Phenolphthalein in Biological pH Imaging and Mapping
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein pH Imaging Background and Objectives
Phenolphthalein, a widely recognized pH indicator, has been a cornerstone in analytical chemistry for over a century. Its unique color-changing properties in response to pH variations have made it an invaluable tool in various scientific disciplines. In recent years, there has been a growing interest in exploring the potential of phenolphthalein for biological pH imaging and mapping, opening up new avenues for research and applications in life sciences.
The evolution of phenolphthalein's use in biological contexts can be traced back to its initial applications in titration experiments and simple pH tests. As technology advanced, researchers began to recognize its potential for more sophisticated pH visualization in living systems. This shift in perspective has led to a surge in studies aimed at adapting phenolphthalein for in vivo and in vitro pH imaging, particularly in cellular and tissue environments.
The primary objective of research on phenolphthalein in biological pH imaging and mapping is to develop novel techniques for real-time, non-invasive visualization of pH dynamics in living organisms. This goal encompasses several key aspects, including enhancing the sensitivity and specificity of phenolphthalein-based pH sensors, improving spatial and temporal resolution of pH measurements, and ensuring biocompatibility for long-term in vivo applications.
One of the main drivers behind this research is the critical role of pH in various biological processes. Intracellular and extracellular pH levels are tightly regulated and play crucial roles in enzyme activity, protein folding, cell signaling, and overall cellular homeostasis. Abnormal pH levels are associated with numerous pathological conditions, including cancer, inflammation, and neurodegenerative diseases. Therefore, the ability to accurately map and monitor pH changes in biological systems could provide valuable insights into disease mechanisms and potentially lead to new diagnostic and therapeutic approaches.
The development of phenolphthalein-based pH imaging techniques also aligns with broader trends in biomedical research, such as the push towards more precise and personalized medicine. By enabling detailed visualization of pH microenvironments within tissues and organs, these techniques could contribute to a more nuanced understanding of disease progression and treatment efficacy at the molecular level.
As research in this field progresses, scientists are exploring various strategies to overcome the limitations of traditional phenolphthalein use. These include chemical modifications to improve its spectral properties, incorporation into nanoparticles or hydrogels for targeted delivery, and integration with advanced imaging technologies such as fluorescence microscopy and magnetic resonance imaging (MRI).
The evolution of phenolphthalein's use in biological contexts can be traced back to its initial applications in titration experiments and simple pH tests. As technology advanced, researchers began to recognize its potential for more sophisticated pH visualization in living systems. This shift in perspective has led to a surge in studies aimed at adapting phenolphthalein for in vivo and in vitro pH imaging, particularly in cellular and tissue environments.
The primary objective of research on phenolphthalein in biological pH imaging and mapping is to develop novel techniques for real-time, non-invasive visualization of pH dynamics in living organisms. This goal encompasses several key aspects, including enhancing the sensitivity and specificity of phenolphthalein-based pH sensors, improving spatial and temporal resolution of pH measurements, and ensuring biocompatibility for long-term in vivo applications.
One of the main drivers behind this research is the critical role of pH in various biological processes. Intracellular and extracellular pH levels are tightly regulated and play crucial roles in enzyme activity, protein folding, cell signaling, and overall cellular homeostasis. Abnormal pH levels are associated with numerous pathological conditions, including cancer, inflammation, and neurodegenerative diseases. Therefore, the ability to accurately map and monitor pH changes in biological systems could provide valuable insights into disease mechanisms and potentially lead to new diagnostic and therapeutic approaches.
The development of phenolphthalein-based pH imaging techniques also aligns with broader trends in biomedical research, such as the push towards more precise and personalized medicine. By enabling detailed visualization of pH microenvironments within tissues and organs, these techniques could contribute to a more nuanced understanding of disease progression and treatment efficacy at the molecular level.
As research in this field progresses, scientists are exploring various strategies to overcome the limitations of traditional phenolphthalein use. These include chemical modifications to improve its spectral properties, incorporation into nanoparticles or hydrogels for targeted delivery, and integration with advanced imaging technologies such as fluorescence microscopy and magnetic resonance imaging (MRI).
Market Demand for Biological pH Imaging
The market demand for biological pH imaging has been steadily growing, driven by advancements in biomedical research, pharmaceutical development, and clinical diagnostics. This technology plays a crucial role in understanding cellular processes, disease mechanisms, and drug efficacy, making it indispensable across various life science sectors.
