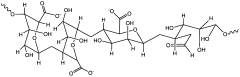
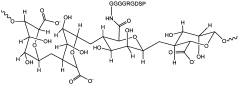
Microcrystalline Cellulose in Advanced Multimodal Therapy Systems for Cancer
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MCC in Cancer Therapy: Background and Objectives
Microcrystalline cellulose (MCC) has emerged as a promising material in the field of advanced cancer therapy systems. The evolution of MCC in this domain can be traced back to its initial applications in pharmaceutical formulations, where it was primarily used as an excipient. Over time, researchers recognized its potential for more sophisticated roles in drug delivery and cancer treatment.
The technological trajectory of MCC in cancer therapy has been marked by significant milestones. Early studies focused on its biocompatibility and ability to encapsulate various therapeutic agents. As research progressed, scientists began exploring MCC's capacity to enhance drug solubility, control release kinetics, and improve the overall efficacy of cancer treatments.
Recent advancements have propelled MCC into the realm of multimodal therapy systems. These systems aim to combine multiple treatment modalities, such as chemotherapy, radiotherapy, and immunotherapy, into a single, cohesive approach. MCC's versatility and adaptability make it an ideal candidate for developing such integrated platforms.
The primary objective of current research on MCC in advanced multimodal therapy systems for cancer is to create more effective, targeted, and personalized treatment options. Researchers are exploring ways to leverage MCC's unique properties to overcome existing limitations in cancer therapy, such as drug resistance, off-target effects, and poor bioavailability.
Key goals include developing MCC-based nanocarriers capable of simultaneously delivering multiple therapeutic agents, designing stimuli-responsive MCC systems for controlled drug release at tumor sites, and creating MCC-based scaffolds for localized therapy and tissue regeneration. Additionally, there is a growing interest in utilizing MCC's potential to enhance the efficacy of immunotherapies and improve the delivery of gene-based treatments.
The technological landscape is rapidly evolving, with researchers investigating novel MCC modifications and functionalization techniques to expand its capabilities in cancer therapy. This includes exploring surface modifications to improve targeting, developing hybrid MCC-based materials with enhanced properties, and integrating MCC with cutting-edge technologies such as theranostics and personalized medicine.
As the field progresses, the ultimate aim is to establish MCC as a cornerstone material in the development of next-generation cancer treatment modalities. This involves not only improving existing therapies but also paving the way for innovative approaches that could revolutionize cancer care, offering patients more effective, less invasive, and better-tolerated treatment options.
The technological trajectory of MCC in cancer therapy has been marked by significant milestones. Early studies focused on its biocompatibility and ability to encapsulate various therapeutic agents. As research progressed, scientists began exploring MCC's capacity to enhance drug solubility, control release kinetics, and improve the overall efficacy of cancer treatments.
Recent advancements have propelled MCC into the realm of multimodal therapy systems. These systems aim to combine multiple treatment modalities, such as chemotherapy, radiotherapy, and immunotherapy, into a single, cohesive approach. MCC's versatility and adaptability make it an ideal candidate for developing such integrated platforms.
The primary objective of current research on MCC in advanced multimodal therapy systems for cancer is to create more effective, targeted, and personalized treatment options. Researchers are exploring ways to leverage MCC's unique properties to overcome existing limitations in cancer therapy, such as drug resistance, off-target effects, and poor bioavailability.
Key goals include developing MCC-based nanocarriers capable of simultaneously delivering multiple therapeutic agents, designing stimuli-responsive MCC systems for controlled drug release at tumor sites, and creating MCC-based scaffolds for localized therapy and tissue regeneration. Additionally, there is a growing interest in utilizing MCC's potential to enhance the efficacy of immunotherapies and improve the delivery of gene-based treatments.
The technological landscape is rapidly evolving, with researchers investigating novel MCC modifications and functionalization techniques to expand its capabilities in cancer therapy. This includes exploring surface modifications to improve targeting, developing hybrid MCC-based materials with enhanced properties, and integrating MCC with cutting-edge technologies such as theranostics and personalized medicine.
