Photodiode-based spectrometry tools in pharmaceutical research
AUG 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Spectrometry Evolution
Spectrometry has undergone significant evolution since its inception, transforming from rudimentary tools to sophisticated instruments capable of precise molecular analysis. The journey began with simple prism-based spectroscopes in the 19th century, which allowed scientists to observe the spectrum of light emitted or absorbed by various substances.
The early 20th century saw the development of more advanced spectrometers, utilizing diffraction gratings to achieve higher resolution. This period marked the transition from qualitative to quantitative analysis, enabling researchers to determine not only the presence of specific elements or compounds but also their concentrations.
The mid-20th century brought about a revolution in spectrometry with the introduction of infrared (IR) and ultraviolet-visible (UV-Vis) spectrophotometers. These instruments expanded the range of analyzable substances and provided deeper insights into molecular structures and interactions. Concurrently, mass spectrometry emerged as a powerful technique for identifying and quantifying molecules based on their mass-to-charge ratios.
The latter half of the 20th century witnessed the integration of computers into spectrometric instruments, leading to automated data collection, analysis, and interpretation. This digital transformation significantly enhanced the speed and accuracy of spectral measurements, paving the way for high-throughput applications in various fields, including pharmaceutical research.
The advent of Fourier Transform techniques in the 1960s and 1970s revolutionized spectrometry once again. Fourier Transform Infrared (FTIR) and Nuclear Magnetic Resonance (NMR) spectroscopy offered unprecedented resolution and sensitivity, enabling the analysis of complex molecular structures with greater precision.
In recent decades, the miniaturization of spectrometric devices has been a key trend. The development of microspectrometers and portable spectrometers has expanded the applications of this technology beyond traditional laboratory settings, allowing for on-site and real-time analysis in various industries, including pharmaceuticals.
The integration of photodiode arrays in spectrometry marks a significant milestone in the field's evolution. These solid-state detectors offer rapid, simultaneous measurement of multiple wavelengths, enhancing the speed and efficiency of spectral analysis. In pharmaceutical research, photodiode-based spectrometry tools have become invaluable for tasks such as drug quality control, formulation analysis, and process monitoring.
As we move into the 21st century, the convergence of spectrometry with other analytical techniques and advanced data processing methods continues to push the boundaries of what is possible in molecular analysis. Machine learning algorithms are being employed to interpret complex spectral data, while hyphenated techniques combine multiple analytical methods for more comprehensive sample characterization.
The early 20th century saw the development of more advanced spectrometers, utilizing diffraction gratings to achieve higher resolution. This period marked the transition from qualitative to quantitative analysis, enabling researchers to determine not only the presence of specific elements or compounds but also their concentrations.
The mid-20th century brought about a revolution in spectrometry with the introduction of infrared (IR) and ultraviolet-visible (UV-Vis) spectrophotometers. These instruments expanded the range of analyzable substances and provided deeper insights into molecular structures and interactions. Concurrently, mass spectrometry emerged as a powerful technique for identifying and quantifying molecules based on their mass-to-charge ratios.
The latter half of the 20th century witnessed the integration of computers into spectrometric instruments, leading to automated data collection, analysis, and interpretation. This digital transformation significantly enhanced the speed and accuracy of spectral measurements, paving the way for high-throughput applications in various fields, including pharmaceutical research.
The advent of Fourier Transform techniques in the 1960s and 1970s revolutionized spectrometry once again. Fourier Transform Infrared (FTIR) and Nuclear Magnetic Resonance (NMR) spectroscopy offered unprecedented resolution and sensitivity, enabling the analysis of complex molecular structures with greater precision.
In recent decades, the miniaturization of spectrometric devices has been a key trend. The development of microspectrometers and portable spectrometers has expanded the applications of this technology beyond traditional laboratory settings, allowing for on-site and real-time analysis in various industries, including pharmaceuticals.
The integration of photodiode arrays in spectrometry marks a significant milestone in the field's evolution. These solid-state detectors offer rapid, simultaneous measurement of multiple wavelengths, enhancing the speed and efficiency of spectral analysis. In pharmaceutical research, photodiode-based spectrometry tools have become invaluable for tasks such as drug quality control, formulation analysis, and process monitoring.
As we move into the 21st century, the convergence of spectrometry with other analytical techniques and advanced data processing methods continues to push the boundaries of what is possible in molecular analysis. Machine learning algorithms are being employed to interpret complex spectral data, while hyphenated techniques combine multiple analytical methods for more comprehensive sample characterization.
Pharma Research Needs
The pharmaceutical industry is experiencing a growing demand for advanced analytical tools, particularly in the realm of spectroscopy. Photodiode-based spectrometry tools have emerged as a crucial technology in pharmaceutical research, offering numerous advantages over traditional spectroscopic methods. These tools provide rapid, non-destructive analysis of pharmaceutical compounds, enabling researchers to obtain detailed information about molecular structures, chemical compositions, and drug interactions.
One of the primary drivers for the adoption of photodiode-based spectrometry in pharmaceutical research is the need for high-throughput screening and quality control processes. As drug development timelines become increasingly compressed, researchers require faster and more efficient analytical methods to evaluate large numbers of compounds. Photodiode-based spectrometers offer the speed and sensitivity necessary to meet these demands, allowing for rapid analysis of multiple samples with minimal sample preparation.
