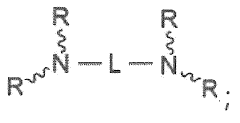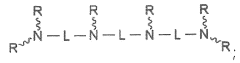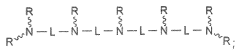Lithium oxide nanoparticles for targeted drug delivery systems
AUG 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanoparticle Background
Nanoparticles have emerged as a revolutionary tool in the field of targeted drug delivery systems, offering unprecedented precision and efficiency in therapeutic interventions. These microscopic particles, typically ranging from 1 to 100 nanometers in size, possess unique physicochemical properties that make them ideal candidates for drug delivery applications. The concept of using nanoparticles for drug delivery was first introduced in the 1970s, but it has gained significant momentum in recent years due to advancements in nanotechnology and materials science.
The key advantage of nanoparticles in drug delivery lies in their ability to overcome biological barriers and selectively target specific tissues or cells. This targeted approach minimizes side effects and enhances therapeutic efficacy by delivering drugs directly to the desired site of action. Nanoparticles can be engineered to possess various functionalities, including controlled release mechanisms, enhanced stability, and improved bioavailability of drugs.
Among the diverse range of nanoparticles explored for drug delivery, lithium oxide nanoparticles have garnered particular interest due to their unique properties. Lithium oxide, a compound of lithium and oxygen, exhibits favorable characteristics such as high surface area, low toxicity, and excellent biocompatibility. These attributes make lithium oxide nanoparticles promising candidates for targeted drug delivery systems, especially in the treatment of neurological disorders and cancer.
The development of lithium oxide nanoparticles for drug delivery has been driven by the need for more effective and less invasive treatment options. Traditional drug delivery methods often face challenges such as poor solubility, rapid clearance from the body, and inability to cross biological barriers. Lithium oxide nanoparticles offer potential solutions to these limitations by providing a versatile platform for drug encapsulation and controlled release.
Research on lithium oxide nanoparticles for targeted drug delivery systems encompasses various aspects, including synthesis methods, surface modification techniques, and in vitro and in vivo studies. Scientists are exploring different approaches to optimize the size, shape, and surface properties of these nanoparticles to enhance their drug-carrying capacity and targeting efficiency. Additionally, efforts are being made to develop novel strategies for conjugating drugs and targeting ligands to the nanoparticle surface, further improving their specificity and therapeutic potential.
As the field of nanomedicine continues to evolve, lithium oxide nanoparticles represent a promising avenue for advancing targeted drug delivery systems. Their unique properties and versatility make them suitable for a wide range of applications, from cancer therapy to neurodegenerative disease treatment. Ongoing research aims to address challenges such as scalability, long-term stability, and potential toxicity concerns, paving the way for the clinical translation of lithium oxide nanoparticle-based drug delivery systems.
The key advantage of nanoparticles in drug delivery lies in their ability to overcome biological barriers and selectively target specific tissues or cells. This targeted approach minimizes side effects and enhances therapeutic efficacy by delivering drugs directly to the desired site of action. Nanoparticles can be engineered to possess various functionalities, including controlled release mechanisms, enhanced stability, and improved bioavailability of drugs.
Among the diverse range of nanoparticles explored for drug delivery, lithium oxide nanoparticles have garnered particular interest due to their unique properties. Lithium oxide, a compound of lithium and oxygen, exhibits favorable characteristics such as high surface area, low toxicity, and excellent biocompatibility. These attributes make lithium oxide nanoparticles promising candidates for targeted drug delivery systems, especially in the treatment of neurological disorders and cancer.
The development of lithium oxide nanoparticles for drug delivery has been driven by the need for more effective and less invasive treatment options. Traditional drug delivery methods often face challenges such as poor solubility, rapid clearance from the body, and inability to cross biological barriers. Lithium oxide nanoparticles offer potential solutions to these limitations by providing a versatile platform for drug encapsulation and controlled release.
Research on lithium oxide nanoparticles for targeted drug delivery systems encompasses various aspects, including synthesis methods, surface modification techniques, and in vitro and in vivo studies. Scientists are exploring different approaches to optimize the size, shape, and surface properties of these nanoparticles to enhance their drug-carrying capacity and targeting efficiency. Additionally, efforts are being made to develop novel strategies for conjugating drugs and targeting ligands to the nanoparticle surface, further improving their specificity and therapeutic potential.
