Neurotransmitter Release Modifiers: Muscimol Insights
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neurotransmitter Modifiers Background and Objectives
Neurotransmitter release modifiers have been a subject of intense research in neuroscience for decades. These compounds play a crucial role in regulating synaptic transmission, influencing various neurological processes and behaviors. The study of these modifiers has evolved significantly, from early observations of neurotransmitter release mechanisms to the development of sophisticated tools for manipulating neural signaling.
The primary objective of research in this field is to understand the intricate mechanisms underlying neurotransmitter release and to develop novel therapeutic approaches for neurological disorders. Muscimol, a potent GABA receptor agonist, has emerged as a key compound in this research, offering valuable insights into inhibitory neurotransmission.
Historically, the investigation of neurotransmitter release modifiers began with the discovery of chemical neurotransmission in the early 20th century. Subsequent research focused on identifying specific neurotransmitters and their release mechanisms. The advent of electrophysiological techniques in the mid-20th century allowed for more detailed studies of synaptic transmission, paving the way for the discovery of compounds that could modulate this process.
In recent years, technological advancements have dramatically expanded our ability to study neurotransmitter release modifiers. High-resolution imaging techniques, optogenetics, and chemogenetics have provided unprecedented insights into the spatial and temporal dynamics of neurotransmitter release. These tools have enabled researchers to manipulate specific neural circuits with remarkable precision, furthering our understanding of how neurotransmitter release modifiers influence brain function.
The study of muscimol as a neurotransmitter release modifier has been particularly illuminating. Isolated from the mushroom Amanita muscaria, muscimol has been instrumental in elucidating the role of GABAergic transmission in various neural processes. Its potent and selective activation of GABA receptors has made it a valuable tool for investigating inhibitory neurotransmission in both basic research and potential therapeutic applications.
Current research goals in this field include developing more selective and efficient neurotransmitter release modifiers, understanding the long-term effects of these compounds on neural plasticity, and exploring their potential in treating neurological and psychiatric disorders. There is also a growing interest in combining neurotransmitter release modifiers with other therapeutic approaches, such as gene therapy and neural implants, to achieve more targeted and effective treatments.
As we look to the future, the study of neurotransmitter release modifiers, including muscimol, promises to yield significant advances in our understanding of brain function and the treatment of neurological disorders. The integration of cutting-edge technologies with molecular and cellular approaches is expected to drive progress in this field, potentially leading to breakthrough therapies for conditions ranging from epilepsy to neurodegenerative diseases.
The primary objective of research in this field is to understand the intricate mechanisms underlying neurotransmitter release and to develop novel therapeutic approaches for neurological disorders. Muscimol, a potent GABA receptor agonist, has emerged as a key compound in this research, offering valuable insights into inhibitory neurotransmission.
Historically, the investigation of neurotransmitter release modifiers began with the discovery of chemical neurotransmission in the early 20th century. Subsequent research focused on identifying specific neurotransmitters and their release mechanisms. The advent of electrophysiological techniques in the mid-20th century allowed for more detailed studies of synaptic transmission, paving the way for the discovery of compounds that could modulate this process.
In recent years, technological advancements have dramatically expanded our ability to study neurotransmitter release modifiers. High-resolution imaging techniques, optogenetics, and chemogenetics have provided unprecedented insights into the spatial and temporal dynamics of neurotransmitter release. These tools have enabled researchers to manipulate specific neural circuits with remarkable precision, furthering our understanding of how neurotransmitter release modifiers influence brain function.
The study of muscimol as a neurotransmitter release modifier has been particularly illuminating. Isolated from the mushroom Amanita muscaria, muscimol has been instrumental in elucidating the role of GABAergic transmission in various neural processes. Its potent and selective activation of GABA receptors has made it a valuable tool for investigating inhibitory neurotransmission in both basic research and potential therapeutic applications.
Current research goals in this field include developing more selective and efficient neurotransmitter release modifiers, understanding the long-term effects of these compounds on neural plasticity, and exploring their potential in treating neurological and psychiatric disorders. There is also a growing interest in combining neurotransmitter release modifiers with other therapeutic approaches, such as gene therapy and neural implants, to achieve more targeted and effective treatments.
As we look to the future, the study of neurotransmitter release modifiers, including muscimol, promises to yield significant advances in our understanding of brain function and the treatment of neurological disorders. The integration of cutting-edge technologies with molecular and cellular approaches is expected to drive progress in this field, potentially leading to breakthrough therapies for conditions ranging from epilepsy to neurodegenerative diseases.
Market Analysis for Neurotransmitter-Based Therapeutics
The neurotransmitter-based therapeutics market has experienced significant growth in recent years, driven by the increasing prevalence of neurological disorders and the growing understanding of neurotransmitter functions. This market segment encompasses a wide range of therapeutic applications, including treatments for depression, anxiety, Parkinson's disease, and epilepsy.
