Plant-Based Muscimol Extracts and Their Effects
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Research Background and Objectives
Muscimol, a psychoactive compound found in certain mushroom species, has garnered significant attention in recent years due to its potential therapeutic applications. The research on plant-based muscimol extracts and their effects has evolved from early ethnobotanical studies to modern scientific investigations, spanning several decades of exploration and discovery.
The historical context of muscimol research dates back to the mid-20th century when scientists first isolated and identified the compound from Amanita muscaria, commonly known as the fly agaric mushroom. This discovery sparked interest in understanding the chemical structure, pharmacological properties, and potential uses of muscimol in various fields, including neuroscience, psychiatry, and pharmacology.
As research progressed, the focus shifted from purely academic pursuits to exploring the therapeutic potential of muscimol. Scientists began investigating its effects on the central nervous system, particularly its interaction with GABA receptors, which play a crucial role in regulating neurotransmission. This led to a growing body of knowledge about muscimol's anxiolytic, sedative, and anticonvulsant properties.
The technological advancements in extraction and analysis techniques have significantly contributed to the evolution of muscimol research. Modern methods such as high-performance liquid chromatography (HPLC) and mass spectrometry have enabled more precise identification and quantification of muscimol in plant materials, facilitating more accurate and reliable studies.
Current research objectives in the field of plant-based muscimol extracts are multifaceted. One primary goal is to develop standardized extraction protocols that ensure consistent and high-quality muscimol yields from natural sources. This is crucial for both research purposes and potential commercial applications, as it would enable more reliable and reproducible results in scientific studies and product development.
Another key objective is to elucidate the full spectrum of muscimol's effects on human physiology and cognition. This includes investigating its potential therapeutic applications in treating various neurological and psychiatric disorders, such as anxiety, epilepsy, and sleep disorders. Researchers are also exploring the possibility of using muscimol as a tool for studying brain function and neurotransmitter systems.
Safety and toxicology studies represent another critical area of focus. As interest in muscimol's therapeutic potential grows, it is essential to thoroughly understand its safety profile, potential side effects, and interactions with other substances. This includes evaluating the long-term effects of muscimol use and establishing appropriate dosing guidelines for different applications.
Furthermore, there is a growing interest in identifying and characterizing other plant sources of muscimol beyond Amanita muscaria. This exploration aims to discover alternative, potentially more sustainable or easily cultivated sources of the compound, which could have significant implications for future research and commercial development.
The historical context of muscimol research dates back to the mid-20th century when scientists first isolated and identified the compound from Amanita muscaria, commonly known as the fly agaric mushroom. This discovery sparked interest in understanding the chemical structure, pharmacological properties, and potential uses of muscimol in various fields, including neuroscience, psychiatry, and pharmacology.
As research progressed, the focus shifted from purely academic pursuits to exploring the therapeutic potential of muscimol. Scientists began investigating its effects on the central nervous system, particularly its interaction with GABA receptors, which play a crucial role in regulating neurotransmission. This led to a growing body of knowledge about muscimol's anxiolytic, sedative, and anticonvulsant properties.
The technological advancements in extraction and analysis techniques have significantly contributed to the evolution of muscimol research. Modern methods such as high-performance liquid chromatography (HPLC) and mass spectrometry have enabled more precise identification and quantification of muscimol in plant materials, facilitating more accurate and reliable studies.
Current research objectives in the field of plant-based muscimol extracts are multifaceted. One primary goal is to develop standardized extraction protocols that ensure consistent and high-quality muscimol yields from natural sources. This is crucial for both research purposes and potential commercial applications, as it would enable more reliable and reproducible results in scientific studies and product development.
Another key objective is to elucidate the full spectrum of muscimol's effects on human physiology and cognition. This includes investigating its potential therapeutic applications in treating various neurological and psychiatric disorders, such as anxiety, epilepsy, and sleep disorders. Researchers are also exploring the possibility of using muscimol as a tool for studying brain function and neurotransmitter systems.
