Muriatic Acid in the Production of Chlorine and Sodium Hydroxide
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chlor-alkali Process Overview and Objectives
The chlor-alkali process is a fundamental industrial method for producing chlorine and sodium hydroxide, two essential chemicals with widespread applications across various industries. This process has evolved significantly since its inception in the late 19th century, with continuous improvements in efficiency, safety, and environmental impact.
The primary objective of the chlor-alkali process is to electrolyze an aqueous solution of sodium chloride (brine) to produce chlorine gas, sodium hydroxide (caustic soda), and hydrogen gas. This electrolysis reaction is the cornerstone of the process, and its optimization has been a key focus of technological advancements in the field.
Historically, the mercury cell process was widely used, but due to environmental concerns, it has been largely phased out in favor of more sustainable methods. The membrane cell technology, introduced in the 1970s, has become the industry standard due to its superior energy efficiency and reduced environmental impact. The diaphragm cell process, while still in use, is gradually being replaced by membrane technology.
The use of muriatic acid, also known as hydrochloric acid, in the chlor-alkali process is an area of ongoing research and development. Muriatic acid is both a byproduct and a potential feedstock in this process, creating opportunities for circular economy approaches in chlor-alkali production.
One of the key objectives in current research is to optimize the utilization of muriatic acid within the chlor-alkali process. This includes exploring methods to convert excess hydrochloric acid back into chlorine, thereby improving overall process efficiency and reducing waste. Additionally, researchers are investigating ways to use muriatic acid as a more cost-effective and environmentally friendly alternative to traditional chlorine production methods.
Another important goal is to enhance the purity and quality of the products obtained from the chlor-alkali process when using muriatic acid as a feedstock. This involves developing advanced purification techniques and improving electrode materials to minimize impurities and increase product yield.
Furthermore, researchers are focusing on reducing the energy consumption of the chlor-alkali process, particularly when incorporating muriatic acid. This aligns with broader industry trends towards sustainability and energy efficiency, aiming to decrease the carbon footprint of chlorine and sodium hydroxide production.
As the chemical industry moves towards more sustainable practices, there is also a growing interest in developing closed-loop systems that integrate muriatic acid recycling within the chlor-alkali process. This approach not only addresses waste management issues but also has the potential to improve the overall economics of chlor-alkali production.
The primary objective of the chlor-alkali process is to electrolyze an aqueous solution of sodium chloride (brine) to produce chlorine gas, sodium hydroxide (caustic soda), and hydrogen gas. This electrolysis reaction is the cornerstone of the process, and its optimization has been a key focus of technological advancements in the field.
Historically, the mercury cell process was widely used, but due to environmental concerns, it has been largely phased out in favor of more sustainable methods. The membrane cell technology, introduced in the 1970s, has become the industry standard due to its superior energy efficiency and reduced environmental impact. The diaphragm cell process, while still in use, is gradually being replaced by membrane technology.
The use of muriatic acid, also known as hydrochloric acid, in the chlor-alkali process is an area of ongoing research and development. Muriatic acid is both a byproduct and a potential feedstock in this process, creating opportunities for circular economy approaches in chlor-alkali production.
One of the key objectives in current research is to optimize the utilization of muriatic acid within the chlor-alkali process. This includes exploring methods to convert excess hydrochloric acid back into chlorine, thereby improving overall process efficiency and reducing waste. Additionally, researchers are investigating ways to use muriatic acid as a more cost-effective and environmentally friendly alternative to traditional chlorine production methods.
Another important goal is to enhance the purity and quality of the products obtained from the chlor-alkali process when using muriatic acid as a feedstock. This involves developing advanced purification techniques and improving electrode materials to minimize impurities and increase product yield.
Furthermore, researchers are focusing on reducing the energy consumption of the chlor-alkali process, particularly when incorporating muriatic acid. This aligns with broader industry trends towards sustainability and energy efficiency, aiming to decrease the carbon footprint of chlorine and sodium hydroxide production.
As the chemical industry moves towards more sustainable practices, there is also a growing interest in developing closed-loop systems that integrate muriatic acid recycling within the chlor-alkali process. This approach not only addresses waste management issues but also has the potential to improve the overall economics of chlor-alkali production.