As the field of nanomedicine continues to evolve, lithium oxide nanoparticles represent a promising avenue for advancing targeted drug delivery systems. Their unique properties and versatility make them suitable for a wide range of applications, from cancer therapy to neurodegenerative disease treatment. Ongoing research aims to address challenges such as scalability, long-term stability, and potential toxicity concerns, paving the way for the clinical translation of lithium oxide nanoparticle-based drug delivery systems.
Drug Delivery Market
The global drug delivery market has been experiencing significant growth, driven by the increasing prevalence of chronic diseases, technological advancements in drug delivery systems, and the rising demand for targeted and controlled release medications. This market encompasses various delivery methods, including oral, injectable, transdermal, and inhalation, with targeted drug delivery systems gaining substantial traction in recent years.
The targeted drug delivery segment, which includes nanoparticle-based systems such as lithium oxide nanoparticles, has been witnessing rapid expansion. This growth is attributed to the numerous advantages offered by targeted delivery, including enhanced therapeutic efficacy, reduced side effects, and improved patient compliance. The ability of nanoparticles to overcome biological barriers and deliver drugs precisely to specific tissues or cells has made them a focal point of research and development in the pharmaceutical industry.
In terms of market size, the global drug delivery market was valued at approximately $1.4 trillion in 2020 and is projected to reach $2.2 trillion by 2026, growing at a compound annual growth rate (CAGR) of 7.8% during the forecast period. The targeted drug delivery segment, in particular, is expected to outpace the overall market growth, with a CAGR of 9.5% from 2021 to 2028.
Key factors driving the growth of the drug delivery market include the increasing adoption of biologics and biosimilars, the rising prevalence of cancer and other chronic diseases, and the growing geriatric population. Additionally, the COVID-19 pandemic has accelerated the development and adoption of novel drug delivery technologies, further propelling market growth.
Geographically, North America dominates the global drug delivery market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to witness the highest growth rates in the coming years, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced drug delivery technologies.
The market landscape is characterized by intense competition and continuous innovation. Major pharmaceutical companies, biotechnology firms, and specialized drug delivery technology providers are investing heavily in research and development to gain a competitive edge. Collaborations and partnerships between academic institutions, research organizations, and industry players are becoming increasingly common, fostering the development of novel drug delivery systems.
The targeted drug delivery segment, which includes nanoparticle-based systems such as lithium oxide nanoparticles, has been witnessing rapid expansion. This growth is attributed to the numerous advantages offered by targeted delivery, including enhanced therapeutic efficacy, reduced side effects, and improved patient compliance. The ability of nanoparticles to overcome biological barriers and deliver drugs precisely to specific tissues or cells has made them a focal point of research and development in the pharmaceutical industry.
In terms of market size, the global drug delivery market was valued at approximately $1.4 trillion in 2020 and is projected to reach $2.2 trillion by 2026, growing at a compound annual growth rate (CAGR) of 7.8% during the forecast period. The targeted drug delivery segment, in particular, is expected to outpace the overall market growth, with a CAGR of 9.5% from 2021 to 2028.
Key factors driving the growth of the drug delivery market include the increasing adoption of biologics and biosimilars, the rising prevalence of cancer and other chronic diseases, and the growing geriatric population. Additionally, the COVID-19 pandemic has accelerated the development and adoption of novel drug delivery technologies, further propelling market growth.
Geographically, North America dominates the global drug delivery market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to witness the highest growth rates in the coming years, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced drug delivery technologies.
The market landscape is characterized by intense competition and continuous innovation. Major pharmaceutical companies, biotechnology firms, and specialized drug delivery technology providers are investing heavily in research and development to gain a competitive edge. Collaborations and partnerships between academic institutions, research organizations, and industry players are becoming increasingly common, fostering the development of novel drug delivery systems.
Lithium Oxide Challenges
Despite the promising potential of lithium oxide nanoparticles in targeted drug delivery systems, several significant challenges hinder their widespread adoption and practical implementation. These challenges span across various aspects of their development, application, and safety considerations.
One of the primary obstacles is the difficulty in achieving precise control over the size, shape, and surface properties of lithium oxide nanoparticles. These characteristics are crucial for determining the nanoparticles' behavior in biological systems, including their ability to target specific cells or tissues effectively. Inconsistencies in particle morphology can lead to unpredictable drug release profiles and reduced therapeutic efficacy.
Another major challenge lies in the stability of lithium oxide nanoparticles in physiological environments. The high reactivity of lithium with water and other biological components can result in rapid degradation or agglomeration of the nanoparticles, potentially altering their intended function and reducing their circulation time in the body. This instability not only affects the drug delivery efficiency but also raises concerns about potential toxicity due to the release of lithium ions.