In the field of biomedical research, there is a significant demand for pH imaging techniques to study intracellular and extracellular pH dynamics. Researchers require these tools to investigate cellular metabolism, signaling pathways, and the microenvironment of tissues. The ability to visualize pH changes in real-time provides valuable insights into physiological and pathological processes, contributing to a deeper understanding of diseases such as cancer, inflammation, and neurodegenerative disorders.
The pharmaceutical industry has also shown increasing interest in biological pH imaging for drug discovery and development. This technology enables researchers to assess the impact of potential drug candidates on cellular pH, evaluate drug uptake and distribution, and optimize drug delivery systems. As the industry moves towards more targeted and personalized therapies, the demand for precise pH imaging tools is expected to rise further.
Clinical diagnostics represent another significant market segment for biological pH imaging. There is a growing need for non-invasive pH monitoring techniques in various medical applications, including cancer detection, wound healing assessment, and gastrointestinal disorder diagnosis. The development of pH-sensitive imaging probes and sensors has opened up new possibilities for early disease detection and treatment monitoring.
The global market for pH imaging in life sciences is projected to expand substantially in the coming years. This growth is fueled by increasing research and development activities in academia and industry, as well as the rising prevalence of chronic diseases that require advanced diagnostic tools. Additionally, the integration of pH imaging with other imaging modalities, such as fluorescence microscopy and magnetic resonance imaging, is creating new opportunities for market expansion.
Emerging applications in environmental monitoring and food safety are also contributing to the market demand for biological pH imaging. These sectors require reliable methods to assess pH levels in ecosystems, water bodies, and food products, driving the development of novel pH-sensitive probes and imaging systems.
As the technology continues to evolve, there is a growing demand for more sensitive, specific, and user-friendly pH imaging solutions. Researchers and clinicians are seeking tools that offer higher spatial and temporal resolution, improved biocompatibility, and the ability to perform multiplexed measurements. This demand is spurring innovation in probe design, imaging instrumentation, and data analysis software, further propelling the market growth for biological pH imaging technologies.
In the field of biomedical research, there is a significant demand for pH imaging techniques to study intracellular and extracellular pH dynamics. Researchers require these tools to investigate cellular metabolism, signaling pathways, and the microenvironment of tissues. The ability to visualize pH changes in real-time provides valuable insights into physiological and pathological processes, contributing to a deeper understanding of diseases such as cancer, inflammation, and neurodegenerative disorders.
The pharmaceutical industry has also shown increasing interest in biological pH imaging for drug discovery and development. This technology enables researchers to assess the impact of potential drug candidates on cellular pH, evaluate drug uptake and distribution, and optimize drug delivery systems. As the industry moves towards more targeted and personalized therapies, the demand for precise pH imaging tools is expected to rise further.
Clinical diagnostics represent another significant market segment for biological pH imaging. There is a growing need for non-invasive pH monitoring techniques in various medical applications, including cancer detection, wound healing assessment, and gastrointestinal disorder diagnosis. The development of pH-sensitive imaging probes and sensors has opened up new possibilities for early disease detection and treatment monitoring.
The global market for pH imaging in life sciences is projected to expand substantially in the coming years. This growth is fueled by increasing research and development activities in academia and industry, as well as the rising prevalence of chronic diseases that require advanced diagnostic tools. Additionally, the integration of pH imaging with other imaging modalities, such as fluorescence microscopy and magnetic resonance imaging, is creating new opportunities for market expansion.
Emerging applications in environmental monitoring and food safety are also contributing to the market demand for biological pH imaging. These sectors require reliable methods to assess pH levels in ecosystems, water bodies, and food products, driving the development of novel pH-sensitive probes and imaging systems.
As the technology continues to evolve, there is a growing demand for more sensitive, specific, and user-friendly pH imaging solutions. Researchers and clinicians are seeking tools that offer higher spatial and temporal resolution, improved biocompatibility, and the ability to perform multiplexed measurements. This demand is spurring innovation in probe design, imaging instrumentation, and data analysis software, further propelling the market growth for biological pH imaging technologies.
Current Challenges in Phenolphthalein-based pH Mapping
Despite the widespread use of phenolphthalein in biological pH imaging and mapping, several challenges persist in its application. One of the primary issues is the limited pH range in which phenolphthalein effectively functions as a pH indicator. The compound typically transitions from colorless to pink in the pH range of 8.2 to 10, which restricts its utility in many biological systems where pH variations often occur within more acidic or neutral ranges.