The global market for neurotransmitter-based therapeutics was valued at approximately $86 billion in 2020 and is projected to reach $130 billion by 2025, with a compound annual growth rate (CAGR) of 8.6%. This growth is attributed to several factors, including the rising incidence of neurological disorders, advancements in drug delivery technologies, and increased investment in research and development.
North America currently dominates the market, accounting for about 40% of the global share, followed by Europe and Asia-Pacific. The United States, in particular, leads in terms of market size and innovation, with a strong presence of pharmaceutical companies and research institutions focused on neurotransmitter-based therapies.
Within this market, GABA receptor modulators, such as muscimol and related compounds, represent a significant segment. The GABA receptor modulator market was valued at $3.2 billion in 2020 and is expected to grow at a CAGR of 5.8% through 2025. This growth is driven by the increasing use of GABA modulators in treating anxiety disorders, epilepsy, and insomnia.
The demand for novel neurotransmitter release modifiers, including muscimol-based therapies, is on the rise. This is due to their potential in treating a variety of neurological and psychiatric disorders with fewer side effects compared to traditional treatments. The market for muscimol and related compounds is expected to grow at a CAGR of 6.2% from 2021 to 2026.
Key market drivers include the growing aging population, which is more susceptible to neurological disorders, and the increasing awareness and diagnosis of mental health conditions. Additionally, the shift towards personalized medicine and targeted therapies is creating new opportunities for neurotransmitter-based therapeutics.
However, the market also faces challenges, such as stringent regulatory requirements and the high cost of drug development. The average cost to bring a new neurotransmitter-based drug to market exceeds $2.5 billion, which can be a significant barrier for smaller companies and startups.
Despite these challenges, the future outlook for the neurotransmitter-based therapeutics market remains positive. Emerging technologies, such as optogenetics and chemogenetics, are opening new avenues for precise manipulation of neurotransmitter release, potentially leading to more effective and targeted therapies. Furthermore, the integration of artificial intelligence and machine learning in drug discovery processes is expected to accelerate the development of novel neurotransmitter-based therapeutics, including muscimol-derived compounds.
The global market for neurotransmitter-based therapeutics was valued at approximately $86 billion in 2020 and is projected to reach $130 billion by 2025, with a compound annual growth rate (CAGR) of 8.6%. This growth is attributed to several factors, including the rising incidence of neurological disorders, advancements in drug delivery technologies, and increased investment in research and development.
North America currently dominates the market, accounting for about 40% of the global share, followed by Europe and Asia-Pacific. The United States, in particular, leads in terms of market size and innovation, with a strong presence of pharmaceutical companies and research institutions focused on neurotransmitter-based therapies.
Within this market, GABA receptor modulators, such as muscimol and related compounds, represent a significant segment. The GABA receptor modulator market was valued at $3.2 billion in 2020 and is expected to grow at a CAGR of 5.8% through 2025. This growth is driven by the increasing use of GABA modulators in treating anxiety disorders, epilepsy, and insomnia.
The demand for novel neurotransmitter release modifiers, including muscimol-based therapies, is on the rise. This is due to their potential in treating a variety of neurological and psychiatric disorders with fewer side effects compared to traditional treatments. The market for muscimol and related compounds is expected to grow at a CAGR of 6.2% from 2021 to 2026.
Key market drivers include the growing aging population, which is more susceptible to neurological disorders, and the increasing awareness and diagnosis of mental health conditions. Additionally, the shift towards personalized medicine and targeted therapies is creating new opportunities for neurotransmitter-based therapeutics.
However, the market also faces challenges, such as stringent regulatory requirements and the high cost of drug development. The average cost to bring a new neurotransmitter-based drug to market exceeds $2.5 billion, which can be a significant barrier for smaller companies and startups.
Despite these challenges, the future outlook for the neurotransmitter-based therapeutics market remains positive. Emerging technologies, such as optogenetics and chemogenetics, are opening new avenues for precise manipulation of neurotransmitter release, potentially leading to more effective and targeted therapies. Furthermore, the integration of artificial intelligence and machine learning in drug discovery processes is expected to accelerate the development of novel neurotransmitter-based therapeutics, including muscimol-derived compounds.
Current Challenges in Neurotransmitter Release Modification
The field of neurotransmitter release modification faces several significant challenges that hinder progress in developing effective therapeutic interventions. One of the primary obstacles is the complexity of the neurotransmitter release process itself. The intricate interplay between various proteins, ion channels, and signaling molecules involved in synaptic vesicle fusion and neurotransmitter exocytosis makes it difficult to target specific components without disrupting the entire system.
Another major challenge lies in achieving selective modulation of neurotransmitter release. Many current approaches lack specificity, affecting multiple neurotransmitter systems simultaneously. This non-selective action often leads to unintended side effects and limits the therapeutic potential of these interventions. Developing compounds that can selectively target specific neurotransmitter systems or even specific synapses remains a significant hurdle.