Another significant market need is the ability to perform real-time monitoring of pharmaceutical processes. Photodiode-based spectrometry tools can be integrated into production lines, enabling continuous monitoring of drug formulations during manufacturing. This capability is essential for ensuring consistent product quality and compliance with regulatory standards, as well as for optimizing production processes.
The pharmaceutical industry also faces increasing pressure to develop more targeted and personalized therapies. This trend has created a demand for analytical tools capable of detecting and quantifying biomarkers and drug metabolites at low concentrations. Photodiode-based spectrometry offers the sensitivity and specificity required for these applications, making it an invaluable tool in the development of precision medicine approaches.
Furthermore, there is a growing need for portable and field-deployable analytical instruments in pharmaceutical research. Photodiode-based spectrometers can be miniaturized and integrated into handheld devices, allowing for on-site analysis of drug samples in various settings, from clinical trials to quality control inspections. This portability is particularly valuable for conducting research in remote locations or for rapid response in emergency situations.
The pharmaceutical industry is also increasingly focused on sustainable practices and reducing environmental impact. Photodiode-based spectrometry aligns with these goals by offering a more energy-efficient and environmentally friendly alternative to traditional analytical methods. These tools typically require smaller sample sizes and generate less waste, contributing to more sustainable research practices.
As the complexity of pharmaceutical compounds continues to increase, researchers require analytical tools capable of providing more detailed and comprehensive spectral information. Photodiode-based spectrometry tools are evolving to meet this need, with advancements in detector technology and data processing algorithms enabling higher resolution and more accurate spectral analysis across a wider range of wavelengths.
One of the primary drivers for the adoption of photodiode-based spectrometry in pharmaceutical research is the need for high-throughput screening and quality control processes. As drug development timelines become increasingly compressed, researchers require faster and more efficient analytical methods to evaluate large numbers of compounds. Photodiode-based spectrometers offer the speed and sensitivity necessary to meet these demands, allowing for rapid analysis of multiple samples with minimal sample preparation.
Another significant market need is the ability to perform real-time monitoring of pharmaceutical processes. Photodiode-based spectrometry tools can be integrated into production lines, enabling continuous monitoring of drug formulations during manufacturing. This capability is essential for ensuring consistent product quality and compliance with regulatory standards, as well as for optimizing production processes.
The pharmaceutical industry also faces increasing pressure to develop more targeted and personalized therapies. This trend has created a demand for analytical tools capable of detecting and quantifying biomarkers and drug metabolites at low concentrations. Photodiode-based spectrometry offers the sensitivity and specificity required for these applications, making it an invaluable tool in the development of precision medicine approaches.
Furthermore, there is a growing need for portable and field-deployable analytical instruments in pharmaceutical research. Photodiode-based spectrometers can be miniaturized and integrated into handheld devices, allowing for on-site analysis of drug samples in various settings, from clinical trials to quality control inspections. This portability is particularly valuable for conducting research in remote locations or for rapid response in emergency situations.
The pharmaceutical industry is also increasingly focused on sustainable practices and reducing environmental impact. Photodiode-based spectrometry aligns with these goals by offering a more energy-efficient and environmentally friendly alternative to traditional analytical methods. These tools typically require smaller sample sizes and generate less waste, contributing to more sustainable research practices.
As the complexity of pharmaceutical compounds continues to increase, researchers require analytical tools capable of providing more detailed and comprehensive spectral information. Photodiode-based spectrometry tools are evolving to meet this need, with advancements in detector technology and data processing algorithms enabling higher resolution and more accurate spectral analysis across a wider range of wavelengths.
Photodiode Challenges
Photodiodes, while widely used in spectrometry tools for pharmaceutical research, face several significant challenges that impact their performance and reliability. One of the primary issues is the limited spectral range of individual photodiodes. Most silicon-based photodiodes are sensitive to wavelengths between 400 and 1100 nm, which may not cover the entire spectrum of interest in pharmaceutical applications. This limitation often necessitates the use of multiple photodiodes or alternative detector technologies for comprehensive spectral analysis.
Another challenge is the inherent noise in photodiode-based systems. Dark current, shot noise, and thermal noise can all contribute to signal degradation, particularly when detecting low-intensity light signals. This becomes especially problematic in pharmaceutical research where trace amounts of compounds need to be accurately measured. Cooling systems and advanced signal processing techniques are often required to mitigate these noise issues, adding complexity and cost to spectrometry tools.
Linearity is another critical concern for photodiodes in spectrometry applications. While photodiodes generally exhibit good linearity over a wide range of light intensities, they can deviate from linear response at very low or very high light levels. This non-linearity can introduce errors in quantitative measurements, which is particularly problematic in pharmaceutical research where precise concentration determinations are crucial.
The response time of photodiodes can also be a limiting factor, especially in high-speed applications such as real-time process monitoring or rapid scanning spectrometry. While many photodiodes offer fast response times, there is often a trade-off between speed and sensitivity that must be carefully balanced based on the specific requirements of the pharmaceutical research application.
Environmental factors pose additional challenges for photodiode-based spectrometry tools. Temperature fluctuations can affect the performance of photodiodes, causing changes in sensitivity and dark current. This necessitates careful temperature control or compensation mechanisms in spectrometry instruments, particularly for field-deployable devices used in pharmaceutical quality control.