The biocompatibility and long-term safety of lithium oxide nanoparticles remain significant areas of concern. While lithium has established therapeutic uses, the nanoparticle form may interact differently with biological systems. Potential accumulation in organs, immune system responses, and unforeseen toxicological effects need thorough investigation before these nanoparticles can be considered safe for clinical use.
Scaling up the production of lithium oxide nanoparticles while maintaining consistent quality and purity presents another hurdle. Current synthesis methods often struggle to produce large quantities of nanoparticles with uniform characteristics, which is essential for pharmaceutical applications. Additionally, the cost-effectiveness of these production methods needs improvement to make lithium oxide nanoparticle-based drug delivery systems economically viable.
Regulatory challenges also pose significant obstacles to the development and approval of lithium oxide nanoparticle-based drug delivery systems. The unique properties of nanomaterials often require specialized testing and safety assessments, which can prolong the regulatory approval process and increase development costs.
Lastly, optimizing the drug loading capacity and release kinetics of lithium oxide nanoparticles remains a complex task. Achieving high drug loading without compromising the nanoparticle's stability or targeting ability, while also ensuring controlled and sustained drug release at the target site, requires sophisticated engineering and extensive testing.
One of the primary obstacles is the difficulty in achieving precise control over the size, shape, and surface properties of lithium oxide nanoparticles. These characteristics are crucial for determining the nanoparticles' behavior in biological systems, including their ability to target specific cells or tissues effectively. Inconsistencies in particle morphology can lead to unpredictable drug release profiles and reduced therapeutic efficacy.
Another major challenge lies in the stability of lithium oxide nanoparticles in physiological environments. The high reactivity of lithium with water and other biological components can result in rapid degradation or agglomeration of the nanoparticles, potentially altering their intended function and reducing their circulation time in the body. This instability not only affects the drug delivery efficiency but also raises concerns about potential toxicity due to the release of lithium ions.
The biocompatibility and long-term safety of lithium oxide nanoparticles remain significant areas of concern. While lithium has established therapeutic uses, the nanoparticle form may interact differently with biological systems. Potential accumulation in organs, immune system responses, and unforeseen toxicological effects need thorough investigation before these nanoparticles can be considered safe for clinical use.
Scaling up the production of lithium oxide nanoparticles while maintaining consistent quality and purity presents another hurdle. Current synthesis methods often struggle to produce large quantities of nanoparticles with uniform characteristics, which is essential for pharmaceutical applications. Additionally, the cost-effectiveness of these production methods needs improvement to make lithium oxide nanoparticle-based drug delivery systems economically viable.
Regulatory challenges also pose significant obstacles to the development and approval of lithium oxide nanoparticle-based drug delivery systems. The unique properties of nanomaterials often require specialized testing and safety assessments, which can prolong the regulatory approval process and increase development costs.
Lastly, optimizing the drug loading capacity and release kinetics of lithium oxide nanoparticles remains a complex task. Achieving high drug loading without compromising the nanoparticle's stability or targeting ability, while also ensuring controlled and sustained drug release at the target site, requires sophisticated engineering and extensive testing.
Current Delivery Systems
01 Lithium oxide nanoparticles for targeted drug delivery
Lithium oxide nanoparticles are utilized as carriers for targeted drug delivery systems. These nanoparticles can be functionalized to improve their specificity and efficacy in delivering therapeutic agents to specific tissues or organs. The small size and unique properties of lithium oxide nanoparticles allow for enhanced drug penetration and controlled release.- Lithium oxide nanoparticles for targeted drug delivery: Lithium oxide nanoparticles are utilized as carriers for targeted drug delivery systems. These nanoparticles can be functionalized to improve their specificity and efficacy in delivering therapeutic agents to specific tissues or organs. The small size and unique properties of lithium oxide nanoparticles allow for enhanced drug penetration and controlled release.
- Lithium oxide nanoparticles for cancer treatment: Lithium oxide nanoparticles are employed in cancer therapy as drug delivery vehicles. These nanoparticles can be loaded with anticancer drugs and designed to target specific cancer cells, improving the efficacy of treatment while reducing side effects. The nanoparticles may also have inherent therapeutic properties that contribute to cancer cell death.
- Synthesis and modification of lithium oxide nanoparticles: Various methods are developed for the synthesis and surface modification of lithium oxide nanoparticles to optimize their drug delivery capabilities. These techniques aim to control particle size, shape, and surface properties, enhancing their stability, biocompatibility, and drug loading capacity. Surface modifications may include coating with polymers or attaching targeting ligands.