Market Analysis for Chlorine and Sodium Hydroxide
The global market for chlorine and sodium hydroxide, key products derived from muriatic acid (hydrochloric acid), has shown significant growth and is expected to continue expanding. These chemicals are fundamental to various industries, including water treatment, chemical manufacturing, paper and pulp production, and textile processing.
Chlorine demand is primarily driven by its use in water treatment and disinfection, as well as in the production of PVC and other chlorinated compounds. The global chlorine market size was valued at over $30 billion in 2020, with a projected compound annual growth rate (CAGR) of around 5% through 2027. Asia-Pacific region, particularly China and India, leads in chlorine consumption due to rapid industrialization and urbanization.
Sodium hydroxide, also known as caustic soda, finds extensive applications in industries such as alumina, paper, textiles, and detergents. The global sodium hydroxide market was valued at approximately $40 billion in 2020, with an expected CAGR of 4-5% until 2027. The Asia-Pacific region again dominates the market, followed by North America and Europe.
The interconnected nature of chlorine and sodium hydroxide production, known as the chlor-alkali process, means that market dynamics for one product significantly impact the other. This symbiotic relationship can lead to supply-demand imbalances, affecting pricing and profitability for producers.
Environmental regulations play a crucial role in shaping the market. Stringent policies regarding chlorine use and production methods have led to increased adoption of membrane cell technology, which is more energy-efficient and environmentally friendly compared to older mercury cell processes.
Emerging trends in the chlorine and sodium hydroxide markets include the growing demand for sustainable and bio-based alternatives, increased focus on recycling and circular economy principles, and the development of new applications in emerging industries such as renewable energy storage and advanced materials.
The COVID-19 pandemic initially disrupted supply chains and manufacturing processes, but also led to increased demand for chlorine-based disinfectants. As economies recover, the market is expected to stabilize and continue its growth trajectory, driven by ongoing industrialization in developing countries and technological advancements in production processes.
Chlorine demand is primarily driven by its use in water treatment and disinfection, as well as in the production of PVC and other chlorinated compounds. The global chlorine market size was valued at over $30 billion in 2020, with a projected compound annual growth rate (CAGR) of around 5% through 2027. Asia-Pacific region, particularly China and India, leads in chlorine consumption due to rapid industrialization and urbanization.
Sodium hydroxide, also known as caustic soda, finds extensive applications in industries such as alumina, paper, textiles, and detergents. The global sodium hydroxide market was valued at approximately $40 billion in 2020, with an expected CAGR of 4-5% until 2027. The Asia-Pacific region again dominates the market, followed by North America and Europe.
The interconnected nature of chlorine and sodium hydroxide production, known as the chlor-alkali process, means that market dynamics for one product significantly impact the other. This symbiotic relationship can lead to supply-demand imbalances, affecting pricing and profitability for producers.
Environmental regulations play a crucial role in shaping the market. Stringent policies regarding chlorine use and production methods have led to increased adoption of membrane cell technology, which is more energy-efficient and environmentally friendly compared to older mercury cell processes.
Emerging trends in the chlorine and sodium hydroxide markets include the growing demand for sustainable and bio-based alternatives, increased focus on recycling and circular economy principles, and the development of new applications in emerging industries such as renewable energy storage and advanced materials.
The COVID-19 pandemic initially disrupted supply chains and manufacturing processes, but also led to increased demand for chlorine-based disinfectants. As economies recover, the market is expected to stabilize and continue its growth trajectory, driven by ongoing industrialization in developing countries and technological advancements in production processes.
Current Challenges in Muriatic Acid Utilization
The utilization of muriatic acid in the production of chlorine and sodium hydroxide faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary issues is the corrosive nature of muriatic acid, which necessitates specialized equipment and handling procedures. This corrosiveness not only increases operational costs but also poses safety risks to workers and the environment.
Another major challenge is the energy-intensive nature of the process. The electrolysis of muriatic acid to produce chlorine and sodium hydroxide requires substantial electrical input, contributing to high operational costs and environmental concerns related to energy consumption. This energy intensity makes the process less attractive in regions with high electricity prices or those striving to reduce their carbon footprint.
