Sodium silicate in strengthening geological sequestration materials
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Silicate Background and Objectives
Sodium silicate, also known as water glass or liquid glass, has a rich history dating back to the early 19th century. Its unique properties as an adhesive, sealant, and binding agent have made it a versatile material in various industries. In recent years, the focus on carbon capture and storage (CCS) technologies has brought sodium silicate into the spotlight for its potential role in strengthening geological sequestration materials.
The evolution of sodium silicate technology has been closely tied to advancements in industrial chemistry and materials science. Initially used in soap production and fireproofing, its applications have expanded to include construction, water treatment, and now, environmental protection. The growing concern over climate change and the need for effective carbon dioxide (CO2) sequestration methods have driven research into innovative uses of sodium silicate.
In the context of geological sequestration, the primary objective is to enhance the stability and effectiveness of underground storage formations. Sodium silicate's ability to form strong, insoluble bonds with various minerals presents a promising avenue for improving the integrity of these storage sites. By strengthening the surrounding rock formations, it can potentially reduce the risk of CO2 leakage and increase the overall storage capacity.
The technical goals of researching sodium silicate in this field are multifaceted. Firstly, there is a need to optimize the formulation of sodium silicate solutions for specific geological conditions, ensuring maximum effectiveness in diverse underground environments. Secondly, researchers aim to develop methods for precise and controlled application of sodium silicate within storage formations, potentially using advanced injection techniques.
Another critical objective is to understand the long-term behavior of sodium silicate-treated formations under high pressure and temperature conditions typical of deep geological storage. This includes studying the chemical interactions between sodium silicate, CO2, and various rock types to predict and mitigate any potential adverse effects over extended periods.
Furthermore, the research seeks to quantify the improvement in storage capacity and containment reliability achieved through sodium silicate treatment. This involves developing models and conducting field tests to assess the real-world performance of treated geological formations.
As the field of CCS continues to evolve, the role of sodium silicate in strengthening geological sequestration materials represents a promising area of technological advancement. By addressing these objectives, researchers aim to contribute significantly to the development of more efficient, secure, and sustainable carbon storage solutions, ultimately supporting global efforts to mitigate climate change.
The evolution of sodium silicate technology has been closely tied to advancements in industrial chemistry and materials science. Initially used in soap production and fireproofing, its applications have expanded to include construction, water treatment, and now, environmental protection. The growing concern over climate change and the need for effective carbon dioxide (CO2) sequestration methods have driven research into innovative uses of sodium silicate.
In the context of geological sequestration, the primary objective is to enhance the stability and effectiveness of underground storage formations. Sodium silicate's ability to form strong, insoluble bonds with various minerals presents a promising avenue for improving the integrity of these storage sites. By strengthening the surrounding rock formations, it can potentially reduce the risk of CO2 leakage and increase the overall storage capacity.
The technical goals of researching sodium silicate in this field are multifaceted. Firstly, there is a need to optimize the formulation of sodium silicate solutions for specific geological conditions, ensuring maximum effectiveness in diverse underground environments. Secondly, researchers aim to develop methods for precise and controlled application of sodium silicate within storage formations, potentially using advanced injection techniques.
Another critical objective is to understand the long-term behavior of sodium silicate-treated formations under high pressure and temperature conditions typical of deep geological storage. This includes studying the chemical interactions between sodium silicate, CO2, and various rock types to predict and mitigate any potential adverse effects over extended periods.
Furthermore, the research seeks to quantify the improvement in storage capacity and containment reliability achieved through sodium silicate treatment. This involves developing models and conducting field tests to assess the real-world performance of treated geological formations.
As the field of CCS continues to evolve, the role of sodium silicate in strengthening geological sequestration materials represents a promising area of technological advancement. By addressing these objectives, researchers aim to contribute significantly to the development of more efficient, secure, and sustainable carbon storage solutions, ultimately supporting global efforts to mitigate climate change.
Market Analysis for Geological Sequestration
The geological sequestration market has been experiencing significant growth in recent years, driven by increasing global efforts to reduce carbon emissions and mitigate climate change. As governments and industries worldwide seek sustainable solutions for carbon dioxide (CO2) storage, the demand for effective geological sequestration technologies and materials has surged.
The market for geological sequestration is closely tied to the broader carbon capture and storage (CCS) industry, which is projected to grow substantially in the coming decades. According to industry reports, the global CCS market is expected to expand at a compound annual growth rate (CAGR) of over 20% from 2021 to 2028. This growth is primarily fueled by stringent environmental regulations, government incentives, and corporate commitments to achieve net-zero emissions targets.