Safety and toxicology studies represent another critical area of focus. As interest in muscimol's therapeutic potential grows, it is essential to thoroughly understand its safety profile, potential side effects, and interactions with other substances. This includes evaluating the long-term effects of muscimol use and establishing appropriate dosing guidelines for different applications.
Furthermore, there is a growing interest in identifying and characterizing other plant sources of muscimol beyond Amanita muscaria. This exploration aims to discover alternative, potentially more sustainable or easily cultivated sources of the compound, which could have significant implications for future research and commercial development.
Market Analysis for Plant-Based Psychoactive Compounds
The market for plant-based psychoactive compounds, including muscimol extracts, has been experiencing significant growth in recent years. This surge is driven by increasing consumer interest in natural alternatives to synthetic drugs, growing acceptance of psychoactive substances for therapeutic purposes, and expanding research into their potential medical applications.
The global market for plant-based psychoactive compounds is diverse, encompassing various sectors such as pharmaceuticals, nutraceuticals, and recreational use. Muscimol, primarily derived from Amanita muscaria mushrooms, represents a niche but growing segment within this market. Its potential applications in anxiety reduction, sleep enhancement, and cognitive improvement have attracted attention from both researchers and consumers.
Consumer demand for plant-based psychoactive compounds is largely fueled by the wellness and mental health trends. As more individuals seek natural remedies for stress, anxiety, and sleep disorders, the market for muscimol and similar compounds has expanded. This trend is particularly pronounced among millennials and Gen Z consumers, who show a strong preference for natural and organic products.
The pharmaceutical industry has also shown increasing interest in plant-based psychoactive compounds. Several companies are exploring the potential of muscimol and related substances for developing new drugs to treat neurological and psychiatric disorders. This research and development activity is expected to drive market growth in the coming years.
Regulatory environments play a crucial role in shaping the market for plant-based psychoactive compounds. While some countries have strict regulations, others are adopting more lenient approaches, particularly for compounds with potential therapeutic benefits. This evolving regulatory landscape is likely to have a significant impact on market accessibility and growth potential.
The market is characterized by a mix of established pharmaceutical companies and innovative startups. These players are investing in research and development to unlock new applications and improve extraction methods for plant-based psychoactive compounds. Collaborations between academic institutions and private companies are becoming more common, accelerating the pace of innovation in this field.
Challenges in the market include ensuring consistent quality and potency of plant-derived compounds, addressing safety concerns, and navigating complex regulatory frameworks. However, advancements in extraction and purification technologies are helping to overcome some of these hurdles, potentially leading to more standardized and reliable products.
The global market for plant-based psychoactive compounds is diverse, encompassing various sectors such as pharmaceuticals, nutraceuticals, and recreational use. Muscimol, primarily derived from Amanita muscaria mushrooms, represents a niche but growing segment within this market. Its potential applications in anxiety reduction, sleep enhancement, and cognitive improvement have attracted attention from both researchers and consumers.
Consumer demand for plant-based psychoactive compounds is largely fueled by the wellness and mental health trends. As more individuals seek natural remedies for stress, anxiety, and sleep disorders, the market for muscimol and similar compounds has expanded. This trend is particularly pronounced among millennials and Gen Z consumers, who show a strong preference for natural and organic products.
The pharmaceutical industry has also shown increasing interest in plant-based psychoactive compounds. Several companies are exploring the potential of muscimol and related substances for developing new drugs to treat neurological and psychiatric disorders. This research and development activity is expected to drive market growth in the coming years.
Regulatory environments play a crucial role in shaping the market for plant-based psychoactive compounds. While some countries have strict regulations, others are adopting more lenient approaches, particularly for compounds with potential therapeutic benefits. This evolving regulatory landscape is likely to have a significant impact on market accessibility and growth potential.
The market is characterized by a mix of established pharmaceutical companies and innovative startups. These players are investing in research and development to unlock new applications and improve extraction methods for plant-based psychoactive compounds. Collaborations between academic institutions and private companies are becoming more common, accelerating the pace of innovation in this field.
