Sodium silicate solubility in carbon sequestration systems
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Sequestration Background and Objectives
Carbon sequestration has emerged as a critical technology in the global effort to mitigate climate change by reducing atmospheric carbon dioxide levels. This process involves capturing CO2 from industrial sources or directly from the air and storing it in geological formations, such as deep saline aquifers or depleted oil and gas reservoirs. The primary objective of carbon sequestration is to prevent large quantities of CO2 from entering the atmosphere, thereby slowing the rate of global warming and its associated environmental impacts.
The concept of carbon sequestration has evolved significantly since its inception in the late 20th century. Initially proposed as a method to enhance oil recovery, it has now become a standalone technology aimed at long-term CO2 storage. The Intergovernmental Panel on Climate Change (IPCC) has identified carbon capture and storage as a crucial component in achieving global climate targets, particularly in scenarios where rapid decarbonization is required.
In recent years, research has focused on improving the efficiency and safety of carbon sequestration systems. One key area of investigation is the interaction between injected CO2 and the geological formations in which it is stored. This includes studying the chemical reactions that occur when CO2 dissolves in formation waters and interacts with rock minerals. Understanding these processes is crucial for predicting the long-term behavior of stored CO2 and ensuring its permanent sequestration.
The solubility of various minerals, including sodium silicate, plays a significant role in these geochemical interactions. Sodium silicate, also known as water glass, is a compound that can form naturally in carbon sequestration systems or be introduced as part of engineered solutions. Its solubility affects the porosity and permeability of the storage formation, which in turn influences the capacity and security of CO2 storage.
Research on sodium silicate solubility in carbon sequestration systems aims to address several critical objectives. Firstly, it seeks to understand how changes in temperature, pressure, and pH - conditions that vary significantly in CO2 storage environments - affect the dissolution and precipitation of sodium silicate. Secondly, it aims to predict how these solubility dynamics might impact the long-term integrity of the storage formation, including potential changes in porosity and the creation of preferential flow paths for CO2.
Furthermore, this research is essential for developing accurate models of CO2 behavior in storage reservoirs. By incorporating detailed knowledge of mineral solubility, including that of sodium silicate, these models can more accurately predict the fate of injected CO2 over extended time periods. This is crucial for risk assessment, regulatory compliance, and public acceptance of carbon sequestration projects.
The concept of carbon sequestration has evolved significantly since its inception in the late 20th century. Initially proposed as a method to enhance oil recovery, it has now become a standalone technology aimed at long-term CO2 storage. The Intergovernmental Panel on Climate Change (IPCC) has identified carbon capture and storage as a crucial component in achieving global climate targets, particularly in scenarios where rapid decarbonization is required.
In recent years, research has focused on improving the efficiency and safety of carbon sequestration systems. One key area of investigation is the interaction between injected CO2 and the geological formations in which it is stored. This includes studying the chemical reactions that occur when CO2 dissolves in formation waters and interacts with rock minerals. Understanding these processes is crucial for predicting the long-term behavior of stored CO2 and ensuring its permanent sequestration.
The solubility of various minerals, including sodium silicate, plays a significant role in these geochemical interactions. Sodium silicate, also known as water glass, is a compound that can form naturally in carbon sequestration systems or be introduced as part of engineered solutions. Its solubility affects the porosity and permeability of the storage formation, which in turn influences the capacity and security of CO2 storage.
Research on sodium silicate solubility in carbon sequestration systems aims to address several critical objectives. Firstly, it seeks to understand how changes in temperature, pressure, and pH - conditions that vary significantly in CO2 storage environments - affect the dissolution and precipitation of sodium silicate. Secondly, it aims to predict how these solubility dynamics might impact the long-term integrity of the storage formation, including potential changes in porosity and the creation of preferential flow paths for CO2.