As the field progresses, the ultimate aim is to establish MCC as a cornerstone material in the development of next-generation cancer treatment modalities. This involves not only improving existing therapies but also paving the way for innovative approaches that could revolutionize cancer care, offering patients more effective, less invasive, and better-tolerated treatment options.
Market Analysis for MCC-based Cancer Therapies
The market for microcrystalline cellulose (MCC)-based cancer therapies is experiencing significant growth, driven by the increasing prevalence of cancer and the demand for more effective, targeted treatment options. MCC, a purified form of cellulose derived from plant sources, has shown promising potential in advanced multimodal therapy systems for cancer due to its biocompatibility, versatility, and ability to enhance drug delivery.
The global cancer therapeutics market, which encompasses MCC-based therapies, was valued at approximately $158 billion in 2020 and is projected to reach $268 billion by 2026, growing at a CAGR of 9.15% during the forecast period. This growth is attributed to factors such as the rising incidence of cancer, advancements in cancer research, and increasing investments in drug development.
MCC-based cancer therapies are particularly gaining traction in the field of targeted drug delivery systems. The market for targeted drug delivery in cancer treatment is expected to grow at a CAGR of 6.5% from 2021 to 2028, with MCC playing a crucial role in this expansion. The ability of MCC to improve drug solubility, enhance bioavailability, and provide controlled release properties makes it an attractive option for pharmaceutical companies developing cancer therapies.
The demand for MCC in cancer treatment is also being driven by the growing focus on personalized medicine and combination therapies. As researchers continue to explore the potential of MCC in advanced multimodal therapy systems, the market is likely to witness the emergence of innovative products and treatment approaches. This trend is expected to create new opportunities for both established pharmaceutical companies and emerging biotech firms.
Geographically, North America currently dominates the market for MCC-based cancer therapies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to exhibit the highest growth rate in the coming years, driven by increasing healthcare expenditure, improving research infrastructure, and rising awareness about advanced cancer treatments.
Despite the promising outlook, the market for MCC-based cancer therapies faces challenges such as stringent regulatory requirements, high development costs, and competition from alternative treatment modalities. Nevertheless, ongoing research and development efforts, coupled with strategic collaborations between academic institutions and industry players, are expected to drive innovation and market growth in the coming years.
The global cancer therapeutics market, which encompasses MCC-based therapies, was valued at approximately $158 billion in 2020 and is projected to reach $268 billion by 2026, growing at a CAGR of 9.15% during the forecast period. This growth is attributed to factors such as the rising incidence of cancer, advancements in cancer research, and increasing investments in drug development.
MCC-based cancer therapies are particularly gaining traction in the field of targeted drug delivery systems. The market for targeted drug delivery in cancer treatment is expected to grow at a CAGR of 6.5% from 2021 to 2028, with MCC playing a crucial role in this expansion. The ability of MCC to improve drug solubility, enhance bioavailability, and provide controlled release properties makes it an attractive option for pharmaceutical companies developing cancer therapies.
The demand for MCC in cancer treatment is also being driven by the growing focus on personalized medicine and combination therapies. As researchers continue to explore the potential of MCC in advanced multimodal therapy systems, the market is likely to witness the emergence of innovative products and treatment approaches. This trend is expected to create new opportunities for both established pharmaceutical companies and emerging biotech firms.
Geographically, North America currently dominates the market for MCC-based cancer therapies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to exhibit the highest growth rate in the coming years, driven by increasing healthcare expenditure, improving research infrastructure, and rising awareness about advanced cancer treatments.
Despite the promising outlook, the market for MCC-based cancer therapies faces challenges such as stringent regulatory requirements, high development costs, and competition from alternative treatment modalities. Nevertheless, ongoing research and development efforts, coupled with strategic collaborations between academic institutions and industry players, are expected to drive innovation and market growth in the coming years.
Current Challenges in MCC-based Cancer Treatment
Despite the promising potential of microcrystalline cellulose (MCC) in advanced multimodal therapy systems for cancer treatment, several significant challenges persist in its practical application. These obstacles span across various aspects of MCC-based cancer therapies, from material properties to clinical implementation.