The blood-brain barrier (BBB) presents a formidable obstacle in the development of neurotransmitter release modifiers. Many promising compounds that show efficacy in vitro fail to cross the BBB effectively, limiting their potential for clinical use. Overcoming this barrier without compromising the integrity of the BBB or causing systemic side effects is a critical challenge in the field.
Temporal control of neurotransmitter release modification is another area of difficulty. Current methods often lack the precision to modulate neurotransmitter release on a millisecond timescale, which is crucial for mimicking natural neural signaling patterns. Developing technologies that allow for rapid and reversible control of neurotransmitter release remains an important goal.
The heterogeneity of neural circuits and the diverse roles of neurotransmitters in different brain regions add another layer of complexity. What may be beneficial in one neural circuit could be detrimental in another, making it challenging to develop broadly applicable therapies. This necessitates a more nuanced approach to neurotransmitter release modification, potentially requiring the development of region-specific or circuit-specific interventions.
Long-term effects and potential compensatory mechanisms pose additional challenges. Chronic modulation of neurotransmitter release may lead to adaptive changes in neural circuits, potentially reducing therapeutic efficacy over time or causing unexpected side effects. Understanding and mitigating these long-term consequences is crucial for developing sustainable therapeutic strategies.
Finally, translating findings from animal models to human applications remains a significant hurdle. The complexity of the human brain and the limitations of current animal models in fully recapitulating human neurological conditions make it challenging to predict the efficacy and safety of neurotransmitter release modifiers in clinical settings. Bridging this translational gap requires innovative approaches in preclinical research and careful design of clinical trials.
Another major challenge lies in achieving selective modulation of neurotransmitter release. Many current approaches lack specificity, affecting multiple neurotransmitter systems simultaneously. This non-selective action often leads to unintended side effects and limits the therapeutic potential of these interventions. Developing compounds that can selectively target specific neurotransmitter systems or even specific synapses remains a significant hurdle.
The blood-brain barrier (BBB) presents a formidable obstacle in the development of neurotransmitter release modifiers. Many promising compounds that show efficacy in vitro fail to cross the BBB effectively, limiting their potential for clinical use. Overcoming this barrier without compromising the integrity of the BBB or causing systemic side effects is a critical challenge in the field.
Temporal control of neurotransmitter release modification is another area of difficulty. Current methods often lack the precision to modulate neurotransmitter release on a millisecond timescale, which is crucial for mimicking natural neural signaling patterns. Developing technologies that allow for rapid and reversible control of neurotransmitter release remains an important goal.
The heterogeneity of neural circuits and the diverse roles of neurotransmitters in different brain regions add another layer of complexity. What may be beneficial in one neural circuit could be detrimental in another, making it challenging to develop broadly applicable therapies. This necessitates a more nuanced approach to neurotransmitter release modification, potentially requiring the development of region-specific or circuit-specific interventions.
Long-term effects and potential compensatory mechanisms pose additional challenges. Chronic modulation of neurotransmitter release may lead to adaptive changes in neural circuits, potentially reducing therapeutic efficacy over time or causing unexpected side effects. Understanding and mitigating these long-term consequences is crucial for developing sustainable therapeutic strategies.
Finally, translating findings from animal models to human applications remains a significant hurdle. The complexity of the human brain and the limitations of current animal models in fully recapitulating human neurological conditions make it challenging to predict the efficacy and safety of neurotransmitter release modifiers in clinical settings. Bridging this translational gap requires innovative approaches in preclinical research and careful design of clinical trials.
Existing Approaches to Neurotransmitter Release Modulation
01 Muscimol's effect on neurotransmitter release
Muscimol, a GABA receptor agonist, influences neurotransmitter release in the central nervous system. It primarily acts by binding to GABA-A receptors, which can lead to inhibition of neuronal activity and modulation of neurotransmitter release. This mechanism is crucial in understanding the pharmacological effects of muscimol and its potential therapeutic applications.- Muscimol's effect on GABA receptors: Muscimol, a potent GABA receptor agonist, influences neurotransmitter release by activating GABA receptors. This activation can lead to inhibition of neuronal activity and modulation of neurotransmitter release in various brain regions. The compound's interaction with GABA receptors plays a crucial role in its effects on neurotransmission.
- Muscimol's impact on glutamate release: Muscimol has been found to affect glutamate release in the brain. By activating GABA receptors, muscimol can indirectly modulate glutamatergic neurotransmission. This interaction between GABAergic and glutamatergic systems is important for understanding the overall effects of muscimol on neurotransmitter release and neuronal excitability.
- Use of muscimol in neurological disorders: Muscimol's ability to modulate neurotransmitter release has led to its investigation in various neurological disorders. Research has explored its potential therapeutic applications in conditions such as epilepsy, anxiety, and sleep disorders. The compound's GABAergic effects may contribute to its potential in treating these conditions.
