impurities, as well as the establishment of appropriate limits. This necessitates the development and validation of sensitive analytical methods capable of detecting and identifying carbonyl-related impurities.
The selection of starting materials and reagents for carbonyl reactions is another critical regulatory aspect. Manufacturers must justify their choices based on safety, quality, and consistency considerations. The use of potentially genotoxic carbonyl compounds or their precursors requires additional risk assessment and control strategies to ensure patient safety.
Process validation is a fundamental regulatory requirement for novel drug synthesis involving carbonyl reactions. Manufacturers must demonstrate that their synthesis processes consistently produce a product meeting predetermined quality attributes. This includes establishing critical process parameters and their acceptable ranges for carbonyl reactions.
Regulatory agencies also focus on the scalability and reproducibility of carbonyl reaction-based syntheses. As drug development progresses from laboratory to commercial scale, manufacturers must provide evidence that the reaction mechanisms and product quality remain consistent across different batch sizes.
Environmental considerations related to carbonyl reactions are increasingly important from a regulatory perspective. Agencies may require assessments of the environmental impact of synthesis processes, including the management of waste streams containing carbonyl compounds or their byproducts.
Lastly, regulatory bodies emphasize the importance of continuous improvement and innovation in synthesis processes. Manufacturers are encouraged to explore new technologies and methodologies that can enhance the efficiency, safety, and sustainability of carbonyl reactions in drug synthesis, provided they can demonstrate equivalence or superiority to existing methods.
Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established comprehensive guidelines for the development and manufacturing of novel drugs. These guidelines encompass various aspects of the synthesis process, including the use of carbonyl reactions. Manufacturers must demonstrate a thorough understanding of the reaction mechanisms and their potential impact on drug safety and efficacy.
One key regulatory consideration is the control of impurities arising from carbonyl reactions. Regulatory agencies require detailed characterization and quantification of impurities, as well as the establishment of appropriate limits. This necessitates the development and validation of sensitive analytical methods capable of detecting and identifying carbonyl-related impurities.
The selection of starting materials and reagents for carbonyl reactions is another critical regulatory aspect. Manufacturers must justify their choices based on safety, quality, and consistency considerations. The use of potentially genotoxic carbonyl compounds or their precursors requires additional risk assessment and control strategies to ensure patient safety.
Process validation is a fundamental regulatory requirement for novel drug synthesis involving carbonyl reactions. Manufacturers must demonstrate that their synthesis processes consistently produce a product meeting predetermined quality attributes. This includes establishing critical process parameters and their acceptable ranges for carbonyl reactions.
Regulatory agencies also focus on the scalability and reproducibility of carbonyl reaction-based syntheses. As drug development progresses from laboratory to commercial scale, manufacturers must provide evidence that the reaction mechanisms and product quality remain consistent across different batch sizes.
Environmental considerations related to carbonyl reactions are increasingly important from a regulatory perspective. Agencies may require assessments of the environmental impact of synthesis processes, including the management of waste streams containing carbonyl compounds or their byproducts.
Lastly, regulatory bodies emphasize the importance of continuous improvement and innovation in synthesis processes. Manufacturers are encouraged to explore new technologies and methodologies that can enhance the efficiency, safety, and sustainability of carbonyl reactions in drug synthesis, provided they can demonstrate equivalence or superiority to existing methods.
Environmental Impact of Carbonyl Reactions
Carbonyl reactions, while essential in advanced drug development, can have significant environmental implications. These reactions often involve the use of organic solvents and catalysts, which may pose risks to ecosystems if not properly managed. The environmental impact of carbonyl reactions primarily stems from the potential release of volatile organic compounds (VOCs) and other hazardous substances during the synthesis process.
One of the main concerns is the emission of VOCs, which can contribute to air pollution and the formation of ground-level ozone. Many carbonyl compounds and their precursors are known to be potent greenhouse gases, potentially exacerbating climate change issues. Additionally, some of these compounds can persist in the environment, leading to long-term ecological effects.
Water pollution is another critical aspect to consider. Effluents from pharmaceutical manufacturing processes that involve carbonyl reactions may contain trace amounts of reactants, intermediates, or byproducts. If not adequately treated, these substances can contaminate water bodies, affecting aquatic ecosystems and potentially entering the food chain.
The disposal of waste products from carbonyl reactions also presents environmental challenges. Hazardous waste management protocols must be strictly followed to prevent soil contamination and protect groundwater resources. Improper disposal can lead to the accumulation of toxic substances in the environment, posing risks to wildlife and human health.
However, the pharmaceutical industry has been making strides in developing more environmentally friendly approaches to carbonyl chemistry. Green chemistry principles are increasingly being applied to minimize the environmental footprint of these reactions. This includes the use of less harmful solvents, catalysts, and reagents, as well as the implementation of more efficient reaction processes that reduce waste generation.
Efforts are also being made to improve the recyclability and reusability of catalysts and solvents used in carbonyl reactions. This not only reduces the environmental impact but also enhances the economic viability of the processes. Furthermore, the development of biocatalytic methods for carbonyl transformations offers a promising avenue for more sustainable drug development practices.
Regulatory bodies worldwide are implementing stricter environmental standards for pharmaceutical manufacturing, including those involving carbonyl chemistry. This has led to increased investment in pollution control technologies and the adoption of cleaner production methods. As a result, the industry is gradually moving towards more sustainable practices in advanced drug development, balancing the need for innovative therapies with environmental stewardship.
One of the main concerns is the emission of VOCs, which can contribute to air pollution and the formation of ground-level ozone. Many carbonyl compounds and their precursors are known to be potent greenhouse gases, potentially exacerbating climate change issues. Additionally, some of these compounds can persist in the environment, leading to long-term ecological effects.
Water pollution is another critical aspect to consider. Effluents from pharmaceutical manufacturing processes that involve carbonyl reactions may contain trace amounts of reactants, intermediates, or byproducts. If not adequately treated, these substances can contaminate water bodies, affecting aquatic ecosystems and potentially entering the food chain.
The disposal of waste products from carbonyl reactions also presents environmental challenges. Hazardous waste management protocols must be strictly followed to prevent soil contamination and protect groundwater resources. Improper disposal can lead to the accumulation of toxic substances in the environment, posing risks to wildlife and human health.
However, the pharmaceutical industry has been making strides in developing more environmentally friendly approaches to carbonyl chemistry. Green chemistry principles are increasingly being applied to minimize the environmental footprint of these reactions. This includes the use of less harmful solvents, catalysts, and reagents, as well as the implementation of more efficient reaction processes that reduce waste generation.
Efforts are also being made to improve the recyclability and reusability of catalysts and solvents used in carbonyl reactions. This not only reduces the environmental impact but also enhances the economic viability of the processes. Furthermore, the development of biocatalytic methods for carbonyl transformations offers a promising avenue for more sustainable drug development practices.
Regulatory bodies worldwide are implementing stricter environmental standards for pharmaceutical manufacturing, including those involving carbonyl chemistry. This has led to increased investment in pollution control technologies and the adoption of cleaner production methods. As a result, the industry is gradually moving towards more sustainable practices in advanced drug development, balancing the need for innovative therapies with environmental stewardship.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!