One of the primary challenges lies in the optimization of MCC's physicochemical properties for specific cancer treatment modalities. While MCC offers excellent biocompatibility and versatility, fine-tuning its characteristics such as particle size, crystallinity, and surface properties to achieve optimal drug loading, release kinetics, and targeted delivery remains a complex task. The heterogeneity of cancer types and microenvironments further complicates this optimization process, necessitating tailored MCC formulations for different cancer scenarios.
Another critical challenge is the development of effective functionalization strategies for MCC to enhance its therapeutic efficacy. Although MCC can be modified with various functional groups, achieving precise and stable functionalization without compromising its inherent properties is technically demanding. This is particularly crucial for targeted drug delivery and multimodal therapies, where MCC needs to interact specifically with cancer cells or respond to external stimuli.
The scalability and reproducibility of MCC-based cancer treatment systems pose significant hurdles in translating laboratory successes to clinical applications. Ensuring consistent quality and performance of MCC formulations at industrial scales, while maintaining cost-effectiveness, remains a major challenge. This is exacerbated by the complex regulatory landscape surrounding novel nanomaterials in cancer therapy.
Furthermore, the long-term safety and biodegradation of MCC in the human body are areas of ongoing concern. While MCC is generally considered safe, its fate and potential long-term effects when used in advanced cancer therapies, especially in combination with other treatment modalities, require extensive investigation. This includes understanding potential interactions with the immune system and assessing any unforeseen toxicities.
Integrating MCC-based systems into existing cancer treatment protocols presents another layer of complexity. Optimizing the synergy between MCC-delivered therapies and conventional treatments like chemotherapy or radiotherapy requires careful consideration of timing, dosing, and potential interactions. Additionally, developing standardized protocols for the clinical use of MCC-based therapies across different cancer types and stages remains a significant challenge.
Lastly, overcoming biological barriers for effective MCC-based drug delivery in cancer treatment continues to be a formidable challenge. This includes navigating the complex tumor microenvironment, achieving sufficient penetration into solid tumors, and addressing issues like multidrug resistance. Developing strategies to enhance the cellular uptake and intracellular trafficking of MCC-based therapeutics while minimizing off-target effects is crucial for improving treatment efficacy.
One of the primary challenges lies in the optimization of MCC's physicochemical properties for specific cancer treatment modalities. While MCC offers excellent biocompatibility and versatility, fine-tuning its characteristics such as particle size, crystallinity, and surface properties to achieve optimal drug loading, release kinetics, and targeted delivery remains a complex task. The heterogeneity of cancer types and microenvironments further complicates this optimization process, necessitating tailored MCC formulations for different cancer scenarios.
Another critical challenge is the development of effective functionalization strategies for MCC to enhance its therapeutic efficacy. Although MCC can be modified with various functional groups, achieving precise and stable functionalization without compromising its inherent properties is technically demanding. This is particularly crucial for targeted drug delivery and multimodal therapies, where MCC needs to interact specifically with cancer cells or respond to external stimuli.
The scalability and reproducibility of MCC-based cancer treatment systems pose significant hurdles in translating laboratory successes to clinical applications. Ensuring consistent quality and performance of MCC formulations at industrial scales, while maintaining cost-effectiveness, remains a major challenge. This is exacerbated by the complex regulatory landscape surrounding novel nanomaterials in cancer therapy.
Furthermore, the long-term safety and biodegradation of MCC in the human body are areas of ongoing concern. While MCC is generally considered safe, its fate and potential long-term effects when used in advanced cancer therapies, especially in combination with other treatment modalities, require extensive investigation. This includes understanding potential interactions with the immune system and assessing any unforeseen toxicities.
Integrating MCC-based systems into existing cancer treatment protocols presents another layer of complexity. Optimizing the synergy between MCC-delivered therapies and conventional treatments like chemotherapy or radiotherapy requires careful consideration of timing, dosing, and potential interactions. Additionally, developing standardized protocols for the clinical use of MCC-based therapies across different cancer types and stages remains a significant challenge.