Within the geological sequestration sector, there is a growing focus on developing and improving materials that can enhance the efficiency and safety of CO2 storage in underground formations. Sodium silicate, in particular, has garnered attention as a promising material for strengthening geological sequestration processes. Its ability to form stable, long-lasting barriers and seal potential leakage pathways has made it an attractive option for researchers and industry professionals alike.
The market demand for sodium silicate in geological sequestration applications is driven by several factors. Firstly, the increasing number of large-scale CCS projects worldwide requires reliable and cost-effective materials for ensuring the long-term integrity of storage sites. Secondly, there is a growing emphasis on minimizing the environmental risks associated with CO2 storage, which has led to a higher demand for advanced sealing and strengthening materials.
Furthermore, the market for geological sequestration materials is influenced by regional policies and initiatives. Countries with significant oil and gas industries, such as the United States, Canada, and Norway, are at the forefront of CCS technology development and implementation. These regions are likely to see a higher demand for materials like sodium silicate in geological sequestration applications.
The potential market size for sodium silicate in geological sequestration is substantial, considering the scale of CO2 emissions that need to be addressed globally. As more countries and industries commit to carbon neutrality goals, the demand for effective sequestration materials is expected to rise. This presents significant opportunities for material suppliers, technology developers, and service providers in the geological sequestration value chain.
However, the market also faces challenges, including the need for further research and development to optimize the use of sodium silicate in various geological settings, as well as the competition from alternative materials and technologies. Additionally, the overall growth of the geological sequestration market is dependent on factors such as carbon pricing mechanisms, regulatory frameworks, and public acceptance of CCS technologies.
The market for geological sequestration is closely tied to the broader carbon capture and storage (CCS) industry, which is projected to grow substantially in the coming decades. According to industry reports, the global CCS market is expected to expand at a compound annual growth rate (CAGR) of over 20% from 2021 to 2028. This growth is primarily fueled by stringent environmental regulations, government incentives, and corporate commitments to achieve net-zero emissions targets.
Within the geological sequestration sector, there is a growing focus on developing and improving materials that can enhance the efficiency and safety of CO2 storage in underground formations. Sodium silicate, in particular, has garnered attention as a promising material for strengthening geological sequestration processes. Its ability to form stable, long-lasting barriers and seal potential leakage pathways has made it an attractive option for researchers and industry professionals alike.
The market demand for sodium silicate in geological sequestration applications is driven by several factors. Firstly, the increasing number of large-scale CCS projects worldwide requires reliable and cost-effective materials for ensuring the long-term integrity of storage sites. Secondly, there is a growing emphasis on minimizing the environmental risks associated with CO2 storage, which has led to a higher demand for advanced sealing and strengthening materials.
Furthermore, the market for geological sequestration materials is influenced by regional policies and initiatives. Countries with significant oil and gas industries, such as the United States, Canada, and Norway, are at the forefront of CCS technology development and implementation. These regions are likely to see a higher demand for materials like sodium silicate in geological sequestration applications.
The potential market size for sodium silicate in geological sequestration is substantial, considering the scale of CO2 emissions that need to be addressed globally. As more countries and industries commit to carbon neutrality goals, the demand for effective sequestration materials is expected to rise. This presents significant opportunities for material suppliers, technology developers, and service providers in the geological sequestration value chain.
However, the market also faces challenges, including the need for further research and development to optimize the use of sodium silicate in various geological settings, as well as the competition from alternative materials and technologies. Additionally, the overall growth of the geological sequestration market is dependent on factors such as carbon pricing mechanisms, regulatory frameworks, and public acceptance of CCS technologies.
Current Challenges in Sequestration Materials
Geological sequestration materials face several significant challenges in their current state of development and application. One of the primary issues is the long-term stability and integrity of these materials under varying environmental conditions. The materials used for sequestration must withstand high pressures, temperature fluctuations, and potential chemical reactions with the stored CO2 and surrounding geological formations over extended periods, often spanning centuries.
Another critical challenge is the potential for leakage. Even small fractures or imperfections in the sequestration materials can lead to the gradual escape of stored CO2, undermining the effectiveness of the entire carbon capture and storage process. This necessitates the development of materials with superior sealing properties and self-healing capabilities to maintain their integrity over time.