Another significant challenge is the potential toxicity of phenolphthalein when used in living systems. While it has been widely employed in various applications, concerns about its safety have emerged, particularly for long-term or high-concentration exposures. This toxicity issue limits its use in certain in vivo imaging applications and necessitates careful consideration of dosage and exposure time in experimental designs.
The stability of phenolphthalein in biological environments poses another hurdle. The compound can be affected by various factors such as temperature, light exposure, and the presence of certain ions or biomolecules. These environmental influences can lead to inconsistencies in pH measurements or false readings, potentially compromising the accuracy and reliability of pH mapping results.
Furthermore, the reversibility of the color change in phenolphthalein-based pH indicators can be problematic in dynamic biological systems. Rapid pH fluctuations may not be accurately captured due to the time required for the indicator to respond and revert to its original state. This limitation can result in missed or inaccurate readings of transient pH changes, which are often crucial in understanding biological processes.
The spatial resolution of phenolphthalein-based pH mapping is another area of concern. While it can provide general pH information, achieving high-resolution mapping at the subcellular level remains challenging. This limitation is particularly evident when attempting to study pH microenvironments within cells or tissues, where precise localization of pH changes is crucial for understanding cellular processes.
Additionally, the interaction of phenolphthalein with other biological molecules and cellular components can lead to interference in pH measurements. Proteins, lipids, and other biomolecules may bind to or alter the behavior of phenolphthalein, potentially skewing pH readings and complicating data interpretation.
Lastly, the development of quantitative pH mapping techniques using phenolphthalein faces challenges in standardization and calibration. Variations in imaging conditions, sample preparation, and instrument sensitivity can lead to inconsistencies in pH measurements across different experiments or laboratories. Establishing robust protocols for quantitative analysis and ensuring reproducibility remains an ongoing challenge in the field.
Another significant challenge is the potential toxicity of phenolphthalein when used in living systems. While it has been widely employed in various applications, concerns about its safety have emerged, particularly for long-term or high-concentration exposures. This toxicity issue limits its use in certain in vivo imaging applications and necessitates careful consideration of dosage and exposure time in experimental designs.
The stability of phenolphthalein in biological environments poses another hurdle. The compound can be affected by various factors such as temperature, light exposure, and the presence of certain ions or biomolecules. These environmental influences can lead to inconsistencies in pH measurements or false readings, potentially compromising the accuracy and reliability of pH mapping results.
Furthermore, the reversibility of the color change in phenolphthalein-based pH indicators can be problematic in dynamic biological systems. Rapid pH fluctuations may not be accurately captured due to the time required for the indicator to respond and revert to its original state. This limitation can result in missed or inaccurate readings of transient pH changes, which are often crucial in understanding biological processes.
The spatial resolution of phenolphthalein-based pH mapping is another area of concern. While it can provide general pH information, achieving high-resolution mapping at the subcellular level remains challenging. This limitation is particularly evident when attempting to study pH microenvironments within cells or tissues, where precise localization of pH changes is crucial for understanding cellular processes.
Additionally, the interaction of phenolphthalein with other biological molecules and cellular components can lead to interference in pH measurements. Proteins, lipids, and other biomolecules may bind to or alter the behavior of phenolphthalein, potentially skewing pH readings and complicating data interpretation.
Lastly, the development of quantitative pH mapping techniques using phenolphthalein faces challenges in standardization and calibration. Variations in imaging conditions, sample preparation, and instrument sensitivity can lead to inconsistencies in pH measurements across different experiments or laboratories. Establishing robust protocols for quantitative analysis and ensuring reproducibility remains an ongoing challenge in the field.
Existing Phenolphthalein pH Imaging Techniques
01 pH range and color change of phenolphthalein
Phenolphthalein is a pH indicator that changes color in different pH ranges. It is colorless in acidic solutions (pH < 8.2) and turns pink to purple in alkaline solutions (pH > 10). This property makes it useful in various applications, including titrations and pH testing.- pH indicator properties of phenolphthalein: Phenolphthalein is widely used as a pH indicator due to its ability to change color at specific pH levels. It is colorless in acidic solutions (pH < 8.2) and turns pink to purple in basic solutions (pH > 10). This property makes it useful in various applications, including titrations and pH testing.
- Phenolphthalein in analytical chemistry: Phenolphthalein is extensively used in analytical chemistry for various purposes. It serves as an indicator in acid-base titrations, helps in determining alkalinity of water samples, and is used in colorimetric analysis. Its sharp color change at specific pH ranges makes it valuable for precise measurements in chemical analysis.