The purity of the muriatic acid feedstock also presents a significant challenge. Impurities in the acid can lead to unwanted side reactions, reduce the efficiency of the electrolysis process, and potentially contaminate the final products. Ensuring a consistent supply of high-purity muriatic acid can be both challenging and costly, particularly for large-scale operations.
Furthermore, the management of by-products and waste streams poses environmental and regulatory challenges. The process generates hydrogen gas as a by-product, which requires careful handling and storage. Additionally, any unreacted acid or impurities must be properly treated and disposed of, adding to the operational complexity and cost.
Market volatility and supply chain issues also present ongoing challenges. The demand for chlorine and sodium hydroxide can fluctuate significantly based on economic conditions and industry trends, making it difficult for producers to maintain consistent production levels and profitability. Moreover, disruptions in the supply chain for muriatic acid can lead to production delays and increased costs.
Regulatory compliance is another critical challenge, particularly as environmental regulations become more stringent globally. Producers must navigate complex regulatory landscapes, often requiring substantial investments in emission control technologies and safety measures to meet evolving standards.
Lastly, the development of alternative technologies and competing processes poses a challenge to the long-term viability of muriatic acid utilization in this context. Emerging technologies that offer more environmentally friendly or cost-effective methods for producing chlorine and sodium hydroxide could potentially displace the traditional muriatic acid-based process, necessitating ongoing innovation and adaptation in the industry.
Another major challenge is the energy-intensive nature of the process. The electrolysis of muriatic acid to produce chlorine and sodium hydroxide requires substantial electrical input, contributing to high operational costs and environmental concerns related to energy consumption. This energy intensity makes the process less attractive in regions with high electricity prices or those striving to reduce their carbon footprint.
The purity of the muriatic acid feedstock also presents a significant challenge. Impurities in the acid can lead to unwanted side reactions, reduce the efficiency of the electrolysis process, and potentially contaminate the final products. Ensuring a consistent supply of high-purity muriatic acid can be both challenging and costly, particularly for large-scale operations.
Furthermore, the management of by-products and waste streams poses environmental and regulatory challenges. The process generates hydrogen gas as a by-product, which requires careful handling and storage. Additionally, any unreacted acid or impurities must be properly treated and disposed of, adding to the operational complexity and cost.
Market volatility and supply chain issues also present ongoing challenges. The demand for chlorine and sodium hydroxide can fluctuate significantly based on economic conditions and industry trends, making it difficult for producers to maintain consistent production levels and profitability. Moreover, disruptions in the supply chain for muriatic acid can lead to production delays and increased costs.
Regulatory compliance is another critical challenge, particularly as environmental regulations become more stringent globally. Producers must navigate complex regulatory landscapes, often requiring substantial investments in emission control technologies and safety measures to meet evolving standards.
Lastly, the development of alternative technologies and competing processes poses a challenge to the long-term viability of muriatic acid utilization in this context. Emerging technologies that offer more environmentally friendly or cost-effective methods for producing chlorine and sodium hydroxide could potentially displace the traditional muriatic acid-based process, necessitating ongoing innovation and adaptation in the industry.
Existing Muriatic Acid-based Production Methods
01 Chemical properties and applications of muriatic acid
Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and commercial applications. It is used in metal cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature makes it effective for removing rust and scale from metals.- Production and purification of muriatic acid: Muriatic acid, also known as hydrochloric acid, can be produced and purified through various industrial processes. These methods may involve the reaction of chlorine with hydrogen or the treatment of salt with sulfuric acid. Purification techniques can include distillation or membrane separation to remove impurities and achieve desired concentrations.
- Applications in metal treatment and surface cleaning: Muriatic acid is widely used in metal treatment processes, such as pickling, etching, and surface cleaning. It can effectively remove rust, scale, and other contaminants from metal surfaces. The acid's properties make it suitable for preparing metal surfaces prior to coating, plating, or other finishing processes.