Challenges in the market include ensuring consistent quality and potency of plant-derived compounds, addressing safety concerns, and navigating complex regulatory frameworks. However, advancements in extraction and purification technologies are helping to overcome some of these hurdles, potentially leading to more standardized and reliable products.
Current State and Challenges in Muscimol Extraction
The current state of muscimol extraction from plant sources presents both significant advancements and notable challenges. Muscimol, a psychoactive compound primarily found in Amanita mushroom species, has gained increasing attention in research due to its potential therapeutic applications. Recent developments in extraction techniques have improved the efficiency and purity of muscimol isolation from natural sources.
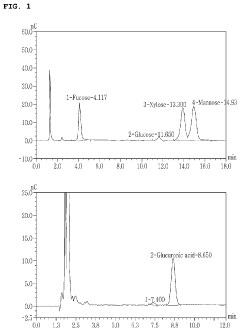
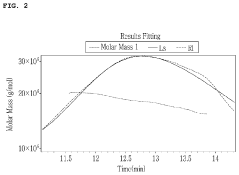
Advanced chromatography methods, particularly high-performance liquid chromatography (HPLC) coupled with mass spectrometry, have enabled more precise identification and quantification of muscimol in plant extracts. This has led to a better understanding of muscimol content variations among different Amanita species and growth conditions. Additionally, novel extraction protocols utilizing supercritical fluid extraction have shown promise in yielding higher muscimol concentrations while minimizing the co-extraction of undesired compounds.
Despite these advancements, several challenges persist in the field of muscimol extraction. One major obstacle is the limited availability of muscimol-containing plant sources. Amanita mushrooms, the primary natural source, are not widely cultivated, and wild harvesting poses risks due to potential misidentification and environmental concerns. This scarcity of raw materials hampers large-scale extraction efforts and limits research capabilities.
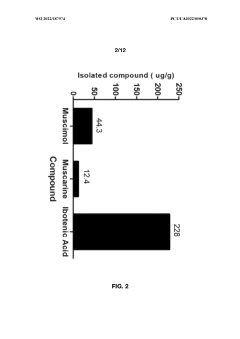
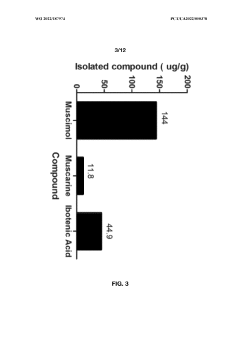
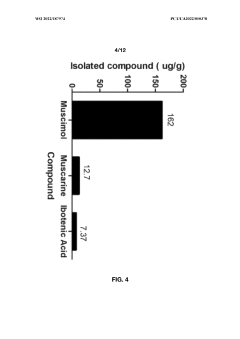
Another significant challenge lies in the complex chemical composition of Amanita mushrooms. Muscimol often coexists with other bioactive compounds, including the toxic ibotenic acid. Developing selective extraction methods that isolate muscimol while eliminating or minimizing the presence of these potentially harmful substances remains a critical area of research. The conversion of ibotenic acid to muscimol during extraction processes adds another layer of complexity to purification efforts.
Regulatory hurdles also impact the progress of muscimol extraction research. The psychoactive nature of muscimol places it under strict control in many jurisdictions, limiting access to raw materials and complicating the approval process for research and potential therapeutic applications. This regulatory landscape necessitates careful navigation and collaboration between researchers, industry, and regulatory bodies.
Scalability of extraction processes presents another challenge. While laboratory-scale extractions have shown promising results, translating these methods to industrial-scale production while maintaining efficiency and cost-effectiveness remains an ongoing concern. Optimizing extraction parameters, such as solvent selection, temperature, and pressure, for large-scale operations requires further investigation and technological innovation.
Lastly, the stability of extracted muscimol poses challenges for long-term storage and formulation development. Muscimol's sensitivity to environmental factors like light, temperature, and pH necessitates the development of robust stabilization techniques to ensure the integrity of the compound throughout the extraction, storage, and potential therapeutic application processes.
