Phenolphthalein in Enhancing Biocompatibility of Medical Implants
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein Biocompatibility Enhancement Goals
The primary goal of enhancing biocompatibility of medical implants using phenolphthalein is to improve the integration and long-term performance of these devices within the human body. This objective stems from the persistent challenges faced in implant technology, where foreign body reactions and immune responses can lead to complications and implant failure.
One key aim is to leverage phenolphthalein's unique properties to create a more favorable interface between the implant surface and surrounding tissues. By modifying the surface chemistry of implants with phenolphthalein-based coatings, researchers hope to reduce inflammatory responses and promote better tissue adhesion. This could potentially lead to faster healing times and improved implant stability.
Another critical goal is to explore phenolphthalein's potential as a bioactive agent. Its pH-sensitive characteristics may be utilized to develop smart implants that can respond to changes in the local physiological environment. This could enable the creation of implants that actively promote tissue regeneration or deliver therapeutic agents in response to specific biological cues.
Researchers also aim to investigate the long-term effects of phenolphthalein-enhanced implants on tissue health and function. This includes studying its impact on cell proliferation, differentiation, and extracellular matrix production. The ultimate objective is to develop implants that not only avoid adverse reactions but also actively contribute to tissue health and regeneration.
Furthermore, there is a focus on optimizing the incorporation of phenolphthalein into various implant materials. This involves developing new synthesis methods and exploring different phenolphthalein derivatives to enhance its compatibility with a wide range of biomaterials, from metals to polymers and ceramics.
Lastly, a crucial goal is to ensure the safety and efficacy of phenolphthalein-enhanced implants. This includes comprehensive toxicological studies to rule out any potential long-term adverse effects and to establish appropriate dosage levels. Researchers aim to develop standardized protocols for phenolphthalein incorporation that can be reliably reproduced in large-scale manufacturing processes.
By achieving these goals, the research on phenolphthalein in enhancing biocompatibility of medical implants seeks to revolutionize implant technology, potentially leading to a new generation of medical devices with superior performance and patient outcomes.
One key aim is to leverage phenolphthalein's unique properties to create a more favorable interface between the implant surface and surrounding tissues. By modifying the surface chemistry of implants with phenolphthalein-based coatings, researchers hope to reduce inflammatory responses and promote better tissue adhesion. This could potentially lead to faster healing times and improved implant stability.
Another critical goal is to explore phenolphthalein's potential as a bioactive agent. Its pH-sensitive characteristics may be utilized to develop smart implants that can respond to changes in the local physiological environment. This could enable the creation of implants that actively promote tissue regeneration or deliver therapeutic agents in response to specific biological cues.
Researchers also aim to investigate the long-term effects of phenolphthalein-enhanced implants on tissue health and function. This includes studying its impact on cell proliferation, differentiation, and extracellular matrix production. The ultimate objective is to develop implants that not only avoid adverse reactions but also actively contribute to tissue health and regeneration.
Furthermore, there is a focus on optimizing the incorporation of phenolphthalein into various implant materials. This involves developing new synthesis methods and exploring different phenolphthalein derivatives to enhance its compatibility with a wide range of biomaterials, from metals to polymers and ceramics.
Lastly, a crucial goal is to ensure the safety and efficacy of phenolphthalein-enhanced implants. This includes comprehensive toxicological studies to rule out any potential long-term adverse effects and to establish appropriate dosage levels. Researchers aim to develop standardized protocols for phenolphthalein incorporation that can be reliably reproduced in large-scale manufacturing processes.
By achieving these goals, the research on phenolphthalein in enhancing biocompatibility of medical implants seeks to revolutionize implant technology, potentially leading to a new generation of medical devices with superior performance and patient outcomes.
Market Demand for Improved Medical Implants
The global medical implants market has been experiencing significant growth, driven by an aging population, increasing prevalence of chronic diseases, and advancements in medical technology. This growth has led to a rising demand for improved biocompatibility in medical implants, creating a substantial market opportunity for innovations like the use of phenolphthalein to enhance implant performance.
The market for medical implants is projected to reach substantial figures in the coming years, with a particular emphasis on biocompatible materials. Patients and healthcare providers are increasingly seeking implants that reduce the risk of rejection, minimize inflammation, and promote better integration with the body's tissues. This demand is particularly strong in orthopedic, cardiovascular, and dental implant sectors.