- Use in construction and building materials: Muriatic acid finds applications in the construction industry, particularly in the treatment of concrete and masonry surfaces. It can be used for cleaning, etching, or preparing surfaces for further treatment. The acid is also employed in the production of certain building materials and in the maintenance of construction equipment.
- Environmental and safety considerations: Handling and disposal of muriatic acid require careful consideration of environmental and safety factors. Proper storage, transportation, and use of the acid are essential to prevent accidents and minimize environmental impact. Neutralization techniques and specialized equipment may be necessary for safe handling and disposal of muriatic acid in industrial settings.
- Alternative applications and formulations: Muriatic acid can be used in various alternative applications beyond its traditional uses. These may include water treatment, pH adjustment in industrial processes, and as a reagent in chemical synthesis. Modified formulations or combinations with other substances can enhance its effectiveness or tailor its properties for specific applications.
02 Use in water treatment and purification
Muriatic acid is employed in water treatment processes for pH adjustment and disinfection. It helps in controlling alkalinity and removing impurities from water. The acid is also used in swimming pool maintenance to balance pH levels and prevent algae growth.Expand Specific Solutions03 Production and handling of muriatic acid
The production of muriatic acid involves various industrial processes, including the reaction of sodium chloride with sulfuric acid. Specialized equipment and safety measures are required for its manufacture, storage, and transportation due to its corrosive nature.Expand Specific Solutions04 Applications in construction and building materials
Muriatic acid is used in the construction industry for cleaning masonry, concrete, and other building materials. It helps in removing efflorescence, mortar stains, and preparing surfaces for further treatment or coating. The acid is also used in the production of certain building materials.Expand Specific Solutions05 Environmental and safety considerations
The use of muriatic acid requires careful handling and disposal due to its corrosive nature and potential environmental impact. Safety measures, such as proper ventilation and protective equipment, are essential when working with this acid. Neutralization and proper disposal methods are important to minimize environmental risks.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The research on the use of Muriatic Acid in the production of Chlorine and Sodium Hydroxide is in a mature stage, with established industrial processes and a significant global market. The technology is well-developed, with major players like BASF Corp., Akzo Nobel Chemicals International BV, and AGC, Inc. leading the field. These companies have extensive experience and advanced capabilities in chemical manufacturing. The market size is substantial, driven by the widespread use of chlorine and sodium hydroxide in various industries. However, there is ongoing research and development focused on improving efficiency, sustainability, and cost-effectiveness of the production processes, particularly by academic institutions like the Institute of Process Engineering, Chinese Academy of Sciences and Beijing University of Technology.
BASF Corp.
Technical Solution: BASF Corp. has developed an advanced membrane cell technology for the production of chlorine and sodium hydroxide using muriatic acid (hydrochloric acid). Their process involves electrolysis of a saturated sodium chloride solution in a membrane cell, where the anode and cathode chambers are separated by an ion-selective membrane. The company has optimized the membrane composition to enhance ion selectivity and durability, resulting in improved efficiency and reduced energy consumption. BASF's technology also incorporates a closed-loop system for recycling unreacted hydrochloric acid, minimizing waste and improving overall process economics[1][3].
Strengths: High efficiency, reduced energy consumption, and minimized waste. Weaknesses: High initial investment costs and potential membrane fouling issues over time.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has pioneered a novel approach to chlorine and sodium hydroxide production using muriatic acid through their proprietary "HCl to Chlorine" (HCl2Cl) technology. This process directly converts hydrochloric acid to chlorine gas and water using an oxygen-depolarized cathode. The company has developed specialized catalysts and electrode materials to enhance the reaction efficiency and selectivity. Their system integrates seamlessly with existing chlor-alkali plants, allowing for flexible production based on market demands. Akzo Nobel's technology also incorporates advanced process control systems to optimize operating conditions and maintain high product quality[2][5].
Strengths: Direct HCl conversion, flexibility in production, and integration with existing plants. Weaknesses: Dependency on specialized catalysts and potential complexity in process control.
Innovative Approaches in Acid Management
A process and an apparatus for obtaining useful products from a sodium chloride-containing waste material
PatentInactiveEP2712354A2
Innovation
- A process involving the reaction of sodium chloride with a carbonaceous material at elevated temperatures, using steam and oxygen to produce hydrogen chloride and sodium oxide, which is then washed with water to obtain caustic soda, reducing energy consumption and waste generation.