Lastly, overcoming biological barriers for effective MCC-based drug delivery in cancer treatment continues to be a formidable challenge. This includes navigating the complex tumor microenvironment, achieving sufficient penetration into solid tumors, and addressing issues like multidrug resistance. Developing strategies to enhance the cellular uptake and intracellular trafficking of MCC-based therapeutics while minimizing off-target effects is crucial for improving treatment efficacy.
Existing MCC-based Multimodal Cancer Therapies
01 Production and modification of microcrystalline cellulose
Various methods are employed to produce and modify microcrystalline cellulose, including chemical treatments, mechanical processing, and enzymatic approaches. These processes aim to enhance the properties of microcrystalline cellulose for specific applications, such as improving its stability, particle size distribution, or functionality.- Production and modification of microcrystalline cellulose: Various methods are employed to produce and modify microcrystalline cellulose, including acid hydrolysis, enzymatic treatment, and mechanical processing. These techniques aim to improve the properties of microcrystalline cellulose for specific applications, such as enhancing its stability, particle size distribution, or functionality.
- Applications in pharmaceutical formulations: Microcrystalline cellulose is widely used in pharmaceutical formulations as an excipient. It serves various functions, including as a binder, disintegrant, and filler in tablet and capsule formulations. Its properties contribute to improved drug release, stability, and overall performance of pharmaceutical products.
- Use in food and cosmetic industries: Microcrystalline cellulose finds applications in food and cosmetic products as a stabilizer, thickener, and texturizing agent. It is used to improve the consistency, mouthfeel, and shelf-life of various food products, as well as in cosmetic formulations to enhance texture and stability.
- Composite materials and reinforcement: Microcrystalline cellulose is utilized in the development of composite materials, where it acts as a reinforcing agent. It can enhance the mechanical properties, thermal stability, and biodegradability of various materials, including plastics, paper, and construction materials.
- Sustainable and eco-friendly applications: As a biodegradable and renewable material, microcrystalline cellulose is being explored for sustainable and eco-friendly applications. This includes its use in biodegradable packaging materials, water treatment processes, and as a replacement for synthetic materials in various industries.
02 Applications in pharmaceutical formulations
Microcrystalline cellulose is widely used in pharmaceutical formulations as an excipient. It serves various functions, including as a binder, disintegrant, and filler in tablet and capsule formulations. Its properties contribute to improved drug release, stability, and overall performance of pharmaceutical products.Expand Specific Solutions03 Use in food and cosmetic industries
Microcrystalline cellulose finds applications in food and cosmetic products as a stabilizer, thickener, and texturizing agent. It is used to improve the consistency, mouthfeel, and shelf-life of various food items and personal care products.Expand Specific Solutions04 Composite materials and reinforcement
Microcrystalline cellulose is utilized in the development of composite materials, where it acts as a reinforcing agent. It can enhance the mechanical properties, thermal stability, and biodegradability of various materials, including plastics, paper, and construction materials.Expand Specific Solutions05 Sustainable and eco-friendly applications
As a biodegradable and renewable material, microcrystalline cellulose is being explored for sustainable and eco-friendly applications. This includes its use in biodegradable packaging materials, water treatment processes, and as a replacement for synthetic materials in various industries.Expand Specific Solutions
Key Players in MCC-based Cancer Treatment Research
The research on microcrystalline cellulose in advanced multimodal therapy systems for cancer is in an early developmental stage, with a growing market potential as cancer treatment innovations advance. The technology's maturity is still evolving, with various companies and institutions contributing to its progress. Key players like Bristol Myers Squibb, Roche, and Pfizer are investing in this area, alongside research institutions such as Memorial Sloan Kettering Cancer Center and the University of Connecticut. The competitive landscape is diverse, with both established pharmaceutical companies and emerging biotech firms exploring applications of microcrystalline cellulose in cancer therapies, indicating a promising but still-developing field.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has developed an advanced multimodal therapy system incorporating MCC for synergistic cancer treatment. Their approach combines MCC-based drug delivery with immunotherapy and radiotherapy. The MCC is used to create a porous, biodegradable scaffold that can be loaded with chemotherapeutic agents and immune-modulating compounds[7]. This scaffold is designed to be implanted at the tumor site post-surgery, providing sustained local drug release. Simultaneously, the scaffold acts as a depot for radioisotopes, enabling localized radiotherapy. The system also incorporates checkpoint inhibitors to enhance the body's immune response against cancer cells[8]. BMS has demonstrated that this multimodal approach can significantly improve treatment outcomes in preclinical models of solid tumors[9].