Orthopedic implants, including joint replacements and spinal devices, represent a significant portion of the market. The growing incidence of osteoarthritis and other degenerative joint diseases, coupled with an active aging population, is fueling the need for implants with improved longevity and reduced complications. Cardiovascular implants, such as stents and heart valves, also require enhanced biocompatibility to minimize the risk of thrombosis and restenosis.
In the dental implant market, there is a strong demand for materials that promote osseointegration and reduce the risk of peri-implantitis. As the global population ages and dental health awareness increases, the market for dental implants with superior biocompatibility is expected to expand significantly.
The potential application of phenolphthalein in enhancing the biocompatibility of medical implants addresses several key market needs. These include reducing the incidence of implant-related infections, improving long-term implant stability, and minimizing adverse immune responses. Such improvements could lead to better patient outcomes, reduced healthcare costs, and increased patient satisfaction.
Furthermore, there is a growing trend towards personalized medicine, which is creating demand for customizable implants with tailored biocompatibility profiles. The ability to fine-tune the properties of implant surfaces using compounds like phenolphthalein could open new avenues for personalized implant solutions.
Regulatory bodies and healthcare systems worldwide are also placing increased emphasis on the safety and efficacy of medical implants. This regulatory environment is driving demand for innovative materials and coatings that can demonstrate improved biocompatibility and reduced long-term complications.
In conclusion, the market demand for improved medical implants with enhanced biocompatibility is robust and growing. The potential application of phenolphthalein in this field aligns well with current market trends and could address significant unmet needs in the medical implant industry.
The market for medical implants is projected to reach substantial figures in the coming years, with a particular emphasis on biocompatible materials. Patients and healthcare providers are increasingly seeking implants that reduce the risk of rejection, minimize inflammation, and promote better integration with the body's tissues. This demand is particularly strong in orthopedic, cardiovascular, and dental implant sectors.
Orthopedic implants, including joint replacements and spinal devices, represent a significant portion of the market. The growing incidence of osteoarthritis and other degenerative joint diseases, coupled with an active aging population, is fueling the need for implants with improved longevity and reduced complications. Cardiovascular implants, such as stents and heart valves, also require enhanced biocompatibility to minimize the risk of thrombosis and restenosis.
In the dental implant market, there is a strong demand for materials that promote osseointegration and reduce the risk of peri-implantitis. As the global population ages and dental health awareness increases, the market for dental implants with superior biocompatibility is expected to expand significantly.
The potential application of phenolphthalein in enhancing the biocompatibility of medical implants addresses several key market needs. These include reducing the incidence of implant-related infections, improving long-term implant stability, and minimizing adverse immune responses. Such improvements could lead to better patient outcomes, reduced healthcare costs, and increased patient satisfaction.
Furthermore, there is a growing trend towards personalized medicine, which is creating demand for customizable implants with tailored biocompatibility profiles. The ability to fine-tune the properties of implant surfaces using compounds like phenolphthalein could open new avenues for personalized implant solutions.
Regulatory bodies and healthcare systems worldwide are also placing increased emphasis on the safety and efficacy of medical implants. This regulatory environment is driving demand for innovative materials and coatings that can demonstrate improved biocompatibility and reduced long-term complications.
In conclusion, the market demand for improved medical implants with enhanced biocompatibility is robust and growing. The potential application of phenolphthalein in this field aligns well with current market trends and could address significant unmet needs in the medical implant industry.
Current Challenges in Implant Biocompatibility
Despite significant advancements in medical implant technology, biocompatibility remains a critical challenge in the field. The human body's immune system often recognizes implants as foreign objects, leading to adverse reactions that can compromise the implant's functionality and patient health. One of the primary issues is the formation of a fibrous capsule around the implant, which can impede its performance and integration with surrounding tissues.
Inflammation and infection are persistent concerns in implant biocompatibility. The implantation process itself can trigger an inflammatory response, potentially leading to chronic inflammation if not properly managed. This prolonged inflammation can result in implant rejection or failure. Additionally, the risk of bacterial colonization on implant surfaces poses a significant threat to patient safety and implant longevity.
Material selection for implants presents another challenge. While materials like titanium and certain polymers have shown good biocompatibility, finding materials that perfectly mimic the properties of natural tissues remains elusive. The mismatch in mechanical properties between implants and surrounding tissues can lead to stress shielding, particularly in orthopedic implants, resulting in bone resorption and implant loosening over time.