Furthermore, this research is essential for developing accurate models of CO2 behavior in storage reservoirs. By incorporating detailed knowledge of mineral solubility, including that of sodium silicate, these models can more accurately predict the fate of injected CO2 over extended time periods. This is crucial for risk assessment, regulatory compliance, and public acceptance of carbon sequestration projects.
Market Analysis for Carbon Capture Technologies
The carbon capture and storage (CCS) market has been experiencing significant growth in recent years, driven by increasing global efforts to mitigate climate change and reduce greenhouse gas emissions. The market for carbon capture technologies is expected to expand rapidly as countries and industries strive to meet their carbon reduction targets and comply with increasingly stringent environmental regulations.
The global CCS market size was valued at approximately $2 billion in 2020 and is projected to reach $7 billion by 2028, growing at a CAGR of over 13% during the forecast period. This growth is primarily attributed to the rising adoption of CCS technologies across various industries, including power generation, oil and gas, cement, and chemical manufacturing.
In the context of sodium silicate solubility in carbon sequestration systems, there is a growing interest in developing more efficient and cost-effective carbon capture solutions. Sodium silicate, also known as water glass, has shown potential in enhancing the efficiency of carbon capture processes, particularly in mineral carbonation techniques.
The demand for sodium silicate-based carbon capture technologies is expected to increase as research continues to demonstrate its effectiveness in improving CO2 absorption rates and reducing overall capture costs. This trend aligns with the broader market demand for innovative and sustainable carbon capture solutions that can be implemented at scale.
Key market drivers for carbon capture technologies, including those involving sodium silicate, include:
1. Stringent government regulations and policies aimed at reducing carbon emissions
2. Increasing corporate commitments to achieve net-zero emissions
3. Growing investments in clean energy and sustainable technologies
4. Rising public awareness and concern about climate change
However, the market also faces challenges, such as high initial capital costs, technological limitations, and the need for extensive infrastructure development. These factors may impact the adoption rate of carbon capture technologies, including those utilizing sodium silicate solutions.
Despite these challenges, the market outlook for carbon capture technologies remains positive, with significant opportunities for growth and innovation. As research on sodium silicate solubility in carbon sequestration systems progresses, it is likely to contribute to the development of more efficient and cost-effective carbon capture solutions, further driving market expansion and technological advancements in the CCS industry.
The global CCS market size was valued at approximately $2 billion in 2020 and is projected to reach $7 billion by 2028, growing at a CAGR of over 13% during the forecast period. This growth is primarily attributed to the rising adoption of CCS technologies across various industries, including power generation, oil and gas, cement, and chemical manufacturing.
In the context of sodium silicate solubility in carbon sequestration systems, there is a growing interest in developing more efficient and cost-effective carbon capture solutions. Sodium silicate, also known as water glass, has shown potential in enhancing the efficiency of carbon capture processes, particularly in mineral carbonation techniques.
The demand for sodium silicate-based carbon capture technologies is expected to increase as research continues to demonstrate its effectiveness in improving CO2 absorption rates and reducing overall capture costs. This trend aligns with the broader market demand for innovative and sustainable carbon capture solutions that can be implemented at scale.
Key market drivers for carbon capture technologies, including those involving sodium silicate, include:
1. Stringent government regulations and policies aimed at reducing carbon emissions
2. Increasing corporate commitments to achieve net-zero emissions
3. Growing investments in clean energy and sustainable technologies
4. Rising public awareness and concern about climate change
However, the market also faces challenges, such as high initial capital costs, technological limitations, and the need for extensive infrastructure development. These factors may impact the adoption rate of carbon capture technologies, including those utilizing sodium silicate solutions.
Despite these challenges, the market outlook for carbon capture technologies remains positive, with significant opportunities for growth and innovation. As research on sodium silicate solubility in carbon sequestration systems progresses, it is likely to contribute to the development of more efficient and cost-effective carbon capture solutions, further driving market expansion and technological advancements in the CCS industry.
