Ion-exchange Strengthening of Borosilicate Glass Sheets
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ion-Exchange Glass Evolution
The evolution of ion-exchange strengthening for borosilicate glass sheets represents a significant advancement in glass technology. This process, which emerged in the mid-20th century, has undergone continuous refinement and improvement over the decades. Initially, the focus was on developing a method to enhance the mechanical properties of glass without compromising its optical clarity.
In the 1960s, researchers discovered that immersing glass in a molten salt bath could lead to ion exchange, resulting in compressive stress on the glass surface. This breakthrough laid the foundation for the modern ion-exchange strengthening process. Early applications were limited due to the high costs and technical challenges associated with the process.
The 1970s and 1980s saw significant progress in understanding the mechanisms of ion exchange. Scientists explored various alkali ions and their effects on different glass compositions. This period marked the transition from purely empirical approaches to more scientifically grounded methodologies. Researchers began to develop mathematical models to predict and optimize the ion-exchange process.
By the 1990s, the focus shifted towards improving the efficiency and scalability of the ion-exchange process. Advancements in furnace technology and temperature control allowed for more precise and uniform strengthening of glass sheets. This era also saw the expansion of ion-exchange strengthening to a wider range of glass compositions, including borosilicate glasses.
The turn of the millennium brought about a renewed interest in ion-exchange strengthening, driven by the growing demand for durable and scratch-resistant glass in consumer electronics. This period witnessed the development of more sophisticated ion-exchange techniques, including double ion-exchange processes and gradient strengthening methods.
In recent years, the evolution of ion-exchange strengthening has been characterized by a focus on sustainability and energy efficiency. Researchers have been exploring ways to reduce the environmental impact of the process, such as developing lower-temperature ion-exchange baths and investigating the use of more environmentally friendly salt compositions.
The latest advancements in ion-exchange strengthening for borosilicate glass sheets involve the integration of nanotechnology and surface engineering. These cutting-edge approaches aim to further enhance the mechanical properties of glass while also imparting additional functionalities, such as self-cleaning or anti-reflective properties.
In the 1960s, researchers discovered that immersing glass in a molten salt bath could lead to ion exchange, resulting in compressive stress on the glass surface. This breakthrough laid the foundation for the modern ion-exchange strengthening process. Early applications were limited due to the high costs and technical challenges associated with the process.
The 1970s and 1980s saw significant progress in understanding the mechanisms of ion exchange. Scientists explored various alkali ions and their effects on different glass compositions. This period marked the transition from purely empirical approaches to more scientifically grounded methodologies. Researchers began to develop mathematical models to predict and optimize the ion-exchange process.
By the 1990s, the focus shifted towards improving the efficiency and scalability of the ion-exchange process. Advancements in furnace technology and temperature control allowed for more precise and uniform strengthening of glass sheets. This era also saw the expansion of ion-exchange strengthening to a wider range of glass compositions, including borosilicate glasses.
The turn of the millennium brought about a renewed interest in ion-exchange strengthening, driven by the growing demand for durable and scratch-resistant glass in consumer electronics. This period witnessed the development of more sophisticated ion-exchange techniques, including double ion-exchange processes and gradient strengthening methods.
In recent years, the evolution of ion-exchange strengthening has been characterized by a focus on sustainability and energy efficiency. Researchers have been exploring ways to reduce the environmental impact of the process, such as developing lower-temperature ion-exchange baths and investigating the use of more environmentally friendly salt compositions.
The latest advancements in ion-exchange strengthening for borosilicate glass sheets involve the integration of nanotechnology and surface engineering. These cutting-edge approaches aim to further enhance the mechanical properties of glass while also imparting additional functionalities, such as self-cleaning or anti-reflective properties.
Market Demand Analysis
The market demand for ion-exchange strengthened borosilicate glass sheets has been steadily increasing across various industries. This growth is primarily driven by the unique properties of borosilicate glass, including its high chemical and thermal resistance, low thermal expansion, and excellent optical clarity. When further enhanced through ion-exchange strengthening, these glass sheets offer superior mechanical strength and durability, making them ideal for a wide range of applications.
In the consumer electronics sector, there is a significant demand for strengthened borosilicate glass in smartphone and tablet screens, smartwatch displays, and laptop covers. The increasing trend towards larger, thinner, and more durable screens has fueled the need for advanced glass solutions that can withstand daily wear and tear while maintaining optical quality.