- Muscimol analogs and derivatives: Researchers have developed and studied various muscimol analogs and derivatives to enhance its effects on neurotransmitter release or improve its pharmacological properties. These modified compounds may offer improved selectivity, potency, or bioavailability compared to the parent compound, potentially leading to new therapeutic applications.
- Muscimol's interaction with other neurotransmitter systems: While primarily known for its effects on GABAergic transmission, muscimol has been found to interact with other neurotransmitter systems. These interactions may involve direct or indirect effects on the release of neurotransmitters such as dopamine, serotonin, or norepinephrine, contributing to the compound's overall impact on brain function and behavior.
02 Muscimol analogs and derivatives for neurotransmitter modulation
Research has focused on developing muscimol analogs and derivatives to enhance its effects on neurotransmitter release or improve its pharmacokinetic properties. These modified compounds may offer improved selectivity, potency, or reduced side effects compared to muscimol itself, potentially leading to new therapeutic agents for neurological disorders.Expand Specific Solutions03 Muscimol in combination therapies
Muscimol is being investigated in combination with other compounds to enhance its effects on neurotransmitter release or to target multiple aspects of neurological disorders. These combination therapies may provide synergistic effects, potentially improving treatment outcomes for conditions such as epilepsy, anxiety, or sleep disorders.Expand Specific Solutions04 Muscimol's role in neuroprotection and neuroplasticity
Studies have explored muscimol's potential neuroprotective effects and its impact on neuroplasticity. By modulating neurotransmitter release, muscimol may help protect neurons from excitotoxicity and promote synaptic plasticity, which could have implications for treating neurodegenerative diseases and promoting recovery after brain injury.Expand Specific Solutions05 Novel delivery methods for muscimol
Researchers are developing innovative delivery methods to enhance the efficacy of muscimol in modulating neurotransmitter release. These may include targeted delivery systems, controlled-release formulations, or novel routes of administration to improve muscimol's bioavailability and reduce potential side effects.Expand Specific Solutions
Key Players in Neuropharmacology and Drug Discovery
The research on neurotransmitter release modifiers, particularly focusing on muscimol insights, is in a developing stage with growing market potential. The field is attracting attention from both established pharmaceutical companies and emerging biotech firms, indicating a competitive landscape. Major players like ACADIA Pharmaceuticals, GlaxoSmithKline, and Abbott Laboratories are investing in this area, leveraging their extensive R&D capabilities. Smaller, specialized companies such as Mind Medicine and Vertex Pharmaceuticals are also making significant contributions, often focusing on novel approaches. The technology's maturity varies, with some companies in clinical trial phases while others are still in preclinical research, suggesting a diverse range of development stages across the industry.
Glaxo Group Ltd.
Technical Solution: Glaxo Group Ltd., part of GlaxoSmithKline (GSK), has been at the forefront of neuroscience research, including studies on neurotransmitter release modifiers. While not specifically focused on muscimol, their work on GABA modulators has contributed to the field. GSK has developed several compounds that target GABA receptors, including positive allosteric modulators that enhance GABA signaling[9]. Their research has also explored the potential of combining GABA modulators with other neurotransmitter system interventions for more comprehensive treatment approaches[10]. GSK's advanced computational modeling techniques have allowed for the prediction of compound interactions with GABA receptors, potentially accelerating the discovery of new muscimol-like substances[11].
Strengths: Comprehensive neuroscience research program, advanced computational modeling capabilities. Weaknesses: Diverse research portfolio may limit resources for specific GABA-A agonist development.
Abbott Laboratories
Technical Solution: Abbott Laboratories has contributed significantly to neurotransmitter release modifier research through its pharmaceutical division (now part of AbbVie). Their work on GABA-related compounds has provided valuable insights into the mechanisms of action for substances like muscimol. Abbott's research has focused on developing novel formulations and delivery methods for neurotransmitter modulators, potentially enhancing the efficacy and reducing side effects of compounds like muscimol[7]. The company has also invested in advanced screening technologies to identify new molecules that can modulate neurotransmitter release with greater specificity and fewer off-target effects[8]. While not directly working on muscimol, their research on related GABAergic compounds has contributed to the broader understanding of neurotransmitter modulation in neurological disorders.
Strengths: Strong expertise in drug formulation and delivery, advanced screening technologies. Weaknesses: Broader focus may dilute resources dedicated to specific neurotransmitter systems.
Muscimol: Mechanism and Therapeutic Potential
Diazabicyclic central nervous system active agents
PatentInactiveUS6809105B2
Innovation
- Development of N-substituted diazabicyclic compounds that selectively control neurotransmitter release by modulating nicotinic acetylcholine receptors, offering therapeutic utility in treating neurological disorders and pain management, potentially used in combination with other medications like opioids or anticonvulsants.