Strengths: Comprehensive treatment approach, potential for reducing tumor recurrence, and minimized systemic side effects. Weaknesses: Invasive implantation procedure and potential complications from local high-dose therapy.
Celgene Corp.
Technical Solution: Celgene has developed an innovative multimodal therapy system for cancer treatment utilizing MCC as a core component. Their approach combines MCC-based drug delivery with epigenetic modulation and targeted protein degradation. The MCC is used to create nanoparticles that encapsulate epigenetic modifiers, such as HDAC inhibitors, along with proteolysis-targeting chimeras (PROTACs)[13]. This system is designed to simultaneously alter gene expression patterns in cancer cells and selectively degrade oncogenic proteins. Celgene's research has demonstrated that this multimodal approach can overcome drug resistance mechanisms in various cancer types[14]. Additionally, they have incorporated stimuli-responsive elements into the MCC nanoparticles, allowing for triggered release of the therapeutic payload in response to specific tumor microenvironment conditions, such as pH or enzyme levels[15].
Strengths: Potential to address multiple cancer hallmarks simultaneously, ability to overcome drug resistance, and targeted delivery to tumor sites. Weaknesses: Complex formulation process and potential off-target effects of epigenetic modulation.
Innovations in MCC for Advanced Cancer Treatment
Diagnosis and therapy of cancer using advanced multifunctional magnetic nanostructures integrated with artificial intelligence technique
PatentPendingIN202341038933A
Innovation
- A framework integrating artificial intelligence algorithms with multifunctional magnetic nanostructures to analyze and predict the efficacy of cancer treatments by comparing patient reports stored in a database, utilizing these nanostructures for diagnosis and therapy.
Scaffolds for cell collection or elimination
PatentWO2009002401A2
Innovation
- A scaffold device with a bioactive composition that captures and eliminates targeted cells through selective filtration and adhesion, using differential permeability, bioactive molecules, and environmental deprivation, allowing for precise targeting and minimal disturbance to non-target cells.
Regulatory Landscape for MCC in Cancer Treatment
The regulatory landscape for microcrystalline cellulose (MCC) in cancer treatment is complex and multifaceted, reflecting the intricate nature of advanced multimodal therapy systems. As a pharmaceutical excipient, MCC is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration (FDA). However, its application in cancer treatment systems necessitates additional regulatory scrutiny.
In the United States, the FDA's Center for Drug Evaluation and Research (CDER) oversees the regulation of MCC-based cancer therapies. These treatments are typically classified as combination products, involving both drug and device components. The FDA's Office of Combination Products (OCP) plays a crucial role in determining the primary mode of action and assigning the appropriate regulatory pathway.
European regulatory bodies, such as the European Medicines Agency (EMA), have similar frameworks for evaluating MCC-based cancer therapies. The EMA's Committee for Medicinal Products for Human Use (CHMP) is responsible for assessing the safety, efficacy, and quality of these treatments. Additionally, the European Commission's Medical Device Regulation (MDR) may apply to certain aspects of MCC-based delivery systems.
Regulatory requirements for MCC in cancer treatment extend beyond traditional pharmaceutical regulations. Good Manufacturing Practice (GMP) guidelines are essential for ensuring the quality and consistency of MCC production. The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q3C on residual solvents, are relevant for MCC purification processes.
Clinical trials involving MCC-based cancer therapies must adhere to stringent ethical and safety standards. Institutional Review Boards (IRBs) and Ethics Committees play vital roles in overseeing these trials. The Declaration of Helsinki serves as a foundational document for ethical considerations in human subject research.