- Modifications and derivatives of phenolphthalein: Researchers have developed modified versions and derivatives of phenolphthalein to enhance its properties or create new applications. These modifications can alter the pH range of color change, improve stability, or introduce additional functionalities. Such developments expand the utility of phenolphthalein-based indicators in various fields.
- Phenolphthalein in polymer and material science: Phenolphthalein has applications in polymer and material science. It can be incorporated into polymers to create pH-sensitive materials or used in the synthesis of certain polymers. These applications leverage the pH-responsive properties of phenolphthalein to develop smart materials or improve existing ones.
- Environmental and biological applications: Phenolphthalein finds use in environmental monitoring and biological research. It can be used to detect alkaline substances in environmental samples, assess soil pH, or as an indicator in biological assays. Its non-toxic nature and clear color change make it suitable for various environmental and biological applications.
02 Phenolphthalein in pH testing devices
Phenolphthalein is incorporated into various pH testing devices and kits. These devices may include test strips, indicator papers, or portable pH meters that use phenolphthalein as the primary indicator for pH measurement in liquids or solutions.Expand Specific Solutions03 Synthesis and production of phenolphthalein
Methods for synthesizing and producing phenolphthalein are described, including various chemical processes and reactions. These methods aim to improve the purity, yield, and efficiency of phenolphthalein production for use in pH indicators and other applications.Expand Specific Solutions04 Phenolphthalein in polymer and resin formulations
Phenolphthalein is used in the development of pH-sensitive polymers and resins. These materials can change color or properties in response to pH changes, making them useful in various applications such as smart packaging, controlled drug delivery, and environmental monitoring.Expand Specific Solutions05 Applications of phenolphthalein beyond pH indication
Phenolphthalein has applications beyond its use as a pH indicator. It is used in forensic science for detecting blood traces, in the production of certain dyes, and in some medical diagnostic tests. Its unique properties make it valuable in various scientific and industrial fields.Expand Specific Solutions
Key Players in Biological Imaging and pH Sensing
The research on phenolphthalein in biological pH imaging and mapping is in its early developmental stage, with a growing market potential due to increasing applications in biomedical research and diagnostics. The technology's maturity is still evolving, with key players like Institut National de la Santé et de la Recherche Médicale, University of Tokyo, and Centre National de la Recherche Scientifique leading the way. These institutions are driving innovation through collaborative efforts, leveraging their expertise in biomedical imaging and chemical sciences. The competitive landscape is characterized by a mix of academic institutions and research organizations, indicating a strong focus on fundamental research and potential for future commercialization.
University of Tokyo
Technical Solution: The University of Tokyo has developed a novel approach for biological pH imaging using phenolphthalein-based fluorescent probes. Their research focuses on creating highly sensitive and selective pH sensors for intracellular and extracellular environments. The team has synthesized phenolphthalein derivatives with improved fluorescence properties and cellular permeability[1]. These probes exhibit a significant increase in fluorescence intensity upon pH changes, allowing for real-time monitoring of pH fluctuations in living cells and tissues[2]. The university has also integrated these probes with advanced microscopy techniques, enabling high-resolution 3D pH mapping of complex biological structures[3].
Strengths: High sensitivity and selectivity for pH changes, improved cellular permeability, and compatibility with advanced imaging techniques. Weaknesses: Potential cytotoxicity at higher concentrations and limited long-term stability in certain biological environments.
The Board of Regents of The University of Texas System
Technical Solution: The University of Texas System has made significant advancements in phenolphthalein-based pH imaging for biological applications. Their research team has developed a series of phenolphthalein-derived fluorescent probes with enhanced photostability and broader pH range sensitivity[4]. These probes are designed to function effectively in various cellular compartments, including lysosomes and mitochondria. The university has also pioneered the use of nanoparticle-encapsulated phenolphthalein derivatives for targeted pH sensing in specific tissues and organs[5]. Additionally, they have created a microfluidic platform that integrates phenolphthalein-based sensors for high-throughput pH analysis of biological samples[6].
Strengths: Wide pH range sensitivity, targeted sensing capabilities, and integration with microfluidic technologies. Weaknesses: Potential interference from other ions in complex biological matrices and challenges in quantitative pH measurements in heterogeneous environments.