Surface modifications of implants have been explored to enhance biocompatibility, but achieving the right balance between promoting cell adhesion and preventing bacterial colonization is complex. Techniques such as coating implants with bioactive materials or creating specific surface topographies have shown promise but also face challenges in long-term stability and effectiveness.
The body's response to implants can vary significantly between individuals, making it difficult to predict and prevent adverse reactions. Factors such as age, overall health, and genetic predisposition play roles in how a patient's body will interact with an implant. This variability necessitates personalized approaches to implant design and treatment, which are currently limited by technological and economic constraints.
Long-term effects of implants on the body remain a concern. Some materials used in implants may degrade over time, potentially releasing particles or chemicals that could have unforeseen effects on the patient's health. The cumulative impact of these materials over decades of use is not fully understood, particularly for newer materials and technologies.
In the context of using phenolphthalein to enhance biocompatibility, researchers face the challenge of fully understanding its mechanisms of action and potential long-term effects. While phenolphthalein shows promise in improving implant integration, its application must be carefully studied to ensure it does not introduce new biocompatibility issues or interfere with the implant's primary function.
Inflammation and infection are persistent concerns in implant biocompatibility. The implantation process itself can trigger an inflammatory response, potentially leading to chronic inflammation if not properly managed. This prolonged inflammation can result in implant rejection or failure. Additionally, the risk of bacterial colonization on implant surfaces poses a significant threat to patient safety and implant longevity.
Material selection for implants presents another challenge. While materials like titanium and certain polymers have shown good biocompatibility, finding materials that perfectly mimic the properties of natural tissues remains elusive. The mismatch in mechanical properties between implants and surrounding tissues can lead to stress shielding, particularly in orthopedic implants, resulting in bone resorption and implant loosening over time.
Surface modifications of implants have been explored to enhance biocompatibility, but achieving the right balance between promoting cell adhesion and preventing bacterial colonization is complex. Techniques such as coating implants with bioactive materials or creating specific surface topographies have shown promise but also face challenges in long-term stability and effectiveness.
The body's response to implants can vary significantly between individuals, making it difficult to predict and prevent adverse reactions. Factors such as age, overall health, and genetic predisposition play roles in how a patient's body will interact with an implant. This variability necessitates personalized approaches to implant design and treatment, which are currently limited by technological and economic constraints.
Long-term effects of implants on the body remain a concern. Some materials used in implants may degrade over time, potentially releasing particles or chemicals that could have unforeseen effects on the patient's health. The cumulative impact of these materials over decades of use is not fully understood, particularly for newer materials and technologies.
In the context of using phenolphthalein to enhance biocompatibility, researchers face the challenge of fully understanding its mechanisms of action and potential long-term effects. While phenolphthalein shows promise in improving implant integration, its application must be carefully studied to ensure it does not introduce new biocompatibility issues or interfere with the implant's primary function.
Existing Phenolphthalein Applications in Biomedicine
01 Biocompatible phenolphthalein derivatives
Development of phenolphthalein derivatives with improved biocompatibility for various applications. These modifications aim to reduce potential toxicity while maintaining the desired properties of phenolphthalein.- Biocompatible polymers containing phenolphthalein: Development of biocompatible polymers incorporating phenolphthalein for various medical and pharmaceutical applications. These polymers are designed to be compatible with biological systems while maintaining the useful properties of phenolphthalein.
- Phenolphthalein in biocompatible drug delivery systems: Incorporation of phenolphthalein into biocompatible drug delivery systems for controlled release of pharmaceuticals. These systems aim to improve drug efficacy while minimizing potential side effects due to the biocompatibility of the carrier materials.
- Biocompatible phenolphthalein derivatives: Synthesis and characterization of phenolphthalein derivatives with enhanced biocompatibility. These modified compounds are designed to retain the useful properties of phenolphthalein while improving their compatibility with biological systems.
- Phenolphthalein in biocompatible diagnostic tools: Development of biocompatible diagnostic tools and assays utilizing phenolphthalein. These applications leverage the indicator properties of phenolphthalein while ensuring compatibility with biological samples and systems.
- Biocompatibility assessment of phenolphthalein-containing materials: Methods and techniques for evaluating the biocompatibility of materials containing phenolphthalein. These assessments aim to ensure the safety and suitability of phenolphthalein-based products for various biological and medical applications.