Advanced chromatography methods, particularly high-performance liquid chromatography (HPLC) coupled with mass spectrometry, have enabled more precise identification and quantification of muscimol in plant extracts. This has led to a better understanding of muscimol content variations among different Amanita species and growth conditions. Additionally, novel extraction protocols utilizing supercritical fluid extraction have shown promise in yielding higher muscimol concentrations while minimizing the co-extraction of undesired compounds.
Despite these advancements, several challenges persist in the field of muscimol extraction. One major obstacle is the limited availability of muscimol-containing plant sources. Amanita mushrooms, the primary natural source, are not widely cultivated, and wild harvesting poses risks due to potential misidentification and environmental concerns. This scarcity of raw materials hampers large-scale extraction efforts and limits research capabilities.
Another significant challenge lies in the complex chemical composition of Amanita mushrooms. Muscimol often coexists with other bioactive compounds, including the toxic ibotenic acid. Developing selective extraction methods that isolate muscimol while eliminating or minimizing the presence of these potentially harmful substances remains a critical area of research. The conversion of ibotenic acid to muscimol during extraction processes adds another layer of complexity to purification efforts.
Regulatory hurdles also impact the progress of muscimol extraction research. The psychoactive nature of muscimol places it under strict control in many jurisdictions, limiting access to raw materials and complicating the approval process for research and potential therapeutic applications. This regulatory landscape necessitates careful navigation and collaboration between researchers, industry, and regulatory bodies.
Scalability of extraction processes presents another challenge. While laboratory-scale extractions have shown promising results, translating these methods to industrial-scale production while maintaining efficiency and cost-effectiveness remains an ongoing concern. Optimizing extraction parameters, such as solvent selection, temperature, and pressure, for large-scale operations requires further investigation and technological innovation.
Lastly, the stability of extracted muscimol poses challenges for long-term storage and formulation development. Muscimol's sensitivity to environmental factors like light, temperature, and pH necessitates the development of robust stabilization techniques to ensure the integrity of the compound throughout the extraction, storage, and potential therapeutic application processes.
Existing Muscimol Extraction Techniques
01 Extraction methods for plant-based muscimol
Various techniques are employed to extract muscimol from plant sources, particularly Amanita mushrooms. These methods may include solvent extraction, chromatography, and other separation processes to isolate and purify the compound. The extraction methods aim to maximize yield and purity of muscimol while minimizing the presence of other potentially harmful compounds.- Extraction methods for plant-based muscimol: Various techniques are employed to extract muscimol from plant sources, particularly Amanita mushrooms. These methods may include solvent extraction, chromatography, and other separation processes to isolate and purify the compound. The extraction procedures are optimized to maximize yield and purity of muscimol while minimizing the presence of other potentially harmful compounds.
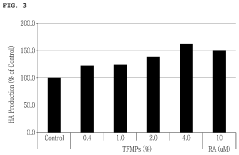
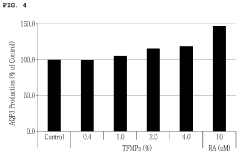
- Pharmacological effects of plant-derived muscimol: Plant-based muscimol extracts exhibit various pharmacological effects, primarily due to their action as a GABA receptor agonist. These effects may include anxiolytic, sedative, and muscle relaxant properties. Research focuses on understanding the mechanisms of action and potential therapeutic applications in neurological and psychiatric disorders.
- Formulation of muscimol-containing products: Muscimol extracts are incorporated into various formulations for different applications. These may include pharmaceutical preparations, nutraceuticals, or cosmetic products. The formulation process considers factors such as bioavailability, stability, and delivery methods to optimize the efficacy and safety of muscimol-based products.
- Safety and toxicity studies of plant-based muscimol: Research is conducted to assess the safety profile and potential toxicity of plant-derived muscimol extracts. This includes studies on acute and chronic toxicity, side effects, and interactions with other substances. The aim is to establish safe dosage ranges and identify any contraindications for muscimol-based products.
- Cultivation and sustainable sourcing of muscimol-containing plants: Methods for cultivating plants rich in muscimol, particularly certain species of Amanita mushrooms, are developed to ensure a sustainable and controlled source of the compound. This includes optimizing growth conditions, developing cultivation techniques, and exploring alternative plant sources that may contain muscimol or similar compounds.