Innovations in Phenolphthalein-based pH Sensors
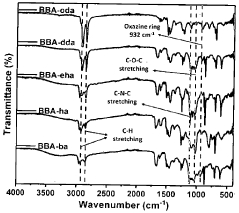
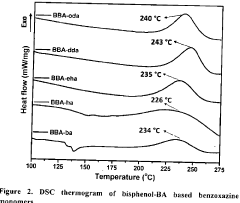
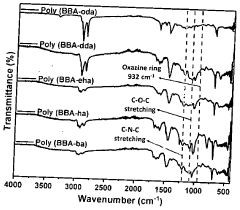
Production of extreme range of PH indicators from benzoxazines
PatentActiveIN202341027342A
Innovation
- Development of bisphenol-BA/aliphatic amine based hydrophobic polybenzoxazines coated on cellulose paper, synthesized through Mannich condensation, which exhibit distinct color changes across a wide pH range from -1.8 to 14, offering thermal stability and repeated use capability.
Biocompatibility and Safety Considerations
The biocompatibility and safety considerations of phenolphthalein in biological pH imaging and mapping are crucial aspects that require thorough examination. Phenolphthalein, while widely used as a pH indicator, presents potential concerns when applied in biological systems.
One primary consideration is the potential toxicity of phenolphthalein when introduced into living organisms. Studies have shown that prolonged exposure or high concentrations of phenolphthalein can lead to adverse effects on cellular functions and tissue integrity. This necessitates careful dosage control and exposure time limitations in biological applications.
The metabolic fate of phenolphthalein within biological systems is another critical factor. Understanding how the compound is processed, distributed, and eliminated from the body is essential for assessing its overall safety profile. Research has indicated that phenolphthalein can be metabolized by liver enzymes, potentially forming reactive intermediates that may interact with cellular components.
Potential allergic reactions or hypersensitivity to phenolphthalein must also be considered. While rare, some individuals may exhibit adverse responses to the compound, ranging from mild skin irritation to more severe systemic reactions. This underscores the importance of thorough screening and precautionary measures in clinical applications.
The long-term effects of phenolphthalein exposure in biological systems remain an area of ongoing research. Chronic low-level exposure may have subtle impacts on cellular processes or organ functions that are not immediately apparent. Longitudinal studies are necessary to fully elucidate these potential long-term consequences.
Interactions between phenolphthalein and other biological molecules or pharmaceutical compounds present another layer of complexity. These interactions could potentially alter the compound's behavior or lead to unexpected biological responses. Comprehensive compatibility testing with common biomolecules and drugs is essential to ensure safe application in diverse biological contexts.
Environmental considerations must also be addressed when using phenolphthalein in biological imaging. The compound's fate in the environment, including its degradation products and potential ecological impacts, should be thoroughly assessed to minimize any adverse effects on ecosystems.
To mitigate these concerns, researchers are exploring various strategies. These include developing modified versions of phenolphthalein with improved biocompatibility profiles, encapsulating the compound in biocompatible carriers to reduce direct tissue contact, and investigating alternative pH-sensitive molecules that offer similar imaging capabilities with enhanced safety profiles.
One primary consideration is the potential toxicity of phenolphthalein when introduced into living organisms. Studies have shown that prolonged exposure or high concentrations of phenolphthalein can lead to adverse effects on cellular functions and tissue integrity. This necessitates careful dosage control and exposure time limitations in biological applications.
The metabolic fate of phenolphthalein within biological systems is another critical factor. Understanding how the compound is processed, distributed, and eliminated from the body is essential for assessing its overall safety profile. Research has indicated that phenolphthalein can be metabolized by liver enzymes, potentially forming reactive intermediates that may interact with cellular components.
Potential allergic reactions or hypersensitivity to phenolphthalein must also be considered. While rare, some individuals may exhibit adverse responses to the compound, ranging from mild skin irritation to more severe systemic reactions. This underscores the importance of thorough screening and precautionary measures in clinical applications.
The long-term effects of phenolphthalein exposure in biological systems remain an area of ongoing research. Chronic low-level exposure may have subtle impacts on cellular processes or organ functions that are not immediately apparent. Longitudinal studies are necessary to fully elucidate these potential long-term consequences.
Interactions between phenolphthalein and other biological molecules or pharmaceutical compounds present another layer of complexity. These interactions could potentially alter the compound's behavior or lead to unexpected biological responses. Comprehensive compatibility testing with common biomolecules and drugs is essential to ensure safe application in diverse biological contexts.