The heterogeneity of geological formations presents an additional hurdle. Sequestration materials must be adaptable to various rock types, porosities, and permeabilities encountered in different storage sites. This variability makes it difficult to develop a one-size-fits-all solution and requires tailored approaches for each specific geological context.
Cost-effectiveness remains a significant barrier to widespread adoption of geological sequestration. The materials used must not only be technically effective but also economically viable for large-scale implementation. This challenge is compounded by the need for materials that can be produced and deployed in substantial quantities without causing undue environmental impact.
The durability of sequestration materials under extreme pH conditions is another area of concern. The interaction between injected CO2 and formation water can create highly acidic environments, potentially compromising the structural integrity of the materials over time. Developing materials that can maintain their properties under these harsh chemical conditions is crucial for long-term storage success.
Furthermore, the ability to monitor and verify the performance of sequestration materials in situ poses a significant technical challenge. Current technologies for real-time monitoring of material integrity and CO2 containment at great depths and over large areas are limited, making it difficult to assess the ongoing effectiveness of the sequestration process.
In the context of sodium silicate as a strengthening agent for geological sequestration materials, specific challenges include optimizing its formulation to enhance long-term stability, improving its resistance to chemical degradation in CO2-rich environments, and developing methods for its uniform distribution within the target geological formations. Additionally, ensuring the compatibility of sodium silicate-based solutions with existing injection technologies and addressing potential environmental impacts of large-scale silicate deployment are areas requiring further research and development.
Another critical challenge is the potential for leakage. Even small fractures or imperfections in the sequestration materials can lead to the gradual escape of stored CO2, undermining the effectiveness of the entire carbon capture and storage process. This necessitates the development of materials with superior sealing properties and self-healing capabilities to maintain their integrity over time.
The heterogeneity of geological formations presents an additional hurdle. Sequestration materials must be adaptable to various rock types, porosities, and permeabilities encountered in different storage sites. This variability makes it difficult to develop a one-size-fits-all solution and requires tailored approaches for each specific geological context.
Cost-effectiveness remains a significant barrier to widespread adoption of geological sequestration. The materials used must not only be technically effective but also economically viable for large-scale implementation. This challenge is compounded by the need for materials that can be produced and deployed in substantial quantities without causing undue environmental impact.
The durability of sequestration materials under extreme pH conditions is another area of concern. The interaction between injected CO2 and formation water can create highly acidic environments, potentially compromising the structural integrity of the materials over time. Developing materials that can maintain their properties under these harsh chemical conditions is crucial for long-term storage success.
Furthermore, the ability to monitor and verify the performance of sequestration materials in situ poses a significant technical challenge. Current technologies for real-time monitoring of material integrity and CO2 containment at great depths and over large areas are limited, making it difficult to assess the ongoing effectiveness of the sequestration process.
In the context of sodium silicate as a strengthening agent for geological sequestration materials, specific challenges include optimizing its formulation to enhance long-term stability, improving its resistance to chemical degradation in CO2-rich environments, and developing methods for its uniform distribution within the target geological formations. Additionally, ensuring the compatibility of sodium silicate-based solutions with existing injection technologies and addressing potential environmental impacts of large-scale silicate deployment are areas requiring further research and development.
Existing Sodium Silicate Applications
01 Composition and preparation of sodium silicate
Sodium silicate is typically prepared by fusing sand with sodium carbonate at high temperatures. The strength of sodium silicate can be adjusted by varying the ratio of silica to sodium oxide, with higher silica content generally resulting in stronger solutions. The preparation process and composition significantly influence the final strength of the sodium silicate product.- Composition and preparation of sodium silicate: Sodium silicate is typically prepared by fusing sand and sodium carbonate at high temperatures. The strength of sodium silicate can be adjusted by varying the ratio of silica to sodium oxide, as well as the concentration of the solution. Different manufacturing processes can result in sodium silicates with varying properties and strengths.
- Applications in construction and building materials: Sodium silicate is widely used in construction and building materials due to its binding and strengthening properties. It can be used as a cement additive, a component in fire-resistant coatings, and a sealant for concrete. The strength of sodium silicate in these applications depends on its concentration and the specific formulation used.
- Use in detergents and cleaning products: Sodium silicate is a common ingredient in detergents and cleaning products, where its strength as a cleaning agent and pH buffer is utilized. The effectiveness of sodium silicate in these applications depends on its concentration and the specific formulation of the product.