Sodium Silicate Solubility Challenges
The solubility of sodium silicate in carbon sequestration systems presents several significant challenges that researchers and engineers must address. One of the primary issues is the complex interaction between sodium silicate and carbon dioxide (CO2) under varying pressure and temperature conditions. As CO2 is injected into geological formations for sequestration, it can alter the pH of the surrounding environment, which in turn affects the solubility and stability of sodium silicate.
The presence of other ions and minerals in the geological formation further complicates the solubility behavior of sodium silicate. These interactions can lead to the formation of secondary minerals or precipitates, potentially reducing the efficiency of carbon sequestration processes. Understanding and predicting these reactions is crucial for optimizing the performance of carbon capture and storage (CCS) systems.
Another challenge lies in the dynamic nature of the subsurface environment. As CO2 is continuously injected, the chemical equilibrium of the system is constantly shifting, making it difficult to maintain consistent sodium silicate solubility. This variability can impact the long-term stability and effectiveness of the sequestration process, potentially leading to reduced storage capacity or increased risk of CO2 leakage.
The high alkalinity of sodium silicate solutions poses additional challenges in carbon sequestration systems. While this alkalinity can be beneficial for CO2 absorption, it may also lead to unwanted side reactions or accelerated corrosion of equipment and infrastructure. Balancing the alkalinity to maximize CO2 uptake while minimizing negative impacts is a delicate and ongoing challenge.
Scale formation is another significant issue related to sodium silicate solubility in CCS systems. As the solubility limits are approached or exceeded, silica-based scales can form, potentially clogging pores in the geological formation or fouling injection equipment. This scaling can reduce the overall efficiency of the sequestration process and increase maintenance requirements and operational costs.
Accurately modeling and predicting sodium silicate solubility under the diverse conditions encountered in carbon sequestration systems remains a complex task. Current models often struggle to account for all the variables involved, including pressure, temperature, pH, and the presence of various ions and minerals. Improving these predictive models is essential for optimizing CCS system design and operation.
Lastly, the potential for sodium silicate to alter the permeability and porosity of the storage formation presents both opportunities and challenges. While controlled precipitation of silicates could potentially enhance CO2 trapping mechanisms, uncontrolled reactions could lead to reduced injectivity or compromised seal integrity. Striking the right balance to leverage the benefits while mitigating the risks requires ongoing research and careful management strategies.
The presence of other ions and minerals in the geological formation further complicates the solubility behavior of sodium silicate. These interactions can lead to the formation of secondary minerals or precipitates, potentially reducing the efficiency of carbon sequestration processes. Understanding and predicting these reactions is crucial for optimizing the performance of carbon capture and storage (CCS) systems.
Another challenge lies in the dynamic nature of the subsurface environment. As CO2 is continuously injected, the chemical equilibrium of the system is constantly shifting, making it difficult to maintain consistent sodium silicate solubility. This variability can impact the long-term stability and effectiveness of the sequestration process, potentially leading to reduced storage capacity or increased risk of CO2 leakage.
The high alkalinity of sodium silicate solutions poses additional challenges in carbon sequestration systems. While this alkalinity can be beneficial for CO2 absorption, it may also lead to unwanted side reactions or accelerated corrosion of equipment and infrastructure. Balancing the alkalinity to maximize CO2 uptake while minimizing negative impacts is a delicate and ongoing challenge.
Scale formation is another significant issue related to sodium silicate solubility in CCS systems. As the solubility limits are approached or exceeded, silica-based scales can form, potentially clogging pores in the geological formation or fouling injection equipment. This scaling can reduce the overall efficiency of the sequestration process and increase maintenance requirements and operational costs.
Accurately modeling and predicting sodium silicate solubility under the diverse conditions encountered in carbon sequestration systems remains a complex task. Current models often struggle to account for all the variables involved, including pressure, temperature, pH, and the presence of various ions and minerals. Improving these predictive models is essential for optimizing CCS system design and operation.