Environmental considerations must also be addressed when using phenolphthalein in biological imaging. The compound's fate in the environment, including its degradation products and potential ecological impacts, should be thoroughly assessed to minimize any adverse effects on ecosystems.
To mitigate these concerns, researchers are exploring various strategies. These include developing modified versions of phenolphthalein with improved biocompatibility profiles, encapsulating the compound in biocompatible carriers to reduce direct tissue contact, and investigating alternative pH-sensitive molecules that offer similar imaging capabilities with enhanced safety profiles.
Applications in Biomedical Research and Diagnostics
Phenolphthalein, traditionally known as a pH indicator in chemistry, has found innovative applications in biomedical research and diagnostics. Its unique color-changing properties at specific pH levels make it a valuable tool for visualizing and mapping biological pH environments.
In cellular biology, phenolphthalein has been utilized to study intracellular pH dynamics. Researchers have developed phenolphthalein-based fluorescent probes that can penetrate cell membranes, allowing for real-time monitoring of pH changes within living cells. This technique has provided insights into cellular processes such as endocytosis, exocytosis, and metabolic activities that involve pH fluctuations.
The application of phenolphthalein in tissue imaging has opened new avenues for understanding disease progression and treatment efficacy. By incorporating phenolphthalein into biocompatible polymers, scientists have created pH-sensitive hydrogels that can be implanted in tissues. These hydrogels change color in response to local pH changes, enabling non-invasive monitoring of tissue microenvironments in conditions such as cancer, where tumor acidity is a key factor.
In the field of wound healing, phenolphthalein-based dressings have been developed to provide visual indicators of wound pH. This allows healthcare professionals to assess wound healing progress and detect early signs of infection without removing the dressing, improving patient care and reducing the risk of complications.
Phenolphthalein has also found applications in gastrointestinal diagnostics. Researchers have designed oral capsules containing phenolphthalein-based sensors that can map the pH along the gastrointestinal tract. This technology offers a non-invasive method for diagnosing conditions such as gastroesophageal reflux disease (GERD) and assessing the effectiveness of acid-suppressing medications.
In neuroscience, phenolphthalein derivatives have been used to study pH changes in the brain associated with neuronal activity and pathological conditions. These pH-sensitive probes have contributed to our understanding of neurotransmitter release, synaptic plasticity, and the role of pH in neurological disorders such as epilepsy and ischemia.
The integration of phenolphthalein into microfluidic devices has enabled high-throughput pH screening of biological samples. This technology has applications in drug discovery, where it can rapidly assess the pH-dependent properties of potential pharmaceutical compounds, as well as in environmental monitoring for detecting pH changes in aquatic ecosystems.
In cellular biology, phenolphthalein has been utilized to study intracellular pH dynamics. Researchers have developed phenolphthalein-based fluorescent probes that can penetrate cell membranes, allowing for real-time monitoring of pH changes within living cells. This technique has provided insights into cellular processes such as endocytosis, exocytosis, and metabolic activities that involve pH fluctuations.
The application of phenolphthalein in tissue imaging has opened new avenues for understanding disease progression and treatment efficacy. By incorporating phenolphthalein into biocompatible polymers, scientists have created pH-sensitive hydrogels that can be implanted in tissues. These hydrogels change color in response to local pH changes, enabling non-invasive monitoring of tissue microenvironments in conditions such as cancer, where tumor acidity is a key factor.
In the field of wound healing, phenolphthalein-based dressings have been developed to provide visual indicators of wound pH. This allows healthcare professionals to assess wound healing progress and detect early signs of infection without removing the dressing, improving patient care and reducing the risk of complications.
Phenolphthalein has also found applications in gastrointestinal diagnostics. Researchers have designed oral capsules containing phenolphthalein-based sensors that can map the pH along the gastrointestinal tract. This technology offers a non-invasive method for diagnosing conditions such as gastroesophageal reflux disease (GERD) and assessing the effectiveness of acid-suppressing medications.
In neuroscience, phenolphthalein derivatives have been used to study pH changes in the brain associated with neuronal activity and pathological conditions. These pH-sensitive probes have contributed to our understanding of neurotransmitter release, synaptic plasticity, and the role of pH in neurological disorders such as epilepsy and ischemia.
The integration of phenolphthalein into microfluidic devices has enabled high-throughput pH screening of biological samples. This technology has applications in drug discovery, where it can rapidly assess the pH-dependent properties of potential pharmaceutical compounds, as well as in environmental monitoring for detecting pH changes in aquatic ecosystems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!