02 Phenolphthalein in biomedical applications
Utilization of phenolphthalein in biomedical fields, including diagnostic tools, drug delivery systems, and medical devices. The focus is on enhancing biocompatibility for safe use in biological environments.Expand Specific Solutions03 Phenolphthalein-based biosensors
Development of biosensors incorporating phenolphthalein for detection of various biological analytes. These sensors are designed to be biocompatible for in vivo or in vitro applications.Expand Specific Solutions04 Biocompatible phenolphthalein formulations
Creation of formulations containing phenolphthalein with improved biocompatibility for pharmaceutical and cosmetic applications. These formulations aim to minimize potential adverse effects while maintaining efficacy.Expand Specific Solutions05 Phenolphthalein in biodegradable materials
Incorporation of phenolphthalein into biodegradable materials for various applications, including environmental monitoring and controlled release systems. The focus is on ensuring biocompatibility and biodegradability of the resulting materials.Expand Specific Solutions
Key Players in Medical Implant Industry
The research on phenolphthalein in enhancing biocompatibility of medical implants is in an early development stage, with a growing market potential as the demand for advanced medical implants increases. The competitive landscape is diverse, involving academic institutions, established medical device companies, and specialized biotech firms. Key players like Straumann Holding AG, Biotronik AG, and Surmodics, Inc. are likely leading commercial efforts, while universities such as ETH Zurich and MIT are contributing to fundamental research. The technology's maturity is still evolving, with ongoing studies focused on improving implant integration and reducing rejection rates.
Straumann Holding AG
Technical Solution: Straumann has developed a phenolphthalein-based surface modification technique for their dental implants to enhance osseointegration and reduce the risk of peri-implantitis. Their approach involves incorporating phenolphthalein into a nano-structured titanium oxide layer, which allows for controlled release of bioactive compounds[2]. This technology aims to create a more favorable environment for bone cell attachment and proliferation. Straumann's research has shown that the phenolphthalein-modified surface can modulate the local pH, potentially inhibiting bacterial growth and promoting a healthier peri-implant environment[4]. The company has also investigated the combination of phenolphthalein with other bioactive molecules to create synergistic effects in improving implant biocompatibility[6].
Strengths: Specific focus on dental implants, potential for improved osseointegration and reduced infection risk. Weaknesses: Limited application to other types of medical implants, potential regulatory challenges for novel surface treatments.
Biotronik AG
Technical Solution: Biotronik has developed a phenolphthalein-based coating technology for cardiovascular implants, particularly focusing on stents and pacemaker leads. Their approach involves incorporating phenolphthalein into a polymer matrix that can be applied to metal surfaces, creating a biocompatible interface between the implant and surrounding tissue[7]. The company's research has shown that this coating can reduce thrombogenicity and improve endothelialization of vascular devices[9]. Biotronik's phenolphthalein-modified surfaces have also demonstrated the ability to modulate local inflammation and promote faster healing in preclinical studies[11]. The company is exploring the potential of this technology to enhance the long-term performance and safety of their cardiovascular implants.
Strengths: Specialized application in cardiovascular implants, potential for reduced thrombogenicity and improved endothelialization. Weaknesses: Limited to specific types of implants, potential challenges in scaling up production for diverse product lines.
Core Innovations in Phenolphthalein-Based Implants
Medical implants and methods of preparation thereof
PatentActiveUS11857409B2
Innovation
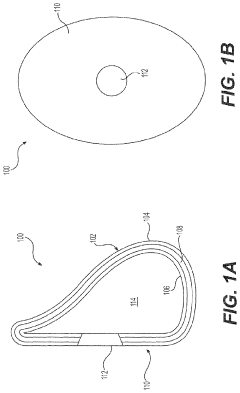
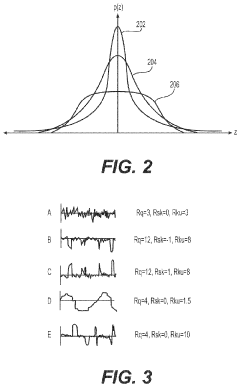
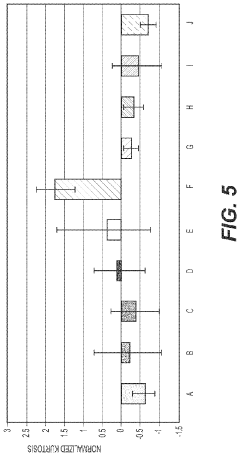
- Development of medical implants with controlled surface characteristics, including specific kurtosis, roughness, and skewness values, using biocompatible materials like silicone, and manufacturing methods that ensure consistent texture and reduced debris, along with features to restrict implant movement and improve fixation.