Long-term stability and aging effects are also significant concerns. Over time, photodiodes may experience degradation in performance due to exposure to light, radiation, or environmental contaminants. This can lead to drift in measurements and require frequent recalibration of spectrometry tools, which is time-consuming and can impact the reliability of long-term studies in pharmaceutical research.
Lastly, the integration of photodiodes into compact, portable spectrometry tools presents design challenges. Miniaturization often involves trade-offs in terms of sensitivity, spectral range, and signal-to-noise ratio. Balancing these factors while maintaining the robustness and accuracy required for pharmaceutical applications remains an ongoing challenge for instrument designers and researchers in the field.
Another challenge is the inherent noise in photodiode-based systems. Dark current, shot noise, and thermal noise can all contribute to signal degradation, particularly when detecting low-intensity light signals. This becomes especially problematic in pharmaceutical research where trace amounts of compounds need to be accurately measured. Cooling systems and advanced signal processing techniques are often required to mitigate these noise issues, adding complexity and cost to spectrometry tools.
Linearity is another critical concern for photodiodes in spectrometry applications. While photodiodes generally exhibit good linearity over a wide range of light intensities, they can deviate from linear response at very low or very high light levels. This non-linearity can introduce errors in quantitative measurements, which is particularly problematic in pharmaceutical research where precise concentration determinations are crucial.
The response time of photodiodes can also be a limiting factor, especially in high-speed applications such as real-time process monitoring or rapid scanning spectrometry. While many photodiodes offer fast response times, there is often a trade-off between speed and sensitivity that must be carefully balanced based on the specific requirements of the pharmaceutical research application.
Environmental factors pose additional challenges for photodiode-based spectrometry tools. Temperature fluctuations can affect the performance of photodiodes, causing changes in sensitivity and dark current. This necessitates careful temperature control or compensation mechanisms in spectrometry instruments, particularly for field-deployable devices used in pharmaceutical quality control.
Long-term stability and aging effects are also significant concerns. Over time, photodiodes may experience degradation in performance due to exposure to light, radiation, or environmental contaminants. This can lead to drift in measurements and require frequent recalibration of spectrometry tools, which is time-consuming and can impact the reliability of long-term studies in pharmaceutical research.