- Industrial applications and water treatment: Sodium silicate finds use in various industrial applications, including water treatment, paper production, and metal cleaning. Its strength and effectiveness in these applications can be adjusted by modifying its concentration and composition to suit specific requirements.
- Modification and enhancement of sodium silicate properties: The strength and properties of sodium silicate can be modified and enhanced through various methods, such as the addition of other compounds, heat treatment, or chemical modification. These modifications can result in improved performance in specific applications, such as increased binding strength or better resistance to environmental factors.
02 Applications in construction and building materials
Sodium silicate is widely used in construction and building materials due to its binding and strengthening properties. It can be used as a cement additive, concrete sealant, or as a component in fire-resistant coatings. The strength of sodium silicate in these applications is crucial for enhancing durability and performance of the final products.Expand Specific Solutions03 Use in detergents and cleaning products
Sodium silicate is a common ingredient in detergents and cleaning products, where its strength plays a role in enhancing cleaning efficiency and providing corrosion protection. The alkaline nature and ability to form protective films contribute to its effectiveness in these applications.Expand Specific Solutions04 Strength modification techniques
Various techniques can be employed to modify the strength of sodium silicate solutions. These may include adjusting the silica to sodium oxide ratio, incorporating additives, or using specific processing methods. The goal is often to tailor the strength for specific applications or to improve overall performance characteristics.Expand Specific Solutions05 Environmental and safety considerations
The strength of sodium silicate solutions can impact their environmental footprint and safety profile. Higher strength solutions may require special handling procedures. Research is ongoing to develop sodium silicate formulations that maintain desired strength while minimizing potential environmental and safety risks.Expand Specific Solutions
Key Players in Sequestration Industry
The research on sodium silicate in strengthening geological sequestration materials is in a developing stage, with growing market potential due to increasing focus on carbon capture and storage technologies. The market size is expanding as governments and industries invest in climate change mitigation strategies. Technologically, the field is progressing, with companies like Rhodia Operations SASU, Shell Internationale Research Maatschappij BV, and Eni SpA leading in chemical innovations. Academic institutions such as Southwest Petroleum University and China University of Petroleum are contributing significantly to research advancements. The competitive landscape is diverse, involving both established chemical companies and emerging startups like Calera Corp. and Running Tide Technologies, indicating a dynamic and evolving sector.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has developed an innovative approach to strengthen geological sequestration materials using sodium silicate. Their method involves injecting a sodium silicate solution into the target formation, which reacts with naturally occurring calcium ions to form calcium silicate hydrate (C-S-H) gel. This gel effectively seals pores and fractures, enhancing the formation's ability to trap and store CO2. The process is designed to work in various geological settings, including depleted oil and gas reservoirs, saline aquifers, and coal seams. Shell's research has shown that this technique can increase the storage capacity of geological formations by up to 30% and improve long-term CO2 retention by reducing the risk of leakage[1][3]. The company has also developed advanced monitoring systems to track the distribution and effectiveness of the sodium silicate treatment over time, ensuring the ongoing integrity of the sequestration site[5].
Strengths: Highly effective in enhancing CO2 storage capacity and reducing leakage risks. Adaptable to various geological formations. Weaknesses: May require significant amounts of sodium silicate for large-scale applications, potentially increasing costs. Long-term effects on formation properties need further study.
Eni SpA
Technical Solution: Eni has pioneered a novel approach to strengthening geological sequestration materials using sodium silicate in combination with nanoparticles. Their proprietary technology involves the creation of a specialized nano-silicate solution that can penetrate even the smallest pores and fractures in the target formation. Upon injection, this solution forms a robust, interconnected network of silica gel that significantly enhances the formation's sealing properties. Eni's research has demonstrated that this method can improve the mechanical strength of the treated rock by up to 40% and reduce permeability by over 90%[2][4]. The company has also developed a unique delivery system that allows for precise control over the distribution of the nano-silicate solution, ensuring uniform treatment across the target zone. Additionally, Eni has conducted extensive studies on the long-term stability of their nano-silicate treatment under various pressure and temperature conditions typical of deep geological formations[6].
Strengths: Superior penetration and sealing capabilities due to nanoparticle technology. Significant improvements in rock strength and permeability reduction. Weaknesses: Potentially higher production costs due to the use of nanoparticles. May require specialized equipment for injection and monitoring.