The automotive industry represents another major market for ion-exchange strengthened borosilicate glass. As vehicle manufacturers focus on lightweight materials to improve fuel efficiency and reduce emissions, there is a growing interest in using these glass sheets for windshields, side windows, and sunroofs. The enhanced strength and safety features of these glass products align well with stringent automotive safety standards.
In the architectural and construction sector, the demand for ion-exchange strengthened borosilicate glass is rising due to its ability to meet both aesthetic and functional requirements. These glass sheets are increasingly used in building facades, interior partitions, and structural glazing applications, offering improved safety, energy efficiency, and design flexibility.
The healthcare and laboratory equipment industry also contributes to the market demand. Borosilicate glass, known for its chemical resistance and thermal stability, is widely used in laboratory glassware, pharmaceutical packaging, and medical devices. The ion-exchange strengthening process further enhances its durability and safety, making it suitable for critical applications in these fields.
Emerging technologies such as solar panels and advanced display systems are creating new opportunities for ion-exchange strengthened borosilicate glass. The renewable energy sector, in particular, is showing increased interest in these materials for photovoltaic modules and concentrated solar power systems, due to their high transmittance and durability under harsh environmental conditions.
Market analysts project a compound annual growth rate (CAGR) for the strengthened glass market, including ion-exchange strengthened borosilicate glass, to be in the high single digits over the next five years. This growth is expected to be driven by technological advancements, increasing adoption in various industries, and the ongoing trend towards more durable and sustainable materials.
In the consumer electronics sector, there is a significant demand for strengthened borosilicate glass in smartphone and tablet screens, smartwatch displays, and laptop covers. The increasing trend towards larger, thinner, and more durable screens has fueled the need for advanced glass solutions that can withstand daily wear and tear while maintaining optical quality.
The automotive industry represents another major market for ion-exchange strengthened borosilicate glass. As vehicle manufacturers focus on lightweight materials to improve fuel efficiency and reduce emissions, there is a growing interest in using these glass sheets for windshields, side windows, and sunroofs. The enhanced strength and safety features of these glass products align well with stringent automotive safety standards.
In the architectural and construction sector, the demand for ion-exchange strengthened borosilicate glass is rising due to its ability to meet both aesthetic and functional requirements. These glass sheets are increasingly used in building facades, interior partitions, and structural glazing applications, offering improved safety, energy efficiency, and design flexibility.
The healthcare and laboratory equipment industry also contributes to the market demand. Borosilicate glass, known for its chemical resistance and thermal stability, is widely used in laboratory glassware, pharmaceutical packaging, and medical devices. The ion-exchange strengthening process further enhances its durability and safety, making it suitable for critical applications in these fields.
Emerging technologies such as solar panels and advanced display systems are creating new opportunities for ion-exchange strengthened borosilicate glass. The renewable energy sector, in particular, is showing increased interest in these materials for photovoltaic modules and concentrated solar power systems, due to their high transmittance and durability under harsh environmental conditions.
Market analysts project a compound annual growth rate (CAGR) for the strengthened glass market, including ion-exchange strengthened borosilicate glass, to be in the high single digits over the next five years. This growth is expected to be driven by technological advancements, increasing adoption in various industries, and the ongoing trend towards more durable and sustainable materials.
Technical Challenges
The ion-exchange strengthening of borosilicate glass sheets presents several technical challenges that researchers and manufacturers must overcome to achieve optimal results. One of the primary difficulties lies in the complex composition of borosilicate glass, which contains both silica and boron oxide. This unique structure makes it more resistant to ion exchange compared to soda-lime glass, requiring more aggressive treatment conditions.
The diffusion process of larger ions, typically potassium, into the glass surface is hindered by the tight network structure of borosilicate glass. This necessitates higher temperatures and longer treatment times, which can lead to thermal stress and potential deformation of the glass sheets. Balancing the treatment parameters to achieve sufficient ion penetration without compromising the glass's dimensional stability is a significant challenge.
Another obstacle is the depth of the ion-exchange layer. Due to the resistance of borosilicate glass to ion diffusion, achieving a deep strengthened layer is difficult. This limitation can result in a relatively thin strengthened surface, which may not provide the desired level of overall strength improvement, especially for applications requiring high impact resistance.