Regulatory bodies are increasingly focusing on patient-centric approaches in cancer treatment. This shift has implications for MCC-based therapies, particularly in terms of patient reported outcomes (PROs) and quality of life assessments. The FDA's Patient-Focused Drug Development (PFDD) initiative exemplifies this trend.
Environmental regulations also impact the development and production of MCC-based cancer therapies. Sustainable sourcing of cellulose and eco-friendly manufacturing processes are becoming increasingly important considerations. The Environmental Protection Agency (EPA) in the U.S. and the European Environment Agency (EEA) provide guidelines on environmental impact assessments.
As nanotechnology advances in cancer treatment, regulatory frameworks are evolving to address the unique challenges posed by nanoparticle-based drug delivery systems. The FDA's Nanotechnology Task Force and the EMA's Expert Group on Nanomedicines are at the forefront of developing appropriate regulatory strategies for these innovative therapies.
In the United States, the FDA's Center for Drug Evaluation and Research (CDER) oversees the regulation of MCC-based cancer therapies. These treatments are typically classified as combination products, involving both drug and device components. The FDA's Office of Combination Products (OCP) plays a crucial role in determining the primary mode of action and assigning the appropriate regulatory pathway.
European regulatory bodies, such as the European Medicines Agency (EMA), have similar frameworks for evaluating MCC-based cancer therapies. The EMA's Committee for Medicinal Products for Human Use (CHMP) is responsible for assessing the safety, efficacy, and quality of these treatments. Additionally, the European Commission's Medical Device Regulation (MDR) may apply to certain aspects of MCC-based delivery systems.
Regulatory requirements for MCC in cancer treatment extend beyond traditional pharmaceutical regulations. Good Manufacturing Practice (GMP) guidelines are essential for ensuring the quality and consistency of MCC production. The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q3C on residual solvents, are relevant for MCC purification processes.
Clinical trials involving MCC-based cancer therapies must adhere to stringent ethical and safety standards. Institutional Review Boards (IRBs) and Ethics Committees play vital roles in overseeing these trials. The Declaration of Helsinki serves as a foundational document for ethical considerations in human subject research.
Regulatory bodies are increasingly focusing on patient-centric approaches in cancer treatment. This shift has implications for MCC-based therapies, particularly in terms of patient reported outcomes (PROs) and quality of life assessments. The FDA's Patient-Focused Drug Development (PFDD) initiative exemplifies this trend.
Environmental regulations also impact the development and production of MCC-based cancer therapies. Sustainable sourcing of cellulose and eco-friendly manufacturing processes are becoming increasingly important considerations. The Environmental Protection Agency (EPA) in the U.S. and the European Environment Agency (EEA) provide guidelines on environmental impact assessments.
As nanotechnology advances in cancer treatment, regulatory frameworks are evolving to address the unique challenges posed by nanoparticle-based drug delivery systems. The FDA's Nanotechnology Task Force and the EMA's Expert Group on Nanomedicines are at the forefront of developing appropriate regulatory strategies for these innovative therapies.
Biocompatibility and Safety Considerations of MCC
Biocompatibility and safety are paramount considerations in the development of microcrystalline cellulose (MCC) for advanced multimodal cancer therapy systems. MCC, derived from natural cellulose sources, has shown promising potential as a versatile carrier for drug delivery and therapeutic applications. However, its integration into complex cancer treatment modalities necessitates a thorough evaluation of its biological interactions and safety profile.
The biocompatibility of MCC is largely attributed to its natural origin and chemical inertness. Numerous studies have demonstrated that MCC exhibits minimal cytotoxicity and does not elicit significant immune responses when introduced into biological systems. This favorable biocompatibility profile is crucial for its application in cancer therapy, where maintaining the integrity of healthy tissues is as important as targeting malignant cells.