Lastly, the potential for sodium silicate to alter the permeability and porosity of the storage formation presents both opportunities and challenges. While controlled precipitation of silicates could potentially enhance CO2 trapping mechanisms, uncontrolled reactions could lead to reduced injectivity or compromised seal integrity. Striking the right balance to leverage the benefits while mitigating the risks requires ongoing research and careful management strategies.
Current Sodium Silicate Solubility Solutions
01 Factors affecting sodium silicate solubility
The solubility of sodium silicate is influenced by various factors, including temperature, pH, and the presence of other ions. Higher temperatures generally increase solubility, while changes in pH can significantly affect the dissolution rate. The presence of certain ions can also impact the solubility of sodium silicate in aqueous solutions.- Factors affecting sodium silicate solubility: The solubility of sodium silicate is influenced by various factors, including temperature, pH, and the presence of other ions. Higher temperatures generally increase solubility, while changes in pH can significantly affect the dissolution rate. The presence of certain ions can also impact the solubility of sodium silicate in aqueous solutions.
- Controlling sodium silicate solubility in industrial applications: In industrial processes, the solubility of sodium silicate is often controlled to achieve desired properties in products such as detergents, adhesives, and water treatment chemicals. This can be done by adjusting the silica-to-alkali ratio, modifying the temperature, or introducing additives that affect solubility.
- Sodium silicate as a solubility enhancer: Sodium silicate can be used to enhance the solubility of other compounds in various applications. Its ability to form complexes with metal ions and organic molecules makes it useful in formulations where increased solubility of certain components is desired.
- Modification of sodium silicate for controlled solubility: Chemical modifications of sodium silicate, such as partial neutralization or reaction with organic compounds, can be used to create products with tailored solubility profiles. These modified forms find applications in areas such as controlled release systems and specialty coatings.
- Measurement and characterization of sodium silicate solubility: Various analytical techniques are employed to measure and characterize the solubility of sodium silicate in different environments. These methods include spectroscopic analysis, gravimetric techniques, and advanced imaging technologies, which help in understanding the dissolution behavior and optimizing its use in different applications.
02 Controlling sodium silicate solubility in industrial applications
In industrial processes, the solubility of sodium silicate is often controlled to achieve desired properties in products such as detergents, adhesives, and water treatment chemicals. This can be done by adjusting the silica-to-alkali ratio, modifying the temperature, or introducing additives that affect solubility.Expand Specific Solutions03 Sodium silicate solubility in geopolymer applications
The solubility of sodium silicate plays a crucial role in geopolymer synthesis and curing processes. Controlling the dissolution rate of sodium silicate affects the formation of the geopolymer network structure, influencing the final mechanical and chemical properties of the material.Expand Specific Solutions04 Enhancing sodium silicate solubility for specific applications
Various methods are employed to enhance the solubility of sodium silicate for specific applications. These may include using surfactants, employing ultrasonic treatment, or combining sodium silicate with other compounds to create more soluble complexes. Enhanced solubility can improve the performance of sodium silicate in applications such as paper production and soil stabilization.Expand Specific Solutions05 Measuring and characterizing sodium silicate solubility
Accurate measurement and characterization of sodium silicate solubility are essential for quality control and product development. Various analytical techniques, including spectrophotometry, titration, and gravimetric analysis, are used to determine the solubility of sodium silicate under different conditions. Understanding these characteristics is crucial for optimizing formulations and processes in industries that utilize sodium silicate.Expand Specific Solutions
Key Players in Carbon Capture Industry
The research on sodium silicate solubility in carbon sequestration systems is in a developing stage, with growing market potential as global efforts to reduce carbon emissions intensify. The technology is advancing but not yet fully mature, as evidenced by ongoing research from major players like Shell, Solvay, and MIT. The competitive landscape is diverse, including oil and gas companies, chemical manufacturers, and academic institutions. While some companies like Cambridge Carbon Capture and Carbonfree Chemicals are focusing specifically on carbon capture technologies, others like Shell and Solvay are leveraging their broader chemical expertise. The market is expected to expand significantly as carbon pricing mechanisms become more widespread, driving innovation and investment in this field.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has developed an innovative approach to carbon sequestration focusing on the solubility of sodium silicate in carbon capture systems. Their method involves injecting CO2 into underground formations containing silicate minerals, where it reacts to form stable carbonate minerals. This process, known as mineral carbonation, enhances the dissolution of silicate minerals, increasing the overall carbon sequestration capacity. Shell's research has shown that the presence of sodium silicate can significantly improve the reaction kinetics and efficiency of the carbonation process[1][3]. They have also developed advanced modeling techniques to predict and optimize the solubility of sodium silicate under various pressure and temperature conditions typical of geological storage sites[2].