The uniformity of the ion-exchange process across the entire surface of large glass sheets poses another technical hurdle. Ensuring consistent ion penetration and stress distribution is crucial for maintaining optical quality and preventing localized weak points. This becomes increasingly challenging as the size of the glass sheets increases, requiring precise control of the molten salt bath and treatment conditions.
Furthermore, the chemical durability of borosilicate glass can be affected by the ion-exchange process. The introduction of larger ions into the surface layer may alter the glass's resistance to chemical attack, potentially compromising its performance in certain applications. Researchers must carefully evaluate and mitigate any changes in chemical properties resulting from the strengthening treatment.
The cost-effectiveness of the ion-exchange process for borosilicate glass is also a significant consideration. The higher temperatures and longer treatment times required increase energy consumption and production costs. Developing more efficient processes that can achieve the desired strengthening effect with reduced energy input and shorter cycle times remains a key challenge for industrial implementation.
Lastly, the integration of ion-exchange strengthening with other glass processing techniques, such as coating applications or surface treatments, presents additional complexities. Ensuring compatibility between the strengthened surface and subsequent processing steps is essential for maintaining the enhanced properties of the glass while meeting specific application requirements.
The diffusion process of larger ions, typically potassium, into the glass surface is hindered by the tight network structure of borosilicate glass. This necessitates higher temperatures and longer treatment times, which can lead to thermal stress and potential deformation of the glass sheets. Balancing the treatment parameters to achieve sufficient ion penetration without compromising the glass's dimensional stability is a significant challenge.
Another obstacle is the depth of the ion-exchange layer. Due to the resistance of borosilicate glass to ion diffusion, achieving a deep strengthened layer is difficult. This limitation can result in a relatively thin strengthened surface, which may not provide the desired level of overall strength improvement, especially for applications requiring high impact resistance.
The uniformity of the ion-exchange process across the entire surface of large glass sheets poses another technical hurdle. Ensuring consistent ion penetration and stress distribution is crucial for maintaining optical quality and preventing localized weak points. This becomes increasingly challenging as the size of the glass sheets increases, requiring precise control of the molten salt bath and treatment conditions.
Furthermore, the chemical durability of borosilicate glass can be affected by the ion-exchange process. The introduction of larger ions into the surface layer may alter the glass's resistance to chemical attack, potentially compromising its performance in certain applications. Researchers must carefully evaluate and mitigate any changes in chemical properties resulting from the strengthening treatment.
The cost-effectiveness of the ion-exchange process for borosilicate glass is also a significant consideration. The higher temperatures and longer treatment times required increase energy consumption and production costs. Developing more efficient processes that can achieve the desired strengthening effect with reduced energy input and shorter cycle times remains a key challenge for industrial implementation.
Lastly, the integration of ion-exchange strengthening with other glass processing techniques, such as coating applications or surface treatments, presents additional complexities. Ensuring compatibility between the strengthened surface and subsequent processing steps is essential for maintaining the enhanced properties of the glass while meeting specific application requirements.
Current Strengthening Methods
01 Chemical composition optimization
Improving the strength of borosilicate glass sheets can be achieved by optimizing the chemical composition. This involves adjusting the ratios of key components such as silica, boron oxide, and alkali oxides to enhance the glass's mechanical properties. The precise balance of these elements can significantly impact the overall strength and durability of the glass sheets.- Chemical composition optimization: Improving the strength of borosilicate glass sheets can be achieved by optimizing the chemical composition. This involves adjusting the ratios of key components such as silica, boron oxide, and alkali oxides to enhance the glass's mechanical properties. The precise balance of these elements can significantly impact the overall strength and durability of the glass sheets.
- Surface treatment techniques: Various surface treatment methods can be applied to borosilicate glass sheets to increase their strength. These techniques may include ion exchange processes, chemical tempering, or the application of protective coatings. Such treatments can create compressive stress on the glass surface, making it more resistant to cracks and breakage.
- Heat treatment and tempering: Heat treatment and tempering processes can significantly enhance the strength of borosilicate glass sheets. These methods involve carefully controlled heating and cooling cycles that induce internal stresses, resulting in increased mechanical strength and improved resistance to thermal shock. The optimized tempering process can lead to glass sheets with superior strength-to-weight ratios.
- Lamination and composite structures: Incorporating borosilicate glass sheets into laminated or composite structures can greatly enhance their overall strength. This may involve bonding multiple layers of glass or combining glass with other materials such as polymers or ceramics. These composite structures can offer improved impact resistance, flexural strength, and overall durability compared to single-layer borosilicate glass sheets.