Lastly, the integration of photodiodes into compact, portable spectrometry tools presents design challenges. Miniaturization often involves trade-offs in terms of sensitivity, spectral range, and signal-to-noise ratio. Balancing these factors while maintaining the robustness and accuracy required for pharmaceutical applications remains an ongoing challenge for instrument designers and researchers in the field.
Current Solutions
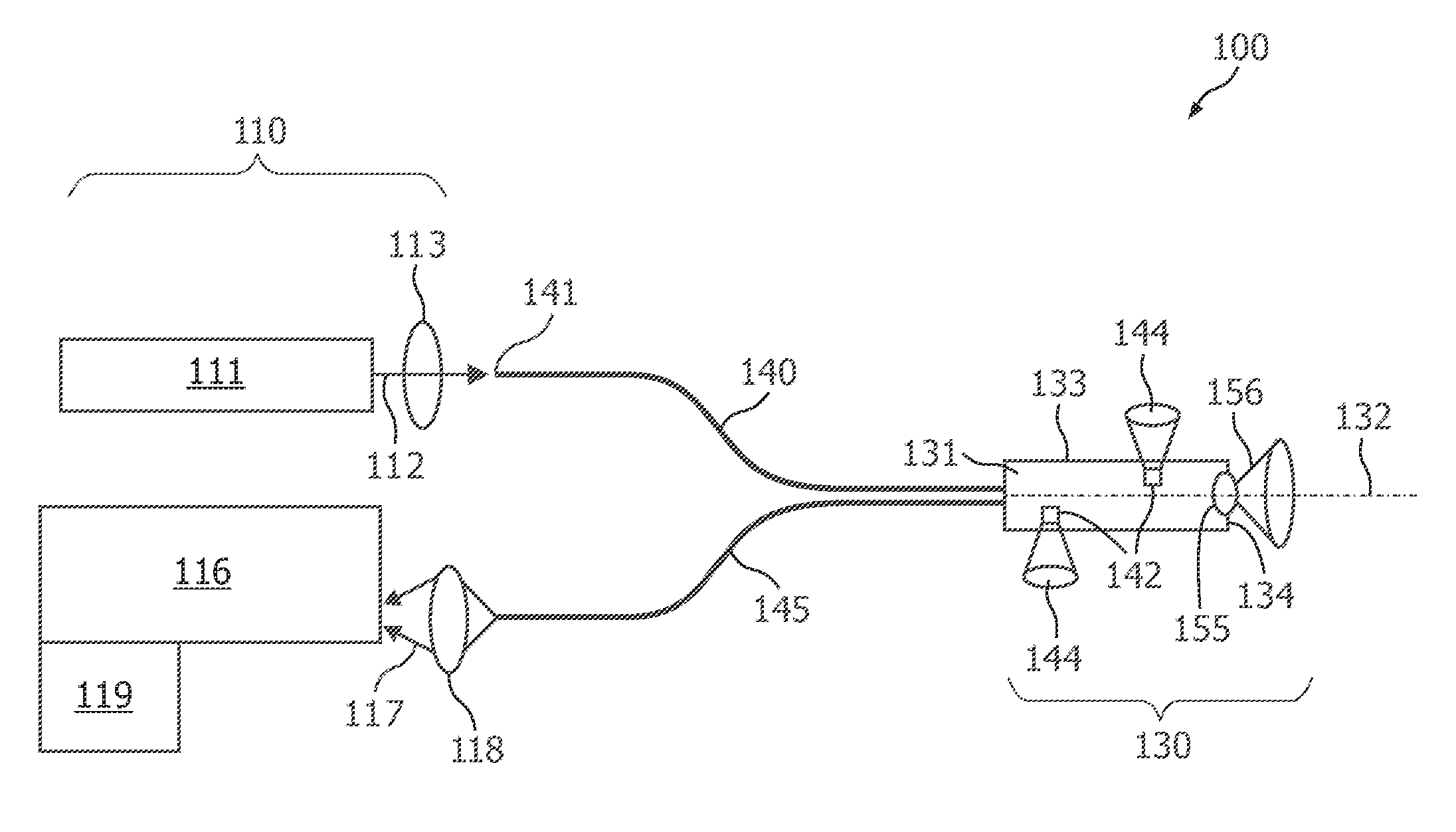
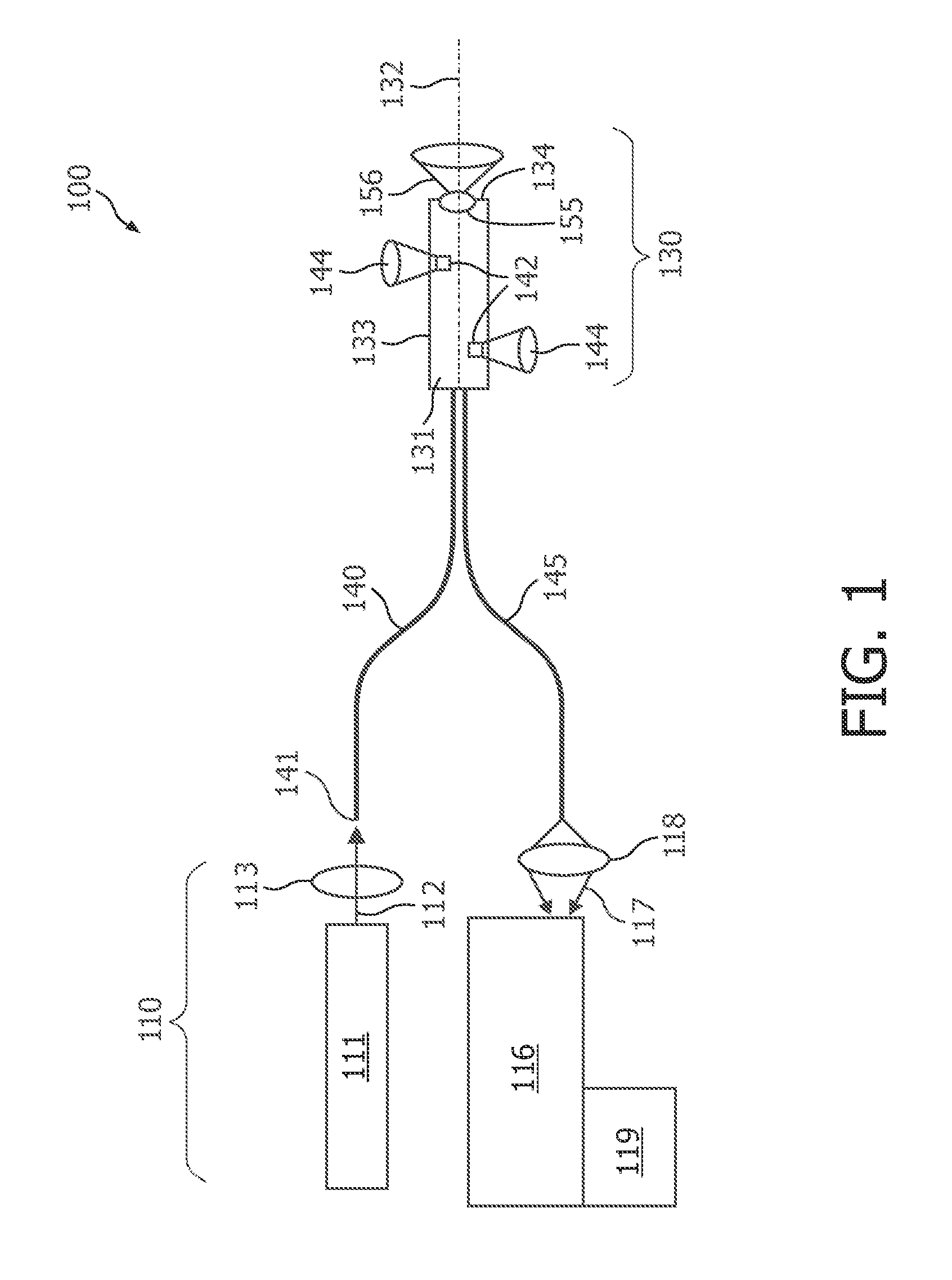
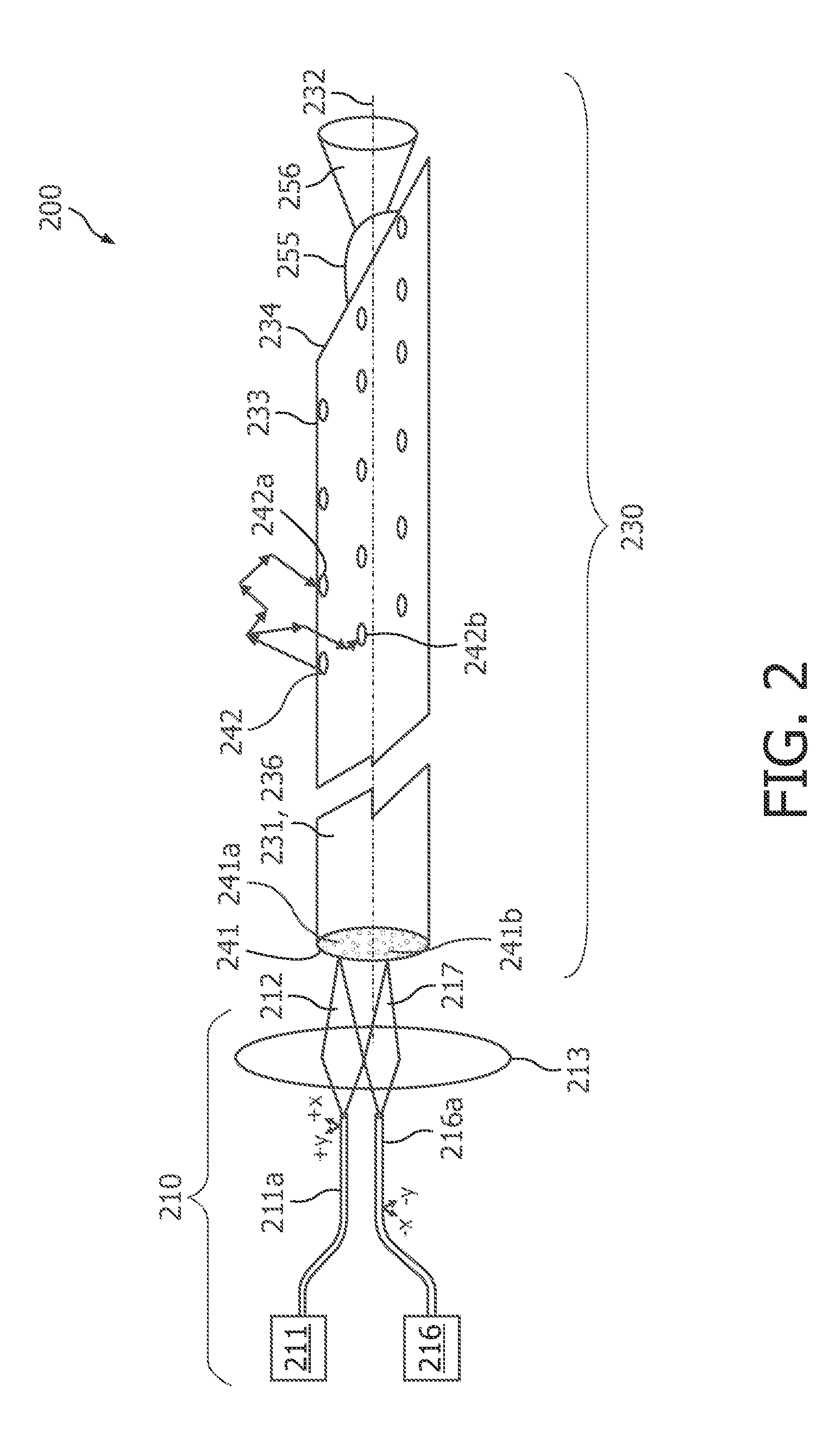
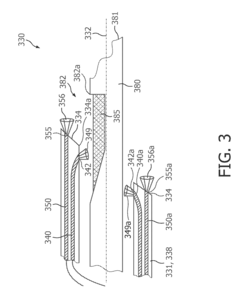
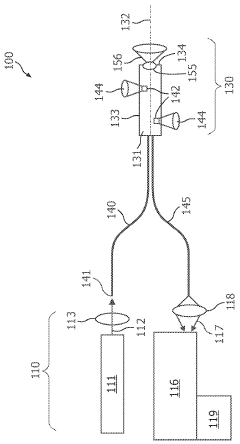
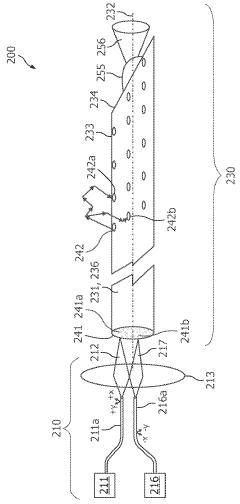
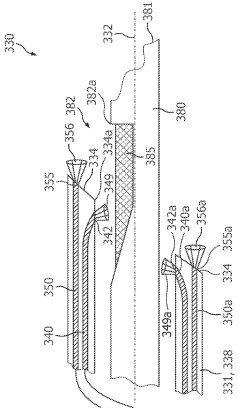
01 Photodiode array structures for spectrometry
Specialized photodiode array structures are designed for use in spectrometry applications. These arrays can include multiple photodiodes arranged in specific configurations to capture light across different wavelengths. The structures may incorporate various materials and fabrication techniques to optimize sensitivity and spectral response.- Photodiode array design for spectrometry: Spectrometry tools utilize specialized photodiode array designs to enhance sensitivity and spectral resolution. These arrays may incorporate multiple photodiodes with different spectral responses or use advanced semiconductor structures to optimize light detection across various wavelengths. The design often includes features to minimize crosstalk between adjacent photodiodes and improve signal-to-noise ratio.