Polyphenol compounds with modulating neurotransmitter release
PatentActiveUS8263643B2
Innovation
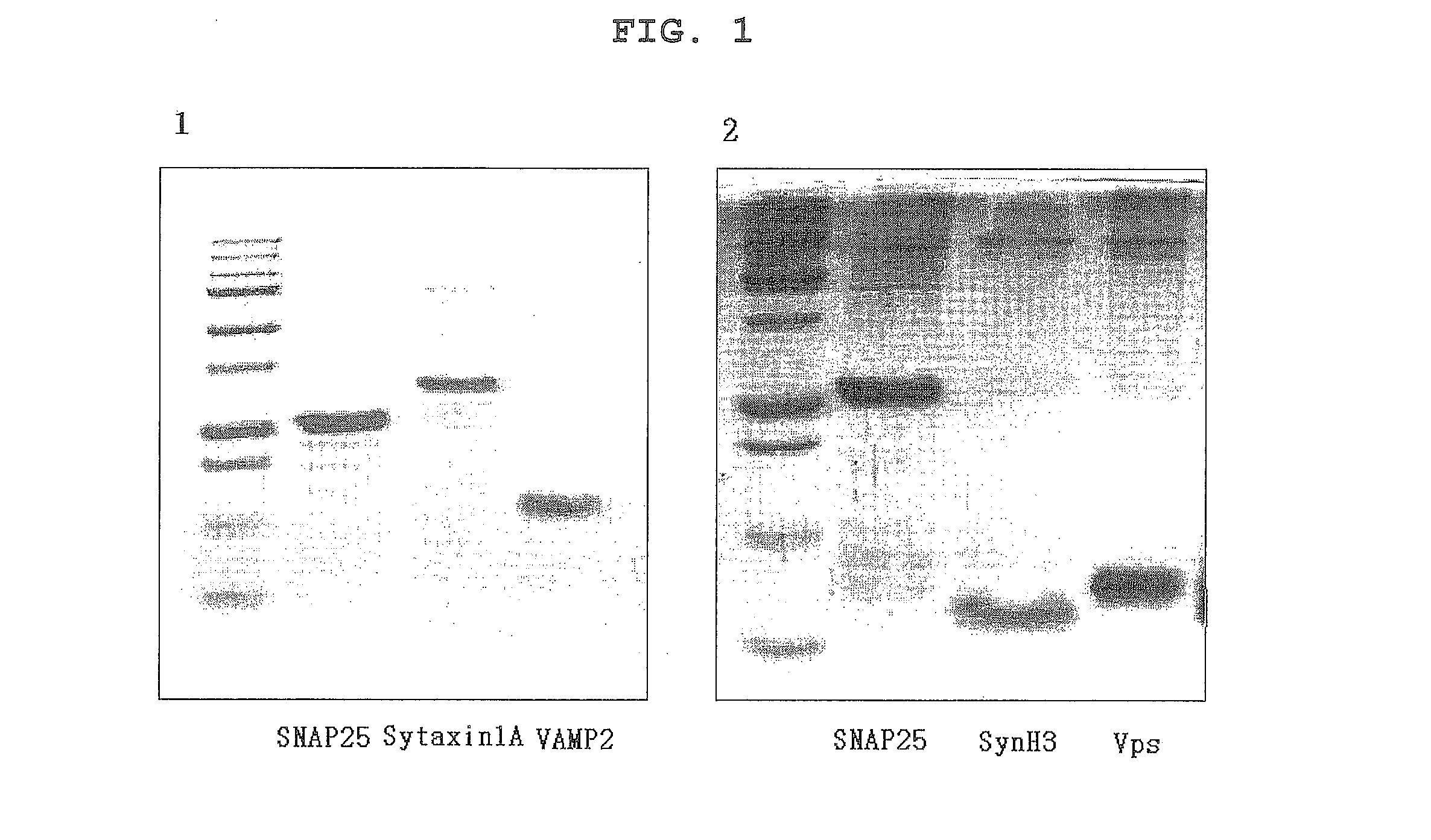
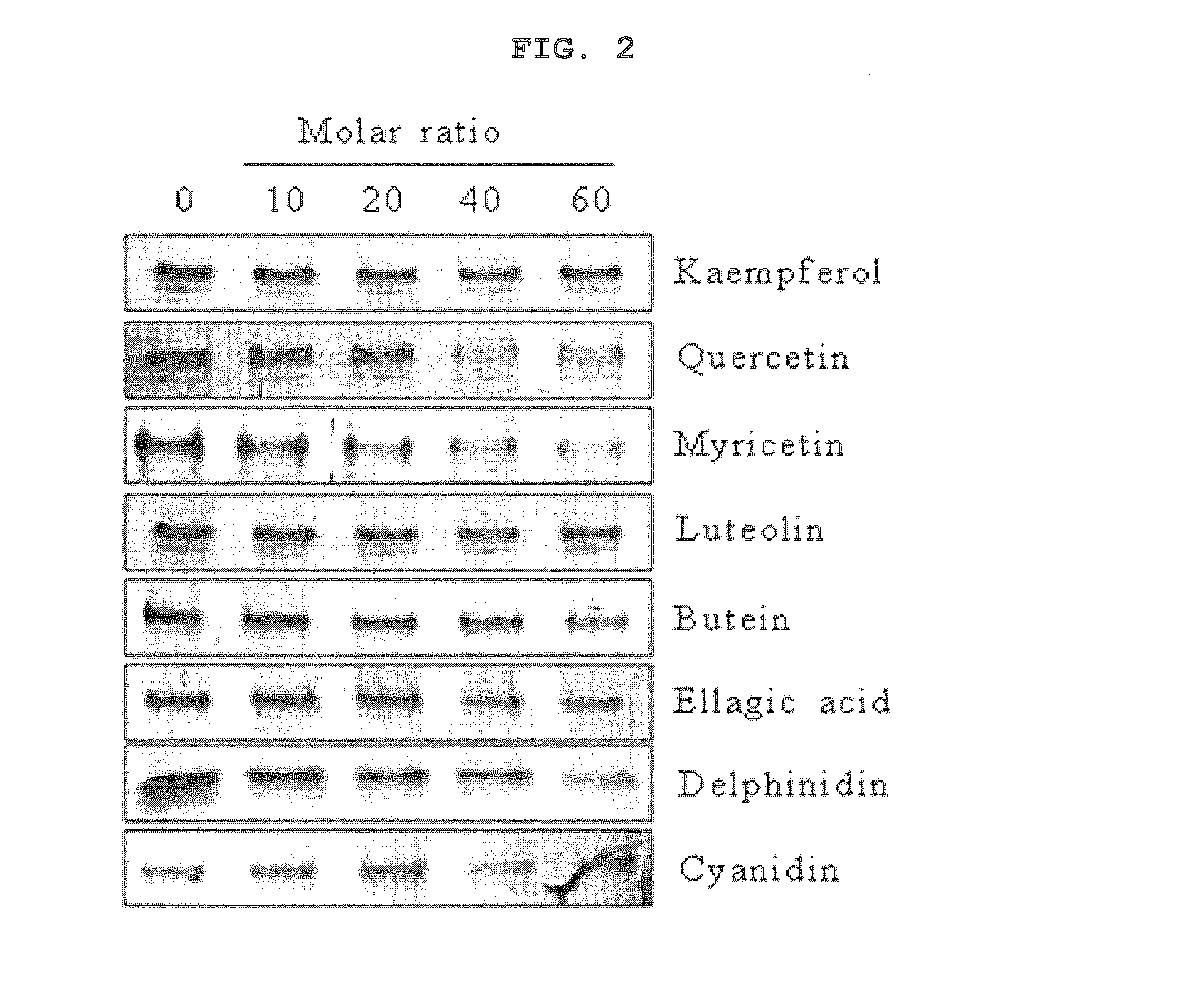
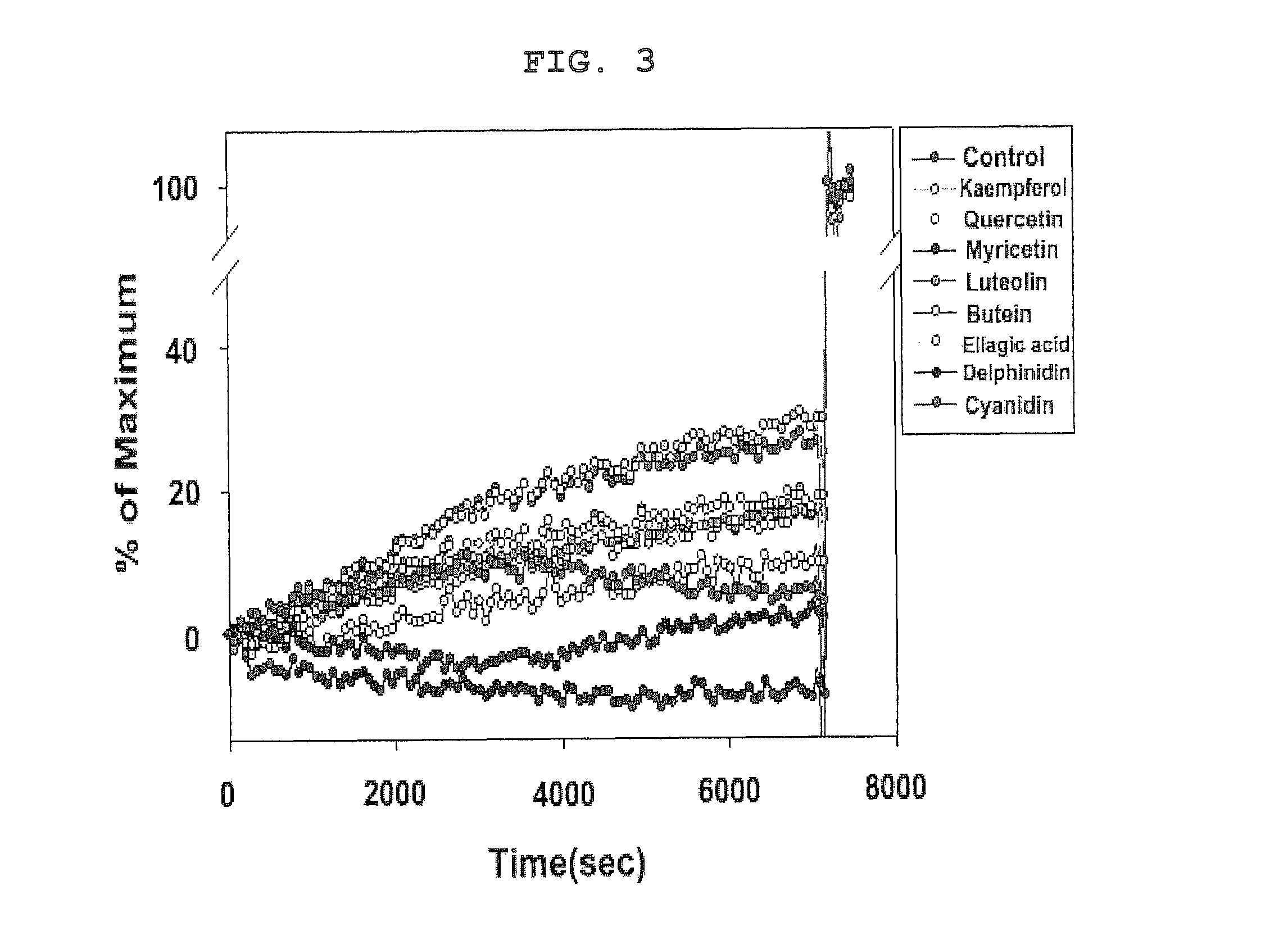
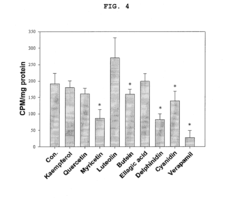
- A composition containing polyphenol compounds like kaempferol, quercetin, myricetin, luteolin, delphinidin, cyanidin, butein, and ellagic acid, which inhibit SNARE complex formation and neurotransmitter release, providing a skin-wrinkle reducing and pain-relieving effect.
Regulatory Framework for Neuropharmaceutical Development
The regulatory framework for neuropharmaceutical development plays a crucial role in ensuring the safety, efficacy, and ethical use of neurotransmitter release modifiers such as muscimol. This framework encompasses various stages of drug development, from preclinical research to clinical trials and post-market surveillance.
At the preclinical stage, researchers must adhere to strict guidelines for animal testing and in vitro studies. These regulations aim to minimize animal suffering while maximizing the validity and reproducibility of results. For muscimol and related compounds, special attention is given to neurotoxicity assessments and potential long-term effects on brain function.