- Nanostructure modification: Modifying the nanostructure of borosilicate glass can lead to significant improvements in strength. This may involve the incorporation of nanoparticles, creation of nanoporous structures, or controlled crystallization at the nanoscale. These modifications can enhance the glass's mechanical properties by altering its microstructure and introducing beneficial stress distributions throughout the material.
02 Heat treatment processes
Various heat treatment processes can be employed to increase the strength of borosilicate glass sheets. These may include tempering, annealing, or ion exchange treatments. Such thermal processes can induce compressive stresses on the glass surface, thereby enhancing its resistance to mechanical and thermal shocks.Expand Specific Solutions03 Surface modification techniques
Enhancing the surface properties of borosilicate glass sheets can lead to improved strength. This can be achieved through techniques such as chemical etching, coating applications, or surface crystallization. These methods can reduce surface flaws, increase scratch resistance, and improve overall durability of the glass sheets.Expand Specific Solutions04 Lamination and composite structures
Incorporating borosilicate glass sheets into laminated or composite structures can significantly enhance their strength. This may involve bonding multiple layers of glass or combining glass with other materials such as polymers or ceramics. Such composite structures can provide improved impact resistance and overall mechanical strength.Expand Specific Solutions05 Nanostructure engineering
Manipulating the nanostructure of borosilicate glass can lead to enhanced strength properties. This may involve the incorporation of nanoparticles, creation of nanoporous structures, or controlled crystallization at the nanoscale. These techniques can improve the glass's resistance to crack propagation and overall mechanical performance.Expand Specific Solutions
Key Industry Players
The research on ion-exchange strengthening of borosilicate glass sheets is in a developing stage, with growing market potential due to increasing demand for durable glass in various industries. The global market for strengthened glass is expanding, driven by applications in electronics, automotive, and construction sectors. Technologically, the process is advancing but still requires further refinement for optimal results. Companies like Corning, Inc., AGC, Inc., and SCHOTT AG are at the forefront of this technology, leveraging their extensive experience in specialty glass manufacturing. Emerging players such as CSG Holding Co., Ltd. and Nippon Electric Glass Co., Ltd. are also making significant strides in this field, contributing to the competitive landscape and driving innovation in ion-exchange strengthening techniques for borosilicate glass.
Corning, Inc.
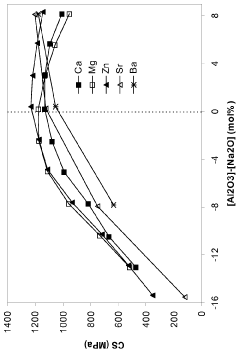
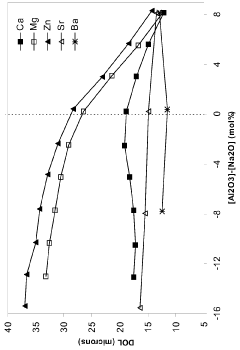
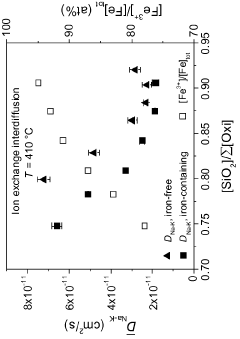
Technical Solution: Corning has developed an advanced ion-exchange strengthening process for borosilicate glass sheets, known as Gorilla Glass. This process involves immersing the glass in a molten potassium salt bath at temperatures around 400°C, allowing potassium ions to replace smaller sodium ions in the glass surface[1]. This creates a compressive stress layer, significantly enhancing the glass's strength and scratch resistance. Corning's process can achieve surface compressive stresses exceeding 700 MPa and depths of layer up to 40 μm[2]. The company has also innovated in developing specialized compositions of borosilicate glass that are more receptive to ion-exchange, allowing for greater strengthening effects[3].
Strengths: Industry-leading expertise in ion-exchange technology, proprietary glass compositions, and established manufacturing processes. Weaknesses: High production costs and energy-intensive process may limit application in some markets.
Nippon Electric Glass Co., Ltd.