- Integration of photodiodes with readout circuitry: Photodiode-based spectrometry tools often integrate photodiodes with readout circuitry on a single chip. This integration can include analog-to-digital converters, amplifiers, and signal processing units. The close integration helps reduce noise, improve speed, and enhance overall system performance. Advanced packaging techniques may be employed to optimize the integration of optical and electronic components.
- Spectral filtering and wavelength selection: Spectrometry tools incorporate various methods for spectral filtering and wavelength selection to work in conjunction with photodiodes. This can include the use of diffraction gratings, prisms, or tunable filters to separate light into its component wavelengths before detection. Some designs may use multiple photodiodes with different spectral sensitivities to achieve wavelength discrimination without additional optical components.
- Miniaturization and portability: There is a trend towards miniaturization of photodiode-based spectrometry tools to enhance portability and enable new applications. This involves the development of compact optical designs, integration of multiple functions on a single chip, and the use of advanced manufacturing techniques. Miniaturized spectrometers can be incorporated into handheld devices or integrated into other systems for in-situ measurements.
- Calibration and error correction techniques: Photodiode-based spectrometry tools employ various calibration and error correction techniques to improve accuracy and reliability. These may include methods for dark current subtraction, temperature compensation, and correction for non-linearity in photodiode response. Some systems use built-in reference standards or self-calibration routines to maintain performance over time and varying environmental conditions.
02 Integration of photodiodes with readout circuits
Photodiodes are integrated with readout circuits to process and amplify the detected signals. This integration can involve CMOS technology, allowing for compact and efficient spectrometry tools. The readout circuits may include analog-to-digital converters and signal processing elements to enhance measurement accuracy.Expand Specific Solutions03 Spectral filtering techniques for photodiode-based spectrometers
Various spectral filtering techniques are employed in photodiode-based spectrometry tools to improve wavelength selectivity. These may include the use of optical filters, gratings, or prisms to separate light into its constituent wavelengths before detection by the photodiodes. Advanced filtering methods can enhance the resolution and accuracy of spectral measurements.Expand Specific Solutions04 Miniaturization of photodiode-based spectrometers
Efforts are made to miniaturize photodiode-based spectrometry tools for portable and integrated applications. This involves developing compact optical designs, reducing component sizes, and optimizing the overall system architecture. Miniaturized spectrometers can be used in various fields, including environmental monitoring and medical diagnostics.Expand Specific Solutions05 Calibration and signal processing for improved accuracy
Advanced calibration techniques and signal processing algorithms are developed to enhance the accuracy of photodiode-based spectrometry tools. These methods can compensate for various sources of error, such as temperature drift and non-linearity in photodiode response. Machine learning and artificial intelligence approaches may be used to improve data analysis and interpretation.Expand Specific Solutions
Industry Leaders
The research on photodiode-based spectrometry tools in pharmaceutical research is in a mature stage of development, with a growing market driven by increasing demand for advanced analytical techniques. The global market for these tools is expanding, fueled by pharmaceutical industry growth and the need for precise drug analysis. Technologically, the field is well-established but continues to evolve, with companies like Hamamatsu Photonics, Agilent Technologies, and Hitachi leading innovation. These firms, along with others such as Koninklijke Philips and Analog Devices, are pushing boundaries in sensitivity, resolution, and miniaturization. Academic institutions like Karlsruhe Institute of Technology and Johns Hopkins University contribute significantly to research advancements, fostering industry-academia collaborations and driving technological progress in this competitive landscape.
Koninklijke Philips NV
Technical Solution: Philips has developed innovative photodiode-based spectrometry tools for pharmaceutical research, focusing on near-infrared (NIR) spectroscopy. Their advanced NIR systems utilize high-performance photodiode arrays for rapid and non-destructive analysis of pharmaceutical compounds[1]. Philips' technology enables real-time monitoring of drug formulation processes, ensuring consistent quality and composition[2]. The company has also introduced miniaturized NIR spectrometers that can be integrated into portable devices, allowing for on-site analysis in various pharmaceutical settings[3]. These systems employ sophisticated algorithms for spectral data processing, enhancing the accuracy and reliability of results in drug development and quality control applications.
Strengths: Non-destructive analysis, real-time monitoring capabilities, and miniaturization for portability. Weaknesses: May have limitations in analyzing certain complex pharmaceutical formulations, potential for interference from environmental factors.