02 Pharmacological effects of plant-derived muscimol
Plant-based muscimol extracts exhibit various pharmacological effects, primarily due to their action as GABA receptor agonists. These effects may include anxiolytic, sedative, and muscle relaxant properties. Research suggests potential therapeutic applications in treating anxiety disorders, sleep disturbances, and certain neurological conditions.Expand Specific Solutions03 Formulation of muscimol-containing products
Muscimol extracts are incorporated into various formulations for different applications. These may include oral dosage forms, topical preparations, and novel drug delivery systems. Formulation strategies focus on enhancing bioavailability, stability, and controlled release of the active compound.Expand Specific Solutions04 Safety and toxicity considerations
Studies on plant-based muscimol extracts address safety concerns and potential toxicity. Research focuses on determining safe dosage ranges, identifying potential side effects, and developing methods to mitigate risks associated with muscimol consumption. This includes investigating interactions with other substances and long-term effects of use.Expand Specific Solutions05 Synthetic production of muscimol analogs
Research explores the synthetic production of muscimol and its analogs to overcome limitations of plant-based extraction. These efforts aim to create more potent or selective compounds with improved pharmacological profiles. Synthetic approaches may also address issues of supply consistency and purity associated with natural extracts.Expand Specific Solutions
Key Players in Muscimol and Psychoactive Research
The research on plant-based muscimol extracts and their effects is in an early developmental stage, characterized by a growing but still limited market size. The competitive landscape is diverse, with players from various sectors including pharmaceutical companies, research institutions, and agricultural universities. Companies like DaebongLS Co., Ltd. and Psyched Wellness Ltd. are exploring the potential of muscimol extracts, while research institutions such as the Korea Research Institute of Bioscience & Biotechnology and universities like Huazhong Agricultural University are contributing to the scientific understanding. The technology is still maturing, with ongoing studies on extraction methods, efficacy, and safety profiles, indicating a nascent but promising field with potential for significant growth and innovation.
Korea Research Institute of Bioscience & Biotechnology
Technical Solution: The Korea Research Institute of Bioscience & Biotechnology (KRIBB) has conducted extensive research on plant-based muscimol extracts, particularly from Amanita muscaria mushrooms. Their approach involves optimizing extraction conditions using various solvents and pH levels to maximize muscimol yield. KRIBB researchers have developed a novel HPLC-based quantification method for muscimol in plant extracts, allowing for more accurate analysis[3]. Additionally, they have investigated the neuroprotective and anti-inflammatory effects of muscimol in cellular and animal models, demonstrating potential therapeutic applications[4].
Strengths: Advanced analytical techniques, comprehensive biological activity studies, government-backed research capabilities. Weaknesses: Focus on basic research rather than product development, potential limitations in commercialization expertise.
Consejo Superior de Investigaciones Científicas
Technical Solution: The Consejo Superior de Investigaciones Científicas (CSIC) has conducted research on alternative plant sources of muscimol beyond Amanita muscaria. Their studies have focused on identifying and characterizing muscimol-like compounds in various plant species, particularly those used in traditional medicine. CSIC researchers have employed advanced metabolomics and molecular docking approaches to screen plant extracts for GABA receptor modulators similar to muscimol[5]. They have also investigated the potential synergistic effects of muscimol with other plant-derived compounds, aiming to enhance therapeutic efficacy while reducing side effects[6].
Strengths: Broad botanical expertise, advanced screening methodologies, focus on synergistic plant compounds. Weaknesses: Less focus on muscimol specifically, potential challenges in translating complex plant extract research to standardized products.