Strengths: Extensive experience in geological storage, advanced modeling capabilities, and global reach for large-scale implementation. Weaknesses: High initial costs and potential long-term monitoring requirements.
Cambridge Carbon Capture Ltd.
Technical Solution: Cambridge Carbon Capture (CCC) has pioneered a unique approach to carbon sequestration utilizing sodium silicate solubility. Their proprietary technology, known as CO2LOC, focuses on enhancing the natural process of mineral carbonation. CCC's method involves the controlled dissolution of silicate-rich materials in the presence of CO2, forming stable carbonate minerals. By optimizing the sodium silicate concentration and pH levels, they have achieved significantly faster carbonation rates compared to natural processes[4]. CCC's research has demonstrated that their technique can sequester up to 0.5 tonnes of CO2 per tonne of silicate material processed[5]. Additionally, they have developed a modular system that can be scaled and adapted to various industrial settings, making it versatile for different carbon capture applications[6].
Strengths: Highly efficient carbonation process, modular and scalable technology, potential for integration with existing industrial processes. Weaknesses: Requires a consistent supply of suitable silicate materials, which may limit application in some regions.
Core Innovations in Silicate Chemistry
Method and system of sequestrating carbon dioxide
PatentInactiveGB2515995A
Innovation
- A method involving the reaction of an alkaline earth silicate-based material with an alkali metal compound, such as sodium or potassium hydroxide, at elevated temperatures (140-220°C) and ambient pressure, to form a hydroxide that is then combined with CO2 to produce a carbonate or bicarbonate, optimizing the process for industrial scalability and cost-effectiveness.
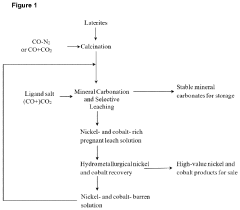
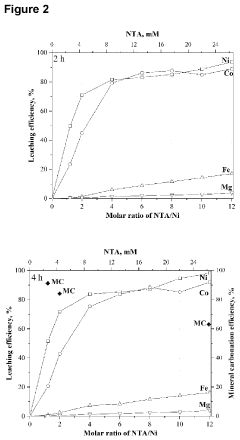
Concerted mineral carbonation and selective leaching of laterites
PatentPendingUS20230407436A1
Innovation
- The process involves calcining hydrated laterite materials to form mineralized silicate materials, which are then subjected to aqueous mineral carbonation in a buffered medium with a selective Ni/Co ligand, facilitating synergistic carbonation and leaching reactions to selectively extract nickel and cobalt while converting magnesium and iron into stable carbonates, thereby forming a pregnant leach solution for subsequent recovery.
Environmental Impact Assessment
The environmental impact assessment of sodium silicate solubility in carbon sequestration systems is a critical aspect of evaluating the overall sustainability and safety of this technology. Carbon sequestration, particularly in geological formations, has gained attention as a potential method to mitigate greenhouse gas emissions. However, the use of sodium silicate in these systems requires careful consideration of its environmental implications.
One of the primary environmental concerns is the potential for groundwater contamination. As sodium silicate dissolves in the carbon sequestration system, it may alter the pH and mineral composition of surrounding aquifers. This could lead to the mobilization of heavy metals or other contaminants, potentially affecting drinking water sources and aquatic ecosystems. Long-term monitoring of groundwater quality in areas surrounding carbon sequestration sites is essential to assess and mitigate these risks.