Technical Solution: Nippon Electric Glass (NEG) has developed a unique approach to ion-exchange strengthening of borosilicate glass sheets, focusing on ultra-thin glass for flexible electronics. Their process involves a two-step ion-exchange treatment, first with sodium ions and then with potassium ions[4]. This method allows for deeper penetration of the strengthening ions, resulting in improved flexibility and durability of the glass. NEG's technology can produce glass sheets as thin as 25 μm while maintaining high strength and flexibility[5]. The company has also developed specialized coatings that enhance the ion-exchange process and provide additional functional properties to the glass surface[6].
Strengths: Expertise in ultra-thin glass strengthening, innovative two-step process, and complementary coating technologies. Weaknesses: Limited application range compared to broader market players, potentially higher production complexity.
Core Ion-Exchange Patents
Aluminosilicate glasses for ion exchange
PatentWO2013130646A1
Innovation
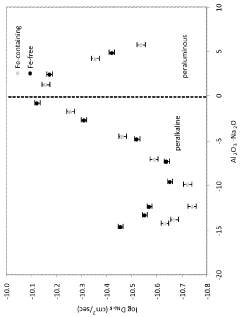
- The development of alkali alumino-silicate glass compositions with specific mol% ranges of Al2O3, SiO2, and other oxides, such as Na2O, K2O, MgO, and ZnO, which are ion exchangeable and capable of producing a compressive stress of at least 1 GPa with a deep depth of layer, either by promoting high compressive stress and depth or reducing the ion exchange time.
Two-step method for strengthening glass
PatentInactiveUS20120052271A1
Innovation
- A two-step ion exchange process where smaller metal cations in alkali aluminoborosilicate glasses are replaced by larger cations, first with sodium to achieve a deep compressive layer, and then with potassium to further increase compressive stress, forming a compressive layer extending from the glass surface to a specific depth.
Environmental Impact
The ion-exchange strengthening process for borosilicate glass sheets has several environmental implications that warrant careful consideration. This technique, while effective in enhancing the mechanical properties of glass, involves the use of various chemicals and energy-intensive processes that can impact the environment.
One of the primary environmental concerns is the use of molten salt baths, typically containing alkali metal ions such as potassium or sodium. These baths require high temperatures to maintain their molten state, resulting in significant energy consumption. The energy-intensive nature of the process contributes to increased carbon emissions, particularly if the energy source is not renewable.
The disposal of spent salt baths presents another environmental challenge. These baths accumulate impurities over time and eventually need replacement. Proper disposal methods must be employed to prevent soil and water contamination, as the salts can contain heavy metals and other potentially harmful substances.
Water usage is another factor to consider. The ion-exchange process often involves washing and rinsing steps to remove excess salts from the glass surface. This can lead to increased water consumption and the generation of wastewater that may require treatment before disposal.
The production of strengthened borosilicate glass also raises concerns about resource depletion. While borosilicate glass is more durable and resistant to thermal shock, its production requires specific raw materials, including boron compounds. The mining and processing of these materials can have environmental impacts, including habitat disruption and energy consumption.
On the positive side, the enhanced durability of ion-exchanged borosilicate glass can lead to longer product lifespans, potentially reducing waste and the need for frequent replacements. This longevity factor could offset some of the environmental costs associated with the production process.
Efforts to mitigate the environmental impact of ion-exchange strengthening are ongoing. Research into more energy-efficient heating methods, such as microwave-assisted ion exchange, shows promise in reducing energy consumption. Additionally, investigations into the recycling of spent salt baths and the development of more environmentally friendly salt compositions are underway.
The glass industry is also exploring ways to optimize the ion-exchange process to minimize material waste and improve energy efficiency. This includes developing precise control mechanisms for the exchange process and investigating alternative strengthening methods that may have a lower environmental footprint.
One of the primary environmental concerns is the use of molten salt baths, typically containing alkali metal ions such as potassium or sodium. These baths require high temperatures to maintain their molten state, resulting in significant energy consumption. The energy-intensive nature of the process contributes to increased carbon emissions, particularly if the energy source is not renewable.
The disposal of spent salt baths presents another environmental challenge. These baths accumulate impurities over time and eventually need replacement. Proper disposal methods must be employed to prevent soil and water contamination, as the salts can contain heavy metals and other potentially harmful substances.
Water usage is another factor to consider. The ion-exchange process often involves washing and rinsing steps to remove excess salts from the glass surface. This can lead to increased water consumption and the generation of wastewater that may require treatment before disposal.
The production of strengthened borosilicate glass also raises concerns about resource depletion. While borosilicate glass is more durable and resistant to thermal shock, its production requires specific raw materials, including boron compounds. The mining and processing of these materials can have environmental impacts, including habitat disruption and energy consumption.