In vitro assessments have shown that MCC particles are well-tolerated by various cell types, including both normal and cancerous cells. The absence of significant cellular damage or alterations in metabolic activity upon exposure to MCC supports its potential for use in drug delivery systems. Furthermore, in vivo studies in animal models have reported minimal systemic toxicity and no apparent organ-specific adverse effects following MCC administration.
However, the safety considerations of MCC extend beyond its inherent biocompatibility. The physical properties of MCC, such as particle size and surface characteristics, can significantly influence its biological interactions and potential risks. Nano-sized MCC particles, while offering enhanced cellular uptake and drug loading capacity, may pose unique safety challenges due to their ability to penetrate biological barriers more readily than larger particles.
The degradation profile of MCC in biological systems is another critical aspect of its safety assessment. While cellulose is generally considered biodegradable, the rate and products of MCC breakdown in vivo need careful evaluation to ensure no accumulation of potentially harmful byproducts. This is particularly important in the context of repeated or long-term administration in cancer therapy regimens.
Interactions between MCC and other components of multimodal therapy systems, such as chemotherapeutic agents, imaging contrast agents, or targeting moieties, must also be thoroughly investigated. These interactions could potentially alter the safety profile of the overall system, necessitating comprehensive compatibility studies.
Regulatory considerations play a crucial role in the development of MCC-based cancer therapy systems. Adherence to Good Manufacturing Practices (GMP) in the production of pharmaceutical-grade MCC is essential to ensure consistent quality and safety. Additionally, regulatory bodies such as the FDA and EMA have specific guidelines for the evaluation of novel biomaterials in medical applications, which must be carefully followed in the development process.
As research in this field progresses, long-term safety studies and post-market surveillance will be vital to identify any rare or delayed adverse effects associated with MCC use in cancer therapy. This ongoing vigilance will be crucial in establishing the safety profile of MCC-based systems and optimizing their therapeutic potential while minimizing risks to patients.
The biocompatibility of MCC is largely attributed to its natural origin and chemical inertness. Numerous studies have demonstrated that MCC exhibits minimal cytotoxicity and does not elicit significant immune responses when introduced into biological systems. This favorable biocompatibility profile is crucial for its application in cancer therapy, where maintaining the integrity of healthy tissues is as important as targeting malignant cells.
In vitro assessments have shown that MCC particles are well-tolerated by various cell types, including both normal and cancerous cells. The absence of significant cellular damage or alterations in metabolic activity upon exposure to MCC supports its potential for use in drug delivery systems. Furthermore, in vivo studies in animal models have reported minimal systemic toxicity and no apparent organ-specific adverse effects following MCC administration.
However, the safety considerations of MCC extend beyond its inherent biocompatibility. The physical properties of MCC, such as particle size and surface characteristics, can significantly influence its biological interactions and potential risks. Nano-sized MCC particles, while offering enhanced cellular uptake and drug loading capacity, may pose unique safety challenges due to their ability to penetrate biological barriers more readily than larger particles.
The degradation profile of MCC in biological systems is another critical aspect of its safety assessment. While cellulose is generally considered biodegradable, the rate and products of MCC breakdown in vivo need careful evaluation to ensure no accumulation of potentially harmful byproducts. This is particularly important in the context of repeated or long-term administration in cancer therapy regimens.
Interactions between MCC and other components of multimodal therapy systems, such as chemotherapeutic agents, imaging contrast agents, or targeting moieties, must also be thoroughly investigated. These interactions could potentially alter the safety profile of the overall system, necessitating comprehensive compatibility studies.
Regulatory considerations play a crucial role in the development of MCC-based cancer therapy systems. Adherence to Good Manufacturing Practices (GMP) in the production of pharmaceutical-grade MCC is essential to ensure consistent quality and safety. Additionally, regulatory bodies such as the FDA and EMA have specific guidelines for the evaluation of novel biomaterials in medical applications, which must be carefully followed in the development process.
As research in this field progresses, long-term safety studies and post-market surveillance will be vital to identify any rare or delayed adverse effects associated with MCC use in cancer therapy. This ongoing vigilance will be crucial in establishing the safety profile of MCC-based systems and optimizing their therapeutic potential while minimizing risks to patients.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!