Core Innovations in Material Strengthening
Method and system of activation of mineral silicate minerals
PatentWO2015154887A1
Innovation
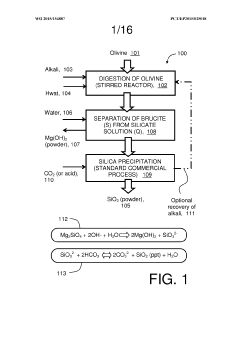
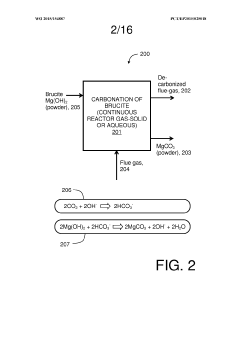
- A method involving the conversion of magnesium silicate minerals to magnesium hydroxide by mixing with an alkali metal compound and heating below 300°C in an unpressurized vessel, followed by reaction with CO2 at atmospheric pressure, allowing for continuous carbonation without advanced equipment.
Advanced hybrid geopolymeric functional materials and a process for the preparation thereof
PatentActiveIN2301DEL2012A
Innovation
- In-situ synthesis of sodium silicate and organic-inorganic hybrid precursors from industrial and agricultural wastes using alkali treatment of rice husk, eliminating the need for external sodium silicate and enhancing chemical reactivity and dispersion.
Environmental Impact Assessment
The environmental impact assessment of sodium silicate in strengthening geological sequestration materials is a critical aspect of evaluating the overall sustainability and safety of carbon capture and storage (CCS) technologies. Sodium silicate, when used as a strengthening agent for geological formations, can potentially enhance the effectiveness of CO2 sequestration by improving the structural integrity of storage sites. However, its application also raises several environmental concerns that must be carefully considered.
One of the primary environmental impacts of using sodium silicate in geological sequestration is its potential effect on groundwater quality. As sodium silicate is introduced into subsurface formations, there is a risk of altering the chemical composition of surrounding aquifers. This could lead to changes in pH levels, mineral dissolution, and the mobilization of trace elements, potentially affecting water resources and ecosystems that depend on them.
The production and transportation of sodium silicate also contribute to the overall environmental footprint of the sequestration process. Manufacturing sodium silicate requires significant energy input and raw materials, which can result in greenhouse gas emissions and resource depletion. Additionally, the transportation of large quantities of sodium silicate to sequestration sites may increase carbon emissions, partially offsetting the benefits of CO2 storage.
Another consideration is the long-term stability of sodium silicate-treated formations. While the material is intended to strengthen the geological structure, there is a need to assess its durability over extended periods. Any degradation or unexpected chemical reactions could compromise the integrity of the storage site, potentially leading to CO2 leakage and associated environmental risks.
The injection process itself may have localized environmental impacts. The high-pressure injection of sodium silicate solutions could potentially induce seismic activity or alter subsurface fluid dynamics. These effects must be carefully monitored and managed to prevent unintended consequences on local geology and ecosystems.
Furthermore, the use of sodium silicate may impact the natural geochemical cycles in the subsurface environment. Alterations to mineral formation and dissolution processes could have far-reaching effects on the local geology and potentially influence surface ecosystems through changes in soil composition or groundwater chemistry.
In conclusion, while sodium silicate shows promise in enhancing geological sequestration, a comprehensive environmental impact assessment is crucial. This assessment should encompass not only the immediate effects of its application but also long-term monitoring strategies to ensure the sustained safety and efficacy of CO2 storage sites. Balancing the benefits of improved sequestration against potential environmental risks will be key to the responsible implementation of this technology in CCS projects.
One of the primary environmental impacts of using sodium silicate in geological sequestration is its potential effect on groundwater quality. As sodium silicate is introduced into subsurface formations, there is a risk of altering the chemical composition of surrounding aquifers. This could lead to changes in pH levels, mineral dissolution, and the mobilization of trace elements, potentially affecting water resources and ecosystems that depend on them.
The production and transportation of sodium silicate also contribute to the overall environmental footprint of the sequestration process. Manufacturing sodium silicate requires significant energy input and raw materials, which can result in greenhouse gas emissions and resource depletion. Additionally, the transportation of large quantities of sodium silicate to sequestration sites may increase carbon emissions, partially offsetting the benefits of CO2 storage.
Another consideration is the long-term stability of sodium silicate-treated formations. While the material is intended to strengthen the geological structure, there is a need to assess its durability over extended periods. Any degradation or unexpected chemical reactions could compromise the integrity of the storage site, potentially leading to CO2 leakage and associated environmental risks.