On the positive side, the enhanced durability of ion-exchanged borosilicate glass can lead to longer product lifespans, potentially reducing waste and the need for frequent replacements. This longevity factor could offset some of the environmental costs associated with the production process.
Efforts to mitigate the environmental impact of ion-exchange strengthening are ongoing. Research into more energy-efficient heating methods, such as microwave-assisted ion exchange, shows promise in reducing energy consumption. Additionally, investigations into the recycling of spent salt baths and the development of more environmentally friendly salt compositions are underway.
The glass industry is also exploring ways to optimize the ion-exchange process to minimize material waste and improve energy efficiency. This includes developing precise control mechanisms for the exchange process and investigating alternative strengthening methods that may have a lower environmental footprint.
Regulatory Compliance
The regulatory landscape for ion-exchange strengthened borosilicate glass sheets is complex and multifaceted, encompassing various standards and guidelines across different industries and applications. In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating the use of these materials in food contact applications and medical devices. The FDA's Code of Federal Regulations (CFR) Title 21, particularly parts 174-179, outlines the requirements for food contact substances, including glass materials.
For the automotive industry, the Federal Motor Vehicle Safety Standards (FMVSS) set forth by the National Highway Traffic Safety Administration (NHTSA) govern the use of strengthened glass in vehicles. FMVSS No. 205 specifically addresses glazing materials, including requirements for impact resistance and optical properties.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation impacts the production and use of ion-exchange strengthened borosilicate glass sheets. Manufacturers must ensure compliance with REACH requirements, particularly concerning the chemicals used in the ion-exchange process.
The construction industry is subject to building codes and standards that regulate the use of glass in structures. The International Building Code (IBC) and its regional adaptations provide guidelines for the use of strengthened glass in buildings, including requirements for safety glazing and impact resistance.
Compliance with environmental regulations is also crucial. The Environmental Protection Agency (EPA) in the United States and similar agencies in other countries regulate the disposal of waste materials and emissions associated with the production of ion-exchange strengthened glass.
For consumer products, safety standards such as those set by the Consumer Product Safety Commission (CPSC) in the United States must be adhered to. These standards often include requirements for impact resistance and breakage characteristics of glass products.
International standards organizations, such as the International Organization for Standardization (ISO) and ASTM International, provide guidelines and testing methods for evaluating the properties of strengthened glass. ISO 20657:2017, for example, specifies test methods for chemically strengthened flat glass.
Manufacturers of ion-exchange strengthened borosilicate glass sheets must navigate this complex regulatory landscape, ensuring compliance with relevant standards across different industries and geographical regions. This often requires extensive testing and documentation to demonstrate that their products meet the necessary safety and performance criteria.
For the automotive industry, the Federal Motor Vehicle Safety Standards (FMVSS) set forth by the National Highway Traffic Safety Administration (NHTSA) govern the use of strengthened glass in vehicles. FMVSS No. 205 specifically addresses glazing materials, including requirements for impact resistance and optical properties.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation impacts the production and use of ion-exchange strengthened borosilicate glass sheets. Manufacturers must ensure compliance with REACH requirements, particularly concerning the chemicals used in the ion-exchange process.
The construction industry is subject to building codes and standards that regulate the use of glass in structures. The International Building Code (IBC) and its regional adaptations provide guidelines for the use of strengthened glass in buildings, including requirements for safety glazing and impact resistance.
Compliance with environmental regulations is also crucial. The Environmental Protection Agency (EPA) in the United States and similar agencies in other countries regulate the disposal of waste materials and emissions associated with the production of ion-exchange strengthened glass.
For consumer products, safety standards such as those set by the Consumer Product Safety Commission (CPSC) in the United States must be adhered to. These standards often include requirements for impact resistance and breakage characteristics of glass products.
International standards organizations, such as the International Organization for Standardization (ISO) and ASTM International, provide guidelines and testing methods for evaluating the properties of strengthened glass. ISO 20657:2017, for example, specifies test methods for chemically strengthened flat glass.
Manufacturers of ion-exchange strengthened borosilicate glass sheets must navigate this complex regulatory landscape, ensuring compliance with relevant standards across different industries and geographical regions. This often requires extensive testing and documentation to demonstrate that their products meet the necessary safety and performance criteria.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!