Hamamatsu Photonics KK
Technical Solution: Hamamatsu Photonics has developed cutting-edge photodiode-based spectrometry tools for pharmaceutical research, leveraging their expertise in optoelectronics. Their mini-spectrometers utilize advanced CMOS linear image sensors, providing high sensitivity and low noise performance for precise spectral analysis[1]. Hamamatsu's C12666MA micro-spectrometer, measuring just 20.1 × 12.5 × 10.1 mm, offers unprecedented miniaturization without compromising performance, enabling integration into compact analytical devices for pharmaceutical applications[2]. The company has also introduced InGaAs linear image sensors for near-infrared (NIR) spectroscopy, extending the spectral range for comprehensive analysis of pharmaceutical compounds[3]. Hamamatsu's spectrometers feature high-speed readout capabilities, allowing for real-time monitoring of fast chemical reactions in drug development processes.
Strengths: Miniaturization capabilities, high sensitivity, and extended spectral range for NIR applications. Weaknesses: May have limitations in analyzing very low concentration samples, potential for higher cost due to advanced technology.
Key Innovations
Obtaining optical tissue properties
PatentInactiveUS20090326385A1
Innovation
- A medical device with integrated optical fibers providing a lateral field of view, allowing for the investigation of tissue properties beyond the longitudinal axis, combined with optical waveguides and reflector elements for enhanced imaging and navigation, enabling detailed optical characterization of tissue properties in three dimensions.
Obtaining optical tissue properties
PatentWO2008068685A1
Innovation
- A medical device with integrated optical fibers providing a lateral field of view, allowing for the investigation of tissue properties beyond the longitudinal axis, combined with optical waveguides and reflector elements for enhanced imaging capabilities, enabling detailed optical characterization of tissue properties and improved navigation during procedures.
Regulatory Compliance
Regulatory compliance is a critical aspect of photodiode-based spectrometry tools in pharmaceutical research. The use of these tools must adhere to strict guidelines set by regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe. These regulations ensure the accuracy, reliability, and safety of pharmaceutical products throughout their development and manufacturing processes.
One of the primary regulatory considerations for photodiode-based spectrometry tools is compliance with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP). These guidelines establish standards for equipment calibration, maintenance, and validation. Manufacturers of spectrometry tools must provide comprehensive documentation on their instruments' performance, accuracy, and precision to meet these requirements.
The FDA's 21 CFR Part 11 regulation is particularly relevant for photodiode-based spectrometry tools used in pharmaceutical research. This regulation outlines the criteria for electronic records and electronic signatures, ensuring data integrity and traceability. Spectrometry tools must incorporate features such as audit trails, user authentication, and data encryption to comply with these standards.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) may apply to certain photodiode-based spectrometry tools, depending on their intended use. These regulations impose stringent requirements on device classification, clinical evaluation, and post-market surveillance.
Manufacturers of photodiode-based spectrometry tools must also consider international standards such as ISO 13485 for quality management systems in medical devices. Compliance with these standards demonstrates a commitment to quality and safety, which is essential for regulatory approval and market acceptance.
Regulatory bodies often require validation of analytical methods using spectrometry tools. This includes method development, optimization, and validation studies to ensure the reliability and reproducibility of results. Pharmaceutical companies must demonstrate that their spectrometry-based methods meet regulatory requirements for specificity, accuracy, precision, and robustness.
As the pharmaceutical industry increasingly adopts continuous manufacturing processes, regulatory agencies are adapting their guidelines to accommodate real-time release testing. Photodiode-based spectrometry tools play a crucial role in this paradigm shift, necessitating updated regulatory frameworks to address the unique challenges of in-line and at-line monitoring.
Regulatory compliance also extends to software used in conjunction with spectrometry tools. Manufacturers must ensure that their software meets regulatory requirements for data integrity, security, and traceability. This includes features such as version control, change management, and system validation.
One of the primary regulatory considerations for photodiode-based spectrometry tools is compliance with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP). These guidelines establish standards for equipment calibration, maintenance, and validation. Manufacturers of spectrometry tools must provide comprehensive documentation on their instruments' performance, accuracy, and precision to meet these requirements.
The FDA's 21 CFR Part 11 regulation is particularly relevant for photodiode-based spectrometry tools used in pharmaceutical research. This regulation outlines the criteria for electronic records and electronic signatures, ensuring data integrity and traceability. Spectrometry tools must incorporate features such as audit trails, user authentication, and data encryption to comply with these standards.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) may apply to certain photodiode-based spectrometry tools, depending on their intended use. These regulations impose stringent requirements on device classification, clinical evaluation, and post-market surveillance.
Manufacturers of photodiode-based spectrometry tools must also consider international standards such as ISO 13485 for quality management systems in medical devices. Compliance with these standards demonstrates a commitment to quality and safety, which is essential for regulatory approval and market acceptance.
Regulatory bodies often require validation of analytical methods using spectrometry tools. This includes method development, optimization, and validation studies to ensure the reliability and reproducibility of results. Pharmaceutical companies must demonstrate that their spectrometry-based methods meet regulatory requirements for specificity, accuracy, precision, and robustness.
As the pharmaceutical industry increasingly adopts continuous manufacturing processes, regulatory agencies are adapting their guidelines to accommodate real-time release testing. Photodiode-based spectrometry tools play a crucial role in this paradigm shift, necessitating updated regulatory frameworks to address the unique challenges of in-line and at-line monitoring.