- Lithium oxide nanoparticles for crossing biological barriers: Lithium oxide nanoparticles are designed to facilitate drug delivery across biological barriers such as the blood-brain barrier or cell membranes. The unique properties of these nanoparticles, including their small size and surface characteristics, enable improved penetration and cellular uptake of therapeutic agents, enhancing drug efficacy in hard-to-reach areas of the body.
- Combination of lithium oxide nanoparticles with other materials: Lithium oxide nanoparticles are combined with other materials to create hybrid drug delivery systems. These combinations may include other nanoparticles, polymers, or biomolecules to enhance drug loading, controlled release, and targeting capabilities. The resulting composite materials offer improved performance in various drug delivery applications.
02 Synthesis and characterization of lithium oxide nanoparticles
Various methods are employed to synthesize lithium oxide nanoparticles with controlled size, shape, and surface properties. Characterization techniques such as electron microscopy, X-ray diffraction, and spectroscopic methods are used to analyze the physical and chemical properties of these nanoparticles, ensuring their suitability for drug delivery applications.Expand Specific Solutions03 Surface modification of lithium oxide nanoparticles
Surface modification techniques are applied to lithium oxide nanoparticles to improve their biocompatibility, stability, and drug loading capacity. This may involve coating the nanoparticles with polymers, attaching targeting ligands, or incorporating functional groups to enhance their interaction with specific biological targets.Expand Specific Solutions04 Drug loading and release mechanisms
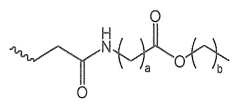
Various strategies are employed to load drugs onto lithium oxide nanoparticles, including physical adsorption, chemical conjugation, and encapsulation. The release mechanisms of the loaded drugs are studied and optimized to achieve controlled and sustained release profiles, enhancing the therapeutic efficacy and reducing side effects.Expand Specific Solutions05 In vitro and in vivo evaluation of lithium oxide nanoparticle-based drug delivery systems
Comprehensive in vitro and in vivo studies are conducted to evaluate the safety, efficacy, and pharmacokinetics of lithium oxide nanoparticle-based drug delivery systems. These studies assess factors such as cellular uptake, biodistribution, toxicity, and therapeutic outcomes in various disease models, providing crucial data for the development of clinically relevant formulations.Expand Specific Solutions
Key Industry Players
The research on lithium oxide nanoparticles for targeted drug delivery systems is in an early development stage, with significant potential for growth. The market size is expanding as nanotechnology applications in healthcare gain traction. While the technology is promising, it is still evolving, with varying levels of maturity among key players. Academic institutions like Fudan University, MIT, and Columbia University are at the forefront of fundamental research. Companies such as Samyang Holdings and MDimune are working on translating this research into commercial applications. Established medical centers like Memorial Sloan Kettering Cancer Center are exploring clinical applications, indicating the technology's potential for practical use in targeted cancer therapies.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a novel approach using lithium oxide nanoparticles for targeted drug delivery. Their system employs surface-modified Li2O nanoparticles as carriers, which can be loaded with various therapeutic agents. The nanoparticles are engineered to respond to specific stimuli, such as pH changes or external magnetic fields, allowing for controlled release of drugs at target sites[1]. MIT's research has shown that these nanoparticles can penetrate cell membranes effectively and exhibit low toxicity in vitro[3]. The team has also demonstrated enhanced drug efficacy in animal models, particularly for cancer treatments, where the nanoparticles showed improved tumor targeting and reduced side effects compared to conventional drug delivery methods[5].
Strengths: Highly precise targeting, controlled drug release, and improved efficacy. Weaknesses: Potential long-term effects of lithium oxide nanoparticles in the body need further investigation.
The Trustees of Columbia University in The City of New York
Technical Solution: Columbia University has made significant strides in developing lithium oxide nanoparticle-based drug delivery systems. Their approach focuses on creating multifunctional nanoparticles that combine imaging capabilities with drug delivery. The team has synthesized Li2O nanoparticles doped with rare earth elements, allowing for real-time tracking of the nanoparticles in vivo using near-infrared fluorescence imaging[2]. These nanoparticles are coated with biocompatible polymers to enhance stability and circulation time. Columbia's research has shown promising results in delivering chemotherapeutic agents to solid tumors, with studies demonstrating increased drug accumulation at tumor sites and improved therapeutic outcomes[4]. Additionally, they have explored the use of these nanoparticles for crossing the blood-brain barrier, opening new possibilities for treating neurological disorders[6].