The impact on soil chemistry is another important consideration. The introduction of sodium silicate into geological formations may alter soil structure and composition, potentially affecting local flora and fauna. Changes in soil pH and mineral content could influence plant growth patterns and microbial communities, with cascading effects on local ecosystems. Studies on soil health and biodiversity in areas adjacent to carbon sequestration sites are necessary to fully understand these impacts.
Furthermore, the production and transportation of sodium silicate for use in carbon sequestration systems must be evaluated for their environmental footprint. The manufacturing process of sodium silicate is energy-intensive and may contribute to greenhouse gas emissions, potentially offsetting some of the benefits of carbon sequestration. Life cycle assessments should be conducted to quantify the net environmental impact of using sodium silicate in these systems.
The potential for induced seismicity is another environmental concern. While the risk is generally low, the injection of fluids into geological formations can, in some cases, trigger small earthquakes. The solubility of sodium silicate and its interaction with rock formations should be carefully studied to minimize this risk and ensure the long-term stability of the sequestration site.
Lastly, the impact on marine environments must be considered, especially for offshore carbon sequestration projects. The potential leakage of sodium silicate-rich solutions into marine ecosystems could alter ocean chemistry, affecting marine life and potentially contributing to ocean acidification. Comprehensive environmental monitoring programs should be implemented to detect and respond to any such impacts.
In conclusion, while sodium silicate solubility in carbon sequestration systems offers promising potential for greenhouse gas mitigation, a thorough environmental impact assessment is crucial. This assessment should encompass groundwater and soil impacts, ecosystem effects, production and transportation considerations, seismic risks, and potential marine impacts. Only through comprehensive evaluation and ongoing monitoring can the environmental sustainability of this technology be ensured.
One of the primary environmental concerns is the potential for groundwater contamination. As sodium silicate dissolves in the carbon sequestration system, it may alter the pH and mineral composition of surrounding aquifers. This could lead to the mobilization of heavy metals or other contaminants, potentially affecting drinking water sources and aquatic ecosystems. Long-term monitoring of groundwater quality in areas surrounding carbon sequestration sites is essential to assess and mitigate these risks.
The impact on soil chemistry is another important consideration. The introduction of sodium silicate into geological formations may alter soil structure and composition, potentially affecting local flora and fauna. Changes in soil pH and mineral content could influence plant growth patterns and microbial communities, with cascading effects on local ecosystems. Studies on soil health and biodiversity in areas adjacent to carbon sequestration sites are necessary to fully understand these impacts.
Furthermore, the production and transportation of sodium silicate for use in carbon sequestration systems must be evaluated for their environmental footprint. The manufacturing process of sodium silicate is energy-intensive and may contribute to greenhouse gas emissions, potentially offsetting some of the benefits of carbon sequestration. Life cycle assessments should be conducted to quantify the net environmental impact of using sodium silicate in these systems.
The potential for induced seismicity is another environmental concern. While the risk is generally low, the injection of fluids into geological formations can, in some cases, trigger small earthquakes. The solubility of sodium silicate and its interaction with rock formations should be carefully studied to minimize this risk and ensure the long-term stability of the sequestration site.
Lastly, the impact on marine environments must be considered, especially for offshore carbon sequestration projects. The potential leakage of sodium silicate-rich solutions into marine ecosystems could alter ocean chemistry, affecting marine life and potentially contributing to ocean acidification. Comprehensive environmental monitoring programs should be implemented to detect and respond to any such impacts.
In conclusion, while sodium silicate solubility in carbon sequestration systems offers promising potential for greenhouse gas mitigation, a thorough environmental impact assessment is crucial. This assessment should encompass groundwater and soil impacts, ecosystem effects, production and transportation considerations, seismic risks, and potential marine impacts. Only through comprehensive evaluation and ongoing monitoring can the environmental sustainability of this technology be ensured.