Regulatory compliance also extends to software used in conjunction with spectrometry tools. Manufacturers must ensure that their software meets regulatory requirements for data integrity, security, and traceability. This includes features such as version control, change management, and system validation.
Data Analysis Methods
Data analysis methods play a crucial role in extracting meaningful information from photodiode-based spectrometry tools in pharmaceutical research. These methods encompass a wide range of techniques, from basic statistical analyses to advanced machine learning algorithms.
One of the fundamental approaches in spectral data analysis is preprocessing. This step involves noise reduction, baseline correction, and normalization of the raw spectral data. Noise reduction techniques, such as Savitzky-Golay filtering or wavelet denoising, help to improve the signal-to-noise ratio. Baseline correction algorithms, including polynomial fitting or asymmetric least squares, remove unwanted background signals. Normalization methods, like standard normal variate (SNV) or multiplicative scatter correction (MSC), account for variations in sample thickness or instrument response.
Multivariate analysis techniques are widely employed to handle the high-dimensional nature of spectral data. Principal Component Analysis (PCA) is often used for dimensionality reduction and exploratory data analysis. It helps identify patterns and groupings in the spectral data, which can be particularly useful for quality control in pharmaceutical manufacturing. Partial Least Squares (PLS) regression is another powerful tool for building predictive models, allowing researchers to correlate spectral features with specific pharmaceutical properties or concentrations.
Advanced chemometric methods, such as Multivariate Curve Resolution (MCR) and Independent Component Analysis (ICA), are utilized to resolve complex spectral mixtures into their individual components. These techniques are invaluable in pharmaceutical research for identifying and quantifying multiple active ingredients in a formulation or detecting impurities in drug products.
Machine learning algorithms have gained significant traction in recent years for spectral data analysis. Support Vector Machines (SVM), Random Forests, and Artificial Neural Networks (ANN) have demonstrated excellent performance in classification tasks, such as identifying counterfeit drugs or determining the crystalline form of pharmaceutical compounds. Deep learning approaches, including Convolutional Neural Networks (CNN) and Long Short-Term Memory (LSTM) networks, have shown promise in handling raw spectral data without extensive preprocessing.
Time-resolved spectroscopy data analysis methods are essential for studying dynamic processes in pharmaceutical research. These include global and target analysis techniques, which can reveal kinetic information about chemical reactions or conformational changes in drug molecules. Singular Value Decomposition (SVD) and Evolving Factor Analysis (EFA) are commonly used to extract time-dependent spectral components from complex datasets.
As the field of spectral data analysis continues to evolve, new methods are emerging to address specific challenges in pharmaceutical research. Hybrid approaches that combine traditional chemometric techniques with machine learning algorithms are gaining popularity. Additionally, the integration of spectral data with other analytical techniques, such as chromatography or mass spectrometry, is driving the development of novel data fusion methods to extract more comprehensive information about pharmaceutical samples.
One of the fundamental approaches in spectral data analysis is preprocessing. This step involves noise reduction, baseline correction, and normalization of the raw spectral data. Noise reduction techniques, such as Savitzky-Golay filtering or wavelet denoising, help to improve the signal-to-noise ratio. Baseline correction algorithms, including polynomial fitting or asymmetric least squares, remove unwanted background signals. Normalization methods, like standard normal variate (SNV) or multiplicative scatter correction (MSC), account for variations in sample thickness or instrument response.
Multivariate analysis techniques are widely employed to handle the high-dimensional nature of spectral data. Principal Component Analysis (PCA) is often used for dimensionality reduction and exploratory data analysis. It helps identify patterns and groupings in the spectral data, which can be particularly useful for quality control in pharmaceutical manufacturing. Partial Least Squares (PLS) regression is another powerful tool for building predictive models, allowing researchers to correlate spectral features with specific pharmaceutical properties or concentrations.
Advanced chemometric methods, such as Multivariate Curve Resolution (MCR) and Independent Component Analysis (ICA), are utilized to resolve complex spectral mixtures into their individual components. These techniques are invaluable in pharmaceutical research for identifying and quantifying multiple active ingredients in a formulation or detecting impurities in drug products.
Machine learning algorithms have gained significant traction in recent years for spectral data analysis. Support Vector Machines (SVM), Random Forests, and Artificial Neural Networks (ANN) have demonstrated excellent performance in classification tasks, such as identifying counterfeit drugs or determining the crystalline form of pharmaceutical compounds. Deep learning approaches, including Convolutional Neural Networks (CNN) and Long Short-Term Memory (LSTM) networks, have shown promise in handling raw spectral data without extensive preprocessing.
Time-resolved spectroscopy data analysis methods are essential for studying dynamic processes in pharmaceutical research. These include global and target analysis techniques, which can reveal kinetic information about chemical reactions or conformational changes in drug molecules. Singular Value Decomposition (SVD) and Evolving Factor Analysis (EFA) are commonly used to extract time-dependent spectral components from complex datasets.
As the field of spectral data analysis continues to evolve, new methods are emerging to address specific challenges in pharmaceutical research. Hybrid approaches that combine traditional chemometric techniques with machine learning algorithms are gaining popularity. Additionally, the integration of spectral data with other analytical techniques, such as chromatography or mass spectrometry, is driving the development of novel data fusion methods to extract more comprehensive information about pharmaceutical samples.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!