The injection process itself may have localized environmental impacts. The high-pressure injection of sodium silicate solutions could potentially induce seismic activity or alter subsurface fluid dynamics. These effects must be carefully monitored and managed to prevent unintended consequences on local geology and ecosystems.
Furthermore, the use of sodium silicate may impact the natural geochemical cycles in the subsurface environment. Alterations to mineral formation and dissolution processes could have far-reaching effects on the local geology and potentially influence surface ecosystems through changes in soil composition or groundwater chemistry.
In conclusion, while sodium silicate shows promise in enhancing geological sequestration, a comprehensive environmental impact assessment is crucial. This assessment should encompass not only the immediate effects of its application but also long-term monitoring strategies to ensure the sustained safety and efficacy of CO2 storage sites. Balancing the benefits of improved sequestration against potential environmental risks will be key to the responsible implementation of this technology in CCS projects.
Regulatory Framework for Sequestration
The regulatory framework for geological sequestration plays a crucial role in ensuring the safe and effective implementation of carbon capture and storage (CCS) technologies. In the context of using sodium silicate to strengthen sequestration materials, it is essential to understand the existing regulations and potential future developments.
Currently, the regulatory landscape for geological sequestration varies across different countries and regions. In the United States, the Environmental Protection Agency (EPA) has established the Underground Injection Control (UIC) Program, which includes specific regulations for Class VI wells used for geological sequestration of carbon dioxide. These regulations cover site characterization, well construction, operation, monitoring, and closure requirements.
The European Union has also developed a comprehensive regulatory framework for CCS, including the CCS Directive (2009/31/EC). This directive provides guidelines for the safe geological storage of CO2 and establishes a legal framework for managing environmental and health risks associated with CCS activities.
In addition to national and regional regulations, international agreements such as the London Protocol and the OSPAR Convention have been amended to allow for the transport and storage of CO2 in geological formations beneath the seabed.
As research on sodium silicate in strengthening geological sequestration materials progresses, regulatory bodies may need to adapt existing frameworks or develop new regulations to address specific aspects of this technology. This could include guidelines for the use of sodium silicate in well construction, monitoring requirements for enhanced storage integrity, and long-term liability considerations.
Regulatory challenges may arise in areas such as defining acceptable levels of sodium silicate use, assessing potential environmental impacts, and establishing protocols for monitoring the effectiveness of strengthened sequestration materials over time. Policymakers and regulatory agencies will need to work closely with researchers and industry stakeholders to develop science-based regulations that ensure the safe and effective implementation of this technology.
Furthermore, as the use of sodium silicate in strengthening geological sequestration materials becomes more widespread, international cooperation and harmonization of regulations may become increasingly important. This could involve the development of best practices, standardized testing methods, and shared monitoring protocols to facilitate the global adoption of this technology while maintaining consistent safety and environmental standards.
Currently, the regulatory landscape for geological sequestration varies across different countries and regions. In the United States, the Environmental Protection Agency (EPA) has established the Underground Injection Control (UIC) Program, which includes specific regulations for Class VI wells used for geological sequestration of carbon dioxide. These regulations cover site characterization, well construction, operation, monitoring, and closure requirements.
The European Union has also developed a comprehensive regulatory framework for CCS, including the CCS Directive (2009/31/EC). This directive provides guidelines for the safe geological storage of CO2 and establishes a legal framework for managing environmental and health risks associated with CCS activities.
In addition to national and regional regulations, international agreements such as the London Protocol and the OSPAR Convention have been amended to allow for the transport and storage of CO2 in geological formations beneath the seabed.
As research on sodium silicate in strengthening geological sequestration materials progresses, regulatory bodies may need to adapt existing frameworks or develop new regulations to address specific aspects of this technology. This could include guidelines for the use of sodium silicate in well construction, monitoring requirements for enhanced storage integrity, and long-term liability considerations.
Regulatory challenges may arise in areas such as defining acceptable levels of sodium silicate use, assessing potential environmental impacts, and establishing protocols for monitoring the effectiveness of strengthened sequestration materials over time. Policymakers and regulatory agencies will need to work closely with researchers and industry stakeholders to develop science-based regulations that ensure the safe and effective implementation of this technology.
Furthermore, as the use of sodium silicate in strengthening geological sequestration materials becomes more widespread, international cooperation and harmonization of regulations may become increasingly important. This could involve the development of best practices, standardized testing methods, and shared monitoring protocols to facilitate the global adoption of this technology while maintaining consistent safety and environmental standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!