Exosome-based biocompatible implant
PatentWO2024076329A1
Innovation
- An exosome-based biocompatible implant is created by spraying exosomes from stem cells onto various implant materials, achieving surface protein adhesion, inflammation prevention, and increased cell integration, mimicking the cellular matrix, and enhancing immune support.
Regulatory Framework for Novel Implant Materials
The regulatory framework for novel implant materials, particularly those incorporating phenolphthalein for enhanced biocompatibility, is a complex and evolving landscape. Regulatory bodies worldwide, such as the FDA in the United States and the EMA in Europe, have established stringent guidelines for the approval and use of medical implants.
These regulatory frameworks typically require extensive pre-clinical and clinical testing to demonstrate the safety and efficacy of new implant materials. For phenolphthalein-enhanced implants, this would include thorough biocompatibility assessments, toxicity studies, and long-term safety evaluations. The regulatory process often involves multiple stages, including initial laboratory testing, animal studies, and human clinical trials.
One key aspect of the regulatory framework is the classification of medical devices. Implants incorporating phenolphthalein would likely fall under Class III devices, which are subject to the most rigorous regulatory scrutiny due to their high-risk nature and long-term implantation in the body. This classification necessitates a premarket approval (PMA) process in the United States, or a CE marking in the European Union, both of which require substantial clinical evidence.
The regulatory framework also emphasizes the importance of quality management systems in the manufacturing process. Manufacturers must adhere to Good Manufacturing Practices (GMP) and implement robust quality control measures to ensure consistency and safety in the production of phenolphthalein-enhanced implants.
Post-market surveillance is another critical component of the regulatory framework. Manufacturers are required to monitor the performance and safety of their implants after market approval, reporting any adverse events or complications to the relevant regulatory authorities. This ongoing surveillance helps identify any long-term effects or unforeseen issues related to the use of phenolphthalein in implants.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), aim to streamline regulatory processes across different countries. However, manufacturers must still navigate country-specific requirements and regulations when seeking approval for their phenolphthalein-enhanced implants in multiple markets.
As research on phenolphthalein in enhancing biocompatibility of medical implants progresses, regulatory frameworks may need to adapt to accommodate this novel approach. Regulatory bodies are likely to closely monitor developments in this field and may update their guidelines and requirements accordingly to ensure patient safety while fostering innovation in implant technology.
These regulatory frameworks typically require extensive pre-clinical and clinical testing to demonstrate the safety and efficacy of new implant materials. For phenolphthalein-enhanced implants, this would include thorough biocompatibility assessments, toxicity studies, and long-term safety evaluations. The regulatory process often involves multiple stages, including initial laboratory testing, animal studies, and human clinical trials.
One key aspect of the regulatory framework is the classification of medical devices. Implants incorporating phenolphthalein would likely fall under Class III devices, which are subject to the most rigorous regulatory scrutiny due to their high-risk nature and long-term implantation in the body. This classification necessitates a premarket approval (PMA) process in the United States, or a CE marking in the European Union, both of which require substantial clinical evidence.
The regulatory framework also emphasizes the importance of quality management systems in the manufacturing process. Manufacturers must adhere to Good Manufacturing Practices (GMP) and implement robust quality control measures to ensure consistency and safety in the production of phenolphthalein-enhanced implants.
Post-market surveillance is another critical component of the regulatory framework. Manufacturers are required to monitor the performance and safety of their implants after market approval, reporting any adverse events or complications to the relevant regulatory authorities. This ongoing surveillance helps identify any long-term effects or unforeseen issues related to the use of phenolphthalein in implants.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), aim to streamline regulatory processes across different countries. However, manufacturers must still navigate country-specific requirements and regulations when seeking approval for their phenolphthalein-enhanced implants in multiple markets.
As research on phenolphthalein in enhancing biocompatibility of medical implants progresses, regulatory frameworks may need to adapt to accommodate this novel approach. Regulatory bodies are likely to closely monitor developments in this field and may update their guidelines and requirements accordingly to ensure patient safety while fostering innovation in implant technology.