Clinical trials for neuropharmaceuticals are subject to rigorous oversight by regulatory bodies such as the FDA in the United States and the EMA in Europe. These agencies require comprehensive documentation of study protocols, safety monitoring plans, and informed consent procedures. Given the potential for muscimol to affect cognitive function, additional safeguards may be necessary to protect vulnerable populations.
The regulatory framework also addresses the manufacturing and quality control of neuropharmaceuticals. Good Manufacturing Practices (GMP) must be followed to ensure consistent product quality and purity. For muscimol-based drugs, specific guidelines may be implemented to monitor for potential contaminants or degradation products that could affect neurological function.
Post-market surveillance is a critical component of the regulatory framework for neuropharmaceuticals. Manufacturers are required to conduct ongoing safety monitoring and report adverse events to regulatory authorities. This is particularly important for drugs like muscimol that may have subtle or long-term effects on brain function.
Ethical considerations are deeply embedded in the regulatory framework for neuropharmaceuticals. This includes guidelines for obtaining informed consent from research participants, protecting patient privacy, and ensuring equitable access to potentially beneficial treatments. For muscimol and other neurotransmitter modifiers, there may be additional ethical considerations related to their potential impact on personality or cognitive abilities.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to streamline regulatory processes across different countries. This is particularly relevant for neuropharmaceuticals, as global collaboration can accelerate the development of new treatments for neurological disorders.
At the preclinical stage, researchers must adhere to strict guidelines for animal testing and in vitro studies. These regulations aim to minimize animal suffering while maximizing the validity and reproducibility of results. For muscimol and related compounds, special attention is given to neurotoxicity assessments and potential long-term effects on brain function.
Clinical trials for neuropharmaceuticals are subject to rigorous oversight by regulatory bodies such as the FDA in the United States and the EMA in Europe. These agencies require comprehensive documentation of study protocols, safety monitoring plans, and informed consent procedures. Given the potential for muscimol to affect cognitive function, additional safeguards may be necessary to protect vulnerable populations.