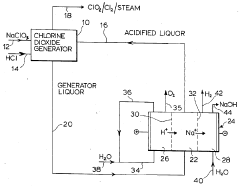
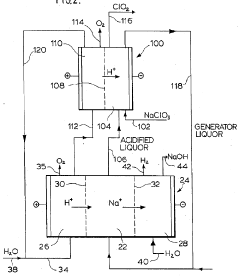
Combined process for production of chlorine dioxide and sodium hydroxide
PatentInactiveUS4806215A
Innovation
- An integrated process that combines a hydrochloric acid-based chlorine dioxide generation with an electrolytic sodium hydroxide production system, using a three-compartment electrolytic cell with cation-exchange membranes to efficiently convert by-product sodium chloride into hydrochloric acid and produce sodium hydroxide without co-producing chlorine.
Environmental Impact and Sustainability Considerations
The production of chlorine and sodium hydroxide using muriatic acid, also known as hydrochloric acid, has significant environmental implications that must be carefully considered. This process, while efficient, generates various waste streams and emissions that can impact air quality, water resources, and soil health if not properly managed.
One of the primary environmental concerns is the potential release of chlorine gas, a highly toxic substance that can cause severe respiratory issues and environmental damage. Stringent safety measures and emission control systems are essential to prevent accidental releases and minimize atmospheric pollution. Additionally, the production process may generate chlorinated organic compounds, which can be persistent environmental pollutants if not adequately treated.
Water usage and wastewater management are critical aspects of the environmental impact assessment. The production process requires substantial amounts of water, and the resulting wastewater often contains high levels of dissolved salts and other contaminants. Advanced treatment technologies, such as membrane filtration and ion exchange, are necessary to ensure that discharged water meets environmental standards and does not harm aquatic ecosystems.
Energy consumption is another significant factor affecting the sustainability of this production method. The electrolysis process used to produce chlorine and sodium hydroxide is energy-intensive, contributing to greenhouse gas emissions if the energy source is not renewable. Implementing energy-efficient technologies and exploring clean energy alternatives can help mitigate this environmental burden.
The handling and disposal of byproducts and waste materials also pose environmental challenges. Proper management of spent catalysts, residual acids, and other chemical wastes is crucial to prevent soil and groundwater contamination. Developing effective recycling and recovery processes for these materials can enhance the overall sustainability of the production cycle.
From a sustainability perspective, there is growing interest in developing alternative production methods that reduce environmental impact. Research into membrane-based technologies and microbial electrolysis cells shows promise for more sustainable chlorine and sodium hydroxide production. These emerging technologies aim to decrease energy consumption, minimize waste generation, and reduce the use of hazardous materials.
Lifecycle assessment (LCA) studies are increasingly being employed to evaluate the full environmental impact of chlorine and sodium hydroxide production. These assessments consider all stages of the production process, from raw material extraction to end-of-life disposal, providing a comprehensive view of the environmental footprint and identifying areas for improvement.
In conclusion, while the use of muriatic acid in chlorine and sodium hydroxide production is an established industrial process, its environmental impact and sustainability considerations are complex and multifaceted. Addressing these challenges requires a combination of technological innovation, stringent regulatory compliance, and a commitment to continuous improvement in environmental performance.
One of the primary environmental concerns is the potential release of chlorine gas, a highly toxic substance that can cause severe respiratory issues and environmental damage. Stringent safety measures and emission control systems are essential to prevent accidental releases and minimize atmospheric pollution. Additionally, the production process may generate chlorinated organic compounds, which can be persistent environmental pollutants if not adequately treated.
Water usage and wastewater management are critical aspects of the environmental impact assessment. The production process requires substantial amounts of water, and the resulting wastewater often contains high levels of dissolved salts and other contaminants. Advanced treatment technologies, such as membrane filtration and ion exchange, are necessary to ensure that discharged water meets environmental standards and does not harm aquatic ecosystems.