Breakthrough Studies on Muscimol Effects
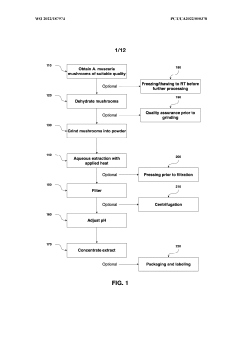
Processes for extracting muscimol from amanita muscaria
PatentWO2022187974A1
Innovation
- Aqueous extraction methods involving heat, pH reduction, and concentration techniques such as distillation and refluxing are employed to decrease ibotenic acid content and increase muscimol content in the extract, including steps like grinding the mushroom biomass, filtering, and acidification to facilitate decarboxylation of ibotenic acid to muscimol.
Composition comprising the extract of tremella fuciformis culture medium
PatentPendingUS20220370337A1
Innovation
- A Tremella fuciformis culture medium extract is developed, utilizing mycelium culture medium polysaccharides with a mannose content of 20-60% by weight, cultured for 6-48 hours at 15-35°C, and extracted with water or C1-C4 alcohols, offering a more viable and effective active ingredient for cosmetic and food compositions.
Regulatory Framework for Psychoactive Compounds
The regulatory framework for psychoactive compounds, including plant-based muscimol extracts, is complex and varies significantly across different jurisdictions. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development, approval, and marketing of psychoactive substances for medical use. The Controlled Substances Act (CSA) also governs the classification and regulation of these compounds, with muscimol currently not specifically scheduled but potentially falling under the Federal Analogue Act.
Internationally, the United Nations Convention on Psychotropic Substances of 1971 provides a global framework for controlling psychoactive substances. However, the status of muscimol and its plant-based extracts remains ambiguous in many countries, leading to varying levels of regulation and control.
In the European Union, the European Medicines Agency (EMA) is responsible for evaluating and monitoring medicines, including those derived from psychoactive compounds. The EU's Novel Food Regulation may also apply to muscimol extracts, depending on their history of consumption and intended use.
Research on plant-based muscimol extracts faces several regulatory challenges. Obtaining approval for clinical trials often requires extensive preclinical data and safety assessments. The sourcing and standardization of plant-based extracts can also present regulatory hurdles, as consistency and quality control are essential for regulatory compliance.
Many countries have implemented specific regulations for traditional and herbal medicines, which may impact the development and marketing of muscimol-containing products. For instance, Canada's Natural Health Products Regulations provide a framework for the approval and sale of natural health products, which could potentially include certain muscimol extracts.
The regulatory landscape for psychedelic compounds is evolving, with some jurisdictions exploring more permissive approaches. Oregon's Measure 109, for example, creates a framework for the therapeutic use of psilocybin, which could set precedents for the regulation of other psychoactive compounds like muscimol.
As research on plant-based muscimol extracts progresses, regulatory bodies will likely need to adapt their frameworks to address the unique challenges posed by these compounds. This may include developing new guidelines for quality control, standardization of plant-based extracts, and assessment of their safety and efficacy profiles.
Internationally, the United Nations Convention on Psychotropic Substances of 1971 provides a global framework for controlling psychoactive substances. However, the status of muscimol and its plant-based extracts remains ambiguous in many countries, leading to varying levels of regulation and control.
In the European Union, the European Medicines Agency (EMA) is responsible for evaluating and monitoring medicines, including those derived from psychoactive compounds. The EU's Novel Food Regulation may also apply to muscimol extracts, depending on their history of consumption and intended use.
Research on plant-based muscimol extracts faces several regulatory challenges. Obtaining approval for clinical trials often requires extensive preclinical data and safety assessments. The sourcing and standardization of plant-based extracts can also present regulatory hurdles, as consistency and quality control are essential for regulatory compliance.
Many countries have implemented specific regulations for traditional and herbal medicines, which may impact the development and marketing of muscimol-containing products. For instance, Canada's Natural Health Products Regulations provide a framework for the approval and sale of natural health products, which could potentially include certain muscimol extracts.
The regulatory landscape for psychedelic compounds is evolving, with some jurisdictions exploring more permissive approaches. Oregon's Measure 109, for example, creates a framework for the therapeutic use of psilocybin, which could set precedents for the regulation of other psychoactive compounds like muscimol.