The regulatory framework also addresses the manufacturing and quality control of neuropharmaceuticals. Good Manufacturing Practices (GMP) must be followed to ensure consistent product quality and purity. For muscimol-based drugs, specific guidelines may be implemented to monitor for potential contaminants or degradation products that could affect neurological function.
Post-market surveillance is a critical component of the regulatory framework for neuropharmaceuticals. Manufacturers are required to conduct ongoing safety monitoring and report adverse events to regulatory authorities. This is particularly important for drugs like muscimol that may have subtle or long-term effects on brain function.
Ethical considerations are deeply embedded in the regulatory framework for neuropharmaceuticals. This includes guidelines for obtaining informed consent from research participants, protecting patient privacy, and ensuring equitable access to potentially beneficial treatments. For muscimol and other neurotransmitter modifiers, there may be additional ethical considerations related to their potential impact on personality or cognitive abilities.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to streamline regulatory processes across different countries. This is particularly relevant for neuropharmaceuticals, as global collaboration can accelerate the development of new treatments for neurological disorders.
Ethical Considerations in Neurotransmitter Manipulation
The ethical considerations surrounding neurotransmitter manipulation, particularly in the context of muscimol research, are complex and multifaceted. As scientific understanding of neurotransmitter release modifiers advances, it becomes increasingly important to address the ethical implications of their use and development.
One primary concern is the potential for misuse or abuse of neurotransmitter-modifying substances. Muscimol, as a potent GABA receptor agonist, has significant effects on neural activity and behavior. While its therapeutic potential is promising, there is a risk of unintended consequences if used improperly or without adequate oversight. This raises questions about responsible access and distribution of such compounds.
The long-term effects of neurotransmitter manipulation on brain function and overall health are not yet fully understood. Ethical research practices must prioritize thorough safety assessments and long-term follow-up studies to ensure that potential risks are identified and mitigated. This is particularly crucial when considering the use of muscimol or similar compounds in clinical applications.
Another ethical consideration is the impact on personal identity and autonomy. Neurotransmitter modifiers can significantly alter mood, cognition, and behavior. This raises philosophical questions about the nature of self and the extent to which artificially modifying brain chemistry affects an individual's authentic experiences and decision-making capacity.
The use of neurotransmitter release modifiers in non-medical contexts, such as cognitive enhancement or recreational use, presents additional ethical challenges. There are concerns about fairness and equality if such substances become widely available, potentially creating disparities in cognitive abilities or opportunities based on access to these compounds.
Privacy and consent are also critical ethical issues in neurotransmitter research. As our understanding of brain chemistry grows more sophisticated, there is a need to protect individuals' neurological data and ensure informed consent in both research and potential therapeutic applications.
The development of muscimol-based therapies or other neurotransmitter modifiers also raises questions about the medicalization of normal human experiences. There is a fine line between treating genuine disorders and potentially pathologizing natural variations in human cognition and emotion.
Lastly, the ethical implications extend to broader societal impacts. The widespread use of neurotransmitter modifiers could potentially alter social dynamics, interpersonal relationships, and cultural norms. It is crucial to consider these wider implications alongside the immediate benefits and risks to individuals.
One primary concern is the potential for misuse or abuse of neurotransmitter-modifying substances. Muscimol, as a potent GABA receptor agonist, has significant effects on neural activity and behavior. While its therapeutic potential is promising, there is a risk of unintended consequences if used improperly or without adequate oversight. This raises questions about responsible access and distribution of such compounds.
The long-term effects of neurotransmitter manipulation on brain function and overall health are not yet fully understood. Ethical research practices must prioritize thorough safety assessments and long-term follow-up studies to ensure that potential risks are identified and mitigated. This is particularly crucial when considering the use of muscimol or similar compounds in clinical applications.
Another ethical consideration is the impact on personal identity and autonomy. Neurotransmitter modifiers can significantly alter mood, cognition, and behavior. This raises philosophical questions about the nature of self and the extent to which artificially modifying brain chemistry affects an individual's authentic experiences and decision-making capacity.
The use of neurotransmitter release modifiers in non-medical contexts, such as cognitive enhancement or recreational use, presents additional ethical challenges. There are concerns about fairness and equality if such substances become widely available, potentially creating disparities in cognitive abilities or opportunities based on access to these compounds.
Privacy and consent are also critical ethical issues in neurotransmitter research. As our understanding of brain chemistry grows more sophisticated, there is a need to protect individuals' neurological data and ensure informed consent in both research and potential therapeutic applications.
The development of muscimol-based therapies or other neurotransmitter modifiers also raises questions about the medicalization of normal human experiences. There is a fine line between treating genuine disorders and potentially pathologizing natural variations in human cognition and emotion.
Lastly, the ethical implications extend to broader societal impacts. The widespread use of neurotransmitter modifiers could potentially alter social dynamics, interpersonal relationships, and cultural norms. It is crucial to consider these wider implications alongside the immediate benefits and risks to individuals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!