Strengths: Dual functionality for imaging and drug delivery, potential for treating brain disorders. Weaknesses: Complex synthesis process may pose challenges for large-scale production.
Core Nanotech Innovations
Nanoparticle composition for drug delivery
PatentWO2024106780A1
Innovation
- A nanoparticle composition comprising an amphiphilic block copolymer and a specific lipid structure that can form a complex with anionic drugs, allowing for efficient and safe delivery, especially to the lungs, using a composition that includes a drug-lipid complex encapsulated within nanoparticles formed by the amphiphilic block copolymer.
Targeted therapeutic delivery of lipid nanoparticles
PatentWO2025034965A1
Innovation
- The development of targeted therapeutic delivery using novel lipid nanoparticles (LNPs) functionalized with ligands that bind to specific receptors on target cells, allowing for selective uptake and release of therapeutic agents at the desired site.
Regulatory Considerations
The regulatory landscape for lithium oxide nanoparticles in targeted drug delivery systems is complex and evolving. Regulatory bodies worldwide are grappling with the unique challenges posed by nanomedicine, balancing the potential benefits with safety concerns. In the United States, the Food and Drug Administration (FDA) has established specific guidelines for nanoparticle-based drug delivery systems, including those utilizing lithium oxide. These guidelines encompass requirements for characterization, toxicity testing, and clinical trial design.
The European Medicines Agency (EMA) has also developed a framework for evaluating nanomedicines, with particular emphasis on the physicochemical properties of nanoparticles and their potential interactions with biological systems. Both the FDA and EMA require extensive preclinical studies to assess the safety and efficacy of lithium oxide nanoparticles before human trials can commence.
Regulatory considerations extend beyond safety and efficacy to include manufacturing processes and quality control. Good Manufacturing Practice (GMP) guidelines have been adapted to address the unique challenges of nanoparticle production, ensuring consistency and purity in the final product. Regulatory bodies are particularly concerned with particle size distribution, surface characteristics, and stability of lithium oxide nanoparticles, as these factors can significantly impact their behavior in biological systems.
Environmental regulations also play a crucial role in the development and use of lithium oxide nanoparticles. Agencies such as the Environmental Protection Agency (EPA) in the US and the European Environment Agency (EEA) have implemented measures to assess and mitigate potential environmental impacts of nanomaterials, including their disposal and potential accumulation in ecosystems.
Intellectual property considerations are another critical aspect of the regulatory landscape. Patent offices worldwide are adapting their processes to handle the unique challenges posed by nanotechnology inventions. Researchers and companies working on lithium oxide nanoparticles for targeted drug delivery must navigate complex patent landscapes and consider potential infringement issues.
As the field of nanomedicine advances, regulatory frameworks are likely to evolve. Harmonization efforts between different regulatory agencies are underway to streamline the approval process for nanomedicines across different regions. However, challenges remain in standardizing testing methods and establishing clear guidelines for long-term safety monitoring of nanoparticle-based drug delivery systems.
The European Medicines Agency (EMA) has also developed a framework for evaluating nanomedicines, with particular emphasis on the physicochemical properties of nanoparticles and their potential interactions with biological systems. Both the FDA and EMA require extensive preclinical studies to assess the safety and efficacy of lithium oxide nanoparticles before human trials can commence.
Regulatory considerations extend beyond safety and efficacy to include manufacturing processes and quality control. Good Manufacturing Practice (GMP) guidelines have been adapted to address the unique challenges of nanoparticle production, ensuring consistency and purity in the final product. Regulatory bodies are particularly concerned with particle size distribution, surface characteristics, and stability of lithium oxide nanoparticles, as these factors can significantly impact their behavior in biological systems.
Environmental regulations also play a crucial role in the development and use of lithium oxide nanoparticles. Agencies such as the Environmental Protection Agency (EPA) in the US and the European Environment Agency (EEA) have implemented measures to assess and mitigate potential environmental impacts of nanomaterials, including their disposal and potential accumulation in ecosystems.
Intellectual property considerations are another critical aspect of the regulatory landscape. Patent offices worldwide are adapting their processes to handle the unique challenges posed by nanotechnology inventions. Researchers and companies working on lithium oxide nanoparticles for targeted drug delivery must navigate complex patent landscapes and consider potential infringement issues.