Techno-economic Analysis
The techno-economic analysis of sodium silicate solubility in carbon sequestration systems reveals significant potential for cost-effective carbon capture and storage (CCS) solutions. The process involves the reaction of sodium silicate with carbon dioxide to form stable carbonate minerals, offering a promising avenue for long-term CO2 sequestration.
Initial capital costs for implementing sodium silicate-based CCS systems are estimated to range from $50 to $100 per ton of CO2 captured, depending on the scale of operation and existing infrastructure. These costs primarily stem from the construction of reaction vessels, pumping systems, and monitoring equipment. However, the operational expenses are comparatively low, with the main recurring cost being the procurement of sodium silicate, which is widely available and relatively inexpensive.
The economic viability of this technology is further enhanced by its potential for integration with existing industrial processes. Many industries, such as glass manufacturing and pulp and paper production, generate sodium silicate as a by-product. Utilizing these waste streams could significantly reduce raw material costs and improve the overall economics of the CCS process.
Energy requirements for the sodium silicate-CO2 reaction are moderate, with most of the energy consumption attributed to pumping and mixing operations. The process operates at near-ambient temperatures and pressures, which translates to lower energy costs compared to other CCS technologies that require high-pressure or high-temperature conditions.
A key economic advantage of this approach is the potential for value-added products. The carbonate minerals formed during the sequestration process have applications in construction materials and soil amendments. This co-product strategy could offset operational costs and potentially generate additional revenue streams, improving the overall economic feasibility of the CCS system.
Sensitivity analyses indicate that the economic performance of sodium silicate-based CCS is most influenced by the cost of sodium silicate, the efficiency of the carbonation reaction, and the market value of the carbonate mineral products. Optimizing these factors through ongoing research and development efforts could further enhance the cost-effectiveness of this technology.
Long-term projections suggest that as carbon pricing mechanisms become more prevalent globally, the economic attractiveness of sodium silicate-based CCS will increase. With potential carbon prices ranging from $30 to $100 per ton of CO2 in various scenarios, this technology could become not only environmentally beneficial but also financially lucrative for adopters.
Initial capital costs for implementing sodium silicate-based CCS systems are estimated to range from $50 to $100 per ton of CO2 captured, depending on the scale of operation and existing infrastructure. These costs primarily stem from the construction of reaction vessels, pumping systems, and monitoring equipment. However, the operational expenses are comparatively low, with the main recurring cost being the procurement of sodium silicate, which is widely available and relatively inexpensive.
The economic viability of this technology is further enhanced by its potential for integration with existing industrial processes. Many industries, such as glass manufacturing and pulp and paper production, generate sodium silicate as a by-product. Utilizing these waste streams could significantly reduce raw material costs and improve the overall economics of the CCS process.
Energy requirements for the sodium silicate-CO2 reaction are moderate, with most of the energy consumption attributed to pumping and mixing operations. The process operates at near-ambient temperatures and pressures, which translates to lower energy costs compared to other CCS technologies that require high-pressure or high-temperature conditions.
A key economic advantage of this approach is the potential for value-added products. The carbonate minerals formed during the sequestration process have applications in construction materials and soil amendments. This co-product strategy could offset operational costs and potentially generate additional revenue streams, improving the overall economic feasibility of the CCS system.
Sensitivity analyses indicate that the economic performance of sodium silicate-based CCS is most influenced by the cost of sodium silicate, the efficiency of the carbonation reaction, and the market value of the carbonate mineral products. Optimizing these factors through ongoing research and development efforts could further enhance the cost-effectiveness of this technology.
Long-term projections suggest that as carbon pricing mechanisms become more prevalent globally, the economic attractiveness of sodium silicate-based CCS will increase. With potential carbon prices ranging from $30 to $100 per ton of CO2 in various scenarios, this technology could become not only environmentally beneficial but also financially lucrative for adopters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!