Safety and Long-term Effects of Phenolphthalein Implants
The safety and long-term effects of phenolphthalein implants are crucial considerations in the research on enhancing biocompatibility of medical implants. While phenolphthalein has shown promise in improving the integration of implants with surrounding tissues, its potential risks and long-term impacts must be thoroughly evaluated.
One primary concern is the potential for phenolphthalein to leach from the implant over time. Studies have shown that phenolphthalein can be released from implants in small quantities, potentially entering the bloodstream and affecting various organs. The rate and extent of this leaching process need to be carefully monitored to ensure that exposure remains within safe limits.
The metabolic fate of phenolphthalein in the body is another important aspect to consider. Research has indicated that phenolphthalein undergoes biotransformation in the liver, producing metabolites that may have different biological activities compared to the parent compound. The long-term effects of these metabolites on various organ systems, including the liver, kidneys, and endocrine system, require extensive investigation.
Carcinogenicity is a significant concern that has been raised regarding phenolphthalein exposure. Some animal studies have suggested a potential link between phenolphthalein and certain types of cancer, particularly in the adrenal glands and ovaries. However, the relevance of these findings to human exposure through medical implants remains uncertain and necessitates further research.
The impact of phenolphthalein on the immune system is another critical area of investigation. While the compound may enhance biocompatibility in the short term, its long-term effects on immune function and potential for triggering autoimmune responses need to be carefully evaluated. This is particularly important for implants that are intended to remain in the body for extended periods.
Reproductive toxicity is an additional concern that warrants attention. Some studies have suggested that phenolphthalein may have estrogenic properties, potentially affecting reproductive function and hormone balance. The implications of these findings for long-term implant use, especially in patients of reproductive age, require thorough examination.
To address these safety concerns, comprehensive long-term studies are essential. These should include both animal models and carefully monitored clinical trials to assess the safety profile of phenolphthalein-enhanced implants over extended periods. Additionally, the development of advanced imaging techniques and biomarkers to monitor the distribution and effects of phenolphthalein in vivo will be crucial for ongoing safety assessments.
In conclusion, while phenolphthalein shows promise in enhancing the biocompatibility of medical implants, its safety and long-term effects must be rigorously evaluated before widespread clinical application. A balanced approach that weighs the potential benefits against the risks is necessary to ensure the development of safe and effective implant technologies.
One primary concern is the potential for phenolphthalein to leach from the implant over time. Studies have shown that phenolphthalein can be released from implants in small quantities, potentially entering the bloodstream and affecting various organs. The rate and extent of this leaching process need to be carefully monitored to ensure that exposure remains within safe limits.
The metabolic fate of phenolphthalein in the body is another important aspect to consider. Research has indicated that phenolphthalein undergoes biotransformation in the liver, producing metabolites that may have different biological activities compared to the parent compound. The long-term effects of these metabolites on various organ systems, including the liver, kidneys, and endocrine system, require extensive investigation.
Carcinogenicity is a significant concern that has been raised regarding phenolphthalein exposure. Some animal studies have suggested a potential link between phenolphthalein and certain types of cancer, particularly in the adrenal glands and ovaries. However, the relevance of these findings to human exposure through medical implants remains uncertain and necessitates further research.
The impact of phenolphthalein on the immune system is another critical area of investigation. While the compound may enhance biocompatibility in the short term, its long-term effects on immune function and potential for triggering autoimmune responses need to be carefully evaluated. This is particularly important for implants that are intended to remain in the body for extended periods.
Reproductive toxicity is an additional concern that warrants attention. Some studies have suggested that phenolphthalein may have estrogenic properties, potentially affecting reproductive function and hormone balance. The implications of these findings for long-term implant use, especially in patients of reproductive age, require thorough examination.
To address these safety concerns, comprehensive long-term studies are essential. These should include both animal models and carefully monitored clinical trials to assess the safety profile of phenolphthalein-enhanced implants over extended periods. Additionally, the development of advanced imaging techniques and biomarkers to monitor the distribution and effects of phenolphthalein in vivo will be crucial for ongoing safety assessments.
In conclusion, while phenolphthalein shows promise in enhancing the biocompatibility of medical implants, its safety and long-term effects must be rigorously evaluated before widespread clinical application. A balanced approach that weighs the potential benefits against the risks is necessary to ensure the development of safe and effective implant technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!