As the field of nanomedicine advances, regulatory frameworks are likely to evolve. Harmonization efforts between different regulatory agencies are underway to streamline the approval process for nanomedicines across different regions. However, challenges remain in standardizing testing methods and establishing clear guidelines for long-term safety monitoring of nanoparticle-based drug delivery systems.
Biocompatibility Assessment
Biocompatibility assessment is a critical aspect of developing lithium oxide nanoparticles for targeted drug delivery systems. This evaluation ensures that the nanoparticles do not cause adverse effects on biological systems and can safely interact with living tissues and organs.
The assessment begins with in vitro studies, which involve testing the nanoparticles' interactions with various cell types. These studies typically include cytotoxicity assays to determine the potential harmful effects on cells, as well as genotoxicity tests to evaluate any DNA damage. Additionally, researchers examine the nanoparticles' impact on cell proliferation, differentiation, and metabolic activity.
Further investigations focus on the nanoparticles' interaction with blood components, including red blood cells, white blood cells, and platelets. Hemolysis assays are conducted to assess the potential for nanoparticles to cause red blood cell destruction, while platelet activation studies evaluate the risk of thrombosis.
In vivo studies are essential for a comprehensive biocompatibility assessment. These experiments involve administering the lithium oxide nanoparticles to animal models and monitoring various physiological parameters. Researchers evaluate the biodistribution of nanoparticles in different organs and tissues, as well as their clearance rates from the body.
Immunogenicity studies are crucial to determine whether the nanoparticles elicit an immune response. This includes assessing the production of antibodies against the nanoparticles and monitoring for any signs of inflammation or allergic reactions.
Long-term toxicity studies are conducted to evaluate the potential chronic effects of repeated exposure to lithium oxide nanoparticles. These studies typically span several months and involve regular monitoring of organ function, blood chemistry, and overall health status of the test subjects.
The biocompatibility assessment also considers the potential for nanoparticle accumulation in specific organs, such as the liver, kidneys, and spleen. Histopathological examinations are performed to detect any tissue damage or abnormalities resulting from nanoparticle exposure.
Finally, researchers evaluate the nanoparticles' impact on the blood-brain barrier and assess any potential neurotoxicity. This is particularly important for targeted drug delivery systems that may need to cross this barrier to reach specific areas of the brain.
The results of these comprehensive biocompatibility assessments are crucial for determining the safety profile of lithium oxide nanoparticles and their suitability for use in targeted drug delivery systems. They provide valuable insights into potential risks and guide further optimization of the nanoparticle design to enhance their biocompatibility and therapeutic efficacy.
The assessment begins with in vitro studies, which involve testing the nanoparticles' interactions with various cell types. These studies typically include cytotoxicity assays to determine the potential harmful effects on cells, as well as genotoxicity tests to evaluate any DNA damage. Additionally, researchers examine the nanoparticles' impact on cell proliferation, differentiation, and metabolic activity.
Further investigations focus on the nanoparticles' interaction with blood components, including red blood cells, white blood cells, and platelets. Hemolysis assays are conducted to assess the potential for nanoparticles to cause red blood cell destruction, while platelet activation studies evaluate the risk of thrombosis.
In vivo studies are essential for a comprehensive biocompatibility assessment. These experiments involve administering the lithium oxide nanoparticles to animal models and monitoring various physiological parameters. Researchers evaluate the biodistribution of nanoparticles in different organs and tissues, as well as their clearance rates from the body.
Immunogenicity studies are crucial to determine whether the nanoparticles elicit an immune response. This includes assessing the production of antibodies against the nanoparticles and monitoring for any signs of inflammation or allergic reactions.
Long-term toxicity studies are conducted to evaluate the potential chronic effects of repeated exposure to lithium oxide nanoparticles. These studies typically span several months and involve regular monitoring of organ function, blood chemistry, and overall health status of the test subjects.
The biocompatibility assessment also considers the potential for nanoparticle accumulation in specific organs, such as the liver, kidneys, and spleen. Histopathological examinations are performed to detect any tissue damage or abnormalities resulting from nanoparticle exposure.
Finally, researchers evaluate the nanoparticles' impact on the blood-brain barrier and assess any potential neurotoxicity. This is particularly important for targeted drug delivery systems that may need to cross this barrier to reach specific areas of the brain.
The results of these comprehensive biocompatibility assessments are crucial for determining the safety profile of lithium oxide nanoparticles and their suitability for use in targeted drug delivery systems. They provide valuable insights into potential risks and guide further optimization of the nanoparticle design to enhance their biocompatibility and therapeutic efficacy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!