As research on plant-based muscimol extracts progresses, regulatory bodies will likely need to adapt their frameworks to address the unique challenges posed by these compounds. This may include developing new guidelines for quality control, standardization of plant-based extracts, and assessment of their safety and efficacy profiles.
Safety and Ethical Considerations in Muscimol Research
The research on plant-based muscimol extracts and their effects necessitates careful consideration of safety and ethical aspects. Muscimol, a potent psychoactive compound found in certain mushroom species, particularly Amanita muscaria, poses significant risks if not handled and studied with utmost caution. The primary safety concern revolves around its potential for toxicity and psychoactive effects, which can lead to severe health consequences if misused or improperly administered.
Researchers must implement stringent laboratory protocols to ensure the safe handling, storage, and disposal of muscimol extracts. This includes the use of appropriate personal protective equipment, controlled access to research facilities, and proper containment measures to prevent accidental exposure or environmental contamination. Additionally, the development of standardized extraction and purification methods is crucial to maintain consistent potency and minimize the risk of contamination with other potentially harmful compounds present in the source plants.
From an ethical standpoint, the use of muscimol in human studies raises complex issues. The psychoactive nature of the compound necessitates rigorous informed consent procedures, ensuring that participants fully understand the potential risks and effects of muscimol exposure. Ethical review boards must carefully scrutinize research protocols to balance the potential scientific benefits against the risks to human subjects. Long-term follow-up studies may be necessary to monitor for any delayed or chronic effects of muscimol exposure.
The potential for abuse and misuse of muscimol-containing products also presents significant ethical challenges. Researchers must consider the implications of their work on public health and safety, particularly in light of the growing interest in psychoactive substances for recreational use. Clear guidelines and regulations regarding the production, distribution, and use of muscimol-derived products are essential to prevent misuse and protect vulnerable populations.
Environmental and ecological considerations are equally important in muscimol research. The harvesting of wild mushrooms for muscimol extraction could potentially impact local ecosystems and biodiversity. Sustainable sourcing practices and cultivation methods should be developed to minimize environmental impact and ensure the long-term viability of research in this field.
Lastly, the cultural and traditional uses of Amanita muscaria in certain indigenous communities must be respected. Researchers should engage in dialogue with these communities and consider the potential impact of their work on traditional practices and beliefs. This approach not only ensures ethical research conduct but may also provide valuable insights into the historical use and effects of muscimol-containing plants.
Researchers must implement stringent laboratory protocols to ensure the safe handling, storage, and disposal of muscimol extracts. This includes the use of appropriate personal protective equipment, controlled access to research facilities, and proper containment measures to prevent accidental exposure or environmental contamination. Additionally, the development of standardized extraction and purification methods is crucial to maintain consistent potency and minimize the risk of contamination with other potentially harmful compounds present in the source plants.
From an ethical standpoint, the use of muscimol in human studies raises complex issues. The psychoactive nature of the compound necessitates rigorous informed consent procedures, ensuring that participants fully understand the potential risks and effects of muscimol exposure. Ethical review boards must carefully scrutinize research protocols to balance the potential scientific benefits against the risks to human subjects. Long-term follow-up studies may be necessary to monitor for any delayed or chronic effects of muscimol exposure.
The potential for abuse and misuse of muscimol-containing products also presents significant ethical challenges. Researchers must consider the implications of their work on public health and safety, particularly in light of the growing interest in psychoactive substances for recreational use. Clear guidelines and regulations regarding the production, distribution, and use of muscimol-derived products are essential to prevent misuse and protect vulnerable populations.
Environmental and ecological considerations are equally important in muscimol research. The harvesting of wild mushrooms for muscimol extraction could potentially impact local ecosystems and biodiversity. Sustainable sourcing practices and cultivation methods should be developed to minimize environmental impact and ensure the long-term viability of research in this field.
Lastly, the cultural and traditional uses of Amanita muscaria in certain indigenous communities must be respected. Researchers should engage in dialogue with these communities and consider the potential impact of their work on traditional practices and beliefs. This approach not only ensures ethical research conduct but may also provide valuable insights into the historical use and effects of muscimol-containing plants.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!