Energy consumption is another significant factor affecting the sustainability of this production method. The electrolysis process used to produce chlorine and sodium hydroxide is energy-intensive, contributing to greenhouse gas emissions if the energy source is not renewable. Implementing energy-efficient technologies and exploring clean energy alternatives can help mitigate this environmental burden.
The handling and disposal of byproducts and waste materials also pose environmental challenges. Proper management of spent catalysts, residual acids, and other chemical wastes is crucial to prevent soil and groundwater contamination. Developing effective recycling and recovery processes for these materials can enhance the overall sustainability of the production cycle.
From a sustainability perspective, there is growing interest in developing alternative production methods that reduce environmental impact. Research into membrane-based technologies and microbial electrolysis cells shows promise for more sustainable chlorine and sodium hydroxide production. These emerging technologies aim to decrease energy consumption, minimize waste generation, and reduce the use of hazardous materials.
Lifecycle assessment (LCA) studies are increasingly being employed to evaluate the full environmental impact of chlorine and sodium hydroxide production. These assessments consider all stages of the production process, from raw material extraction to end-of-life disposal, providing a comprehensive view of the environmental footprint and identifying areas for improvement.
In conclusion, while the use of muriatic acid in chlorine and sodium hydroxide production is an established industrial process, its environmental impact and sustainability considerations are complex and multifaceted. Addressing these challenges requires a combination of technological innovation, stringent regulatory compliance, and a commitment to continuous improvement in environmental performance.
Regulatory Framework for Chemical Manufacturing
The regulatory framework for chemical manufacturing, particularly in the production of chlorine and sodium hydroxide using muriatic acid, is complex and multifaceted. At the federal level in the United States, the Environmental Protection Agency (EPA) plays a crucial role in overseeing and regulating the chemical industry. The EPA enforces several key regulations, including the Toxic Substances Control Act (TSCA) and the Clean Air Act, which directly impact the production processes involving muriatic acid.
The Occupational Safety and Health Administration (OSHA) is another significant regulatory body, focusing on worker safety in chemical manufacturing facilities. OSHA's Process Safety Management (PSM) standard is particularly relevant, as it addresses the handling of highly hazardous chemicals, including those used in chlorine and sodium hydroxide production.
At the state level, regulations can vary, but most states have their own environmental protection agencies that work in conjunction with federal regulations. These state-level agencies often impose additional requirements or stricter standards on chemical manufacturers, particularly in areas with higher population densities or sensitive ecosystems.
Internationally, the production of chlorine and sodium hydroxide is subject to various treaties and agreements. The Stockholm Convention on Persistent Organic Pollutants, for instance, impacts chlorine production due to its potential to generate harmful byproducts. The Montreal Protocol, while primarily focused on ozone-depleting substances, also has implications for certain chlorine-based compounds.
Compliance with these regulations requires extensive documentation, regular inspections, and adherence to strict safety protocols. Manufacturers must implement robust environmental management systems, conduct regular risk assessments, and maintain detailed records of their production processes and chemical handling procedures.
The regulatory landscape is not static; it continually evolves in response to new scientific findings, technological advancements, and changing environmental concerns. For instance, recent years have seen an increased focus on the circular economy and sustainable chemistry principles, which are beginning to influence regulations in the chemical manufacturing sector.
Manufacturers must also navigate product-specific regulations. For example, the use of chlorine in water treatment is subject to the Safe Drinking Water Act, which sets standards for chlorine levels in public water systems. Similarly, the use of sodium hydroxide in various consumer products falls under the purview of consumer safety regulations enforced by agencies like the Consumer Product Safety Commission.
In conclusion, the regulatory framework for chemical manufacturing, especially in the context of using muriatic acid for chlorine and sodium hydroxide production, is a comprehensive system that spans multiple levels of government and international agreements. It requires constant vigilance and adaptation from manufacturers to ensure compliance and maintain safe, environmentally responsible operations.
The Occupational Safety and Health Administration (OSHA) is another significant regulatory body, focusing on worker safety in chemical manufacturing facilities. OSHA's Process Safety Management (PSM) standard is particularly relevant, as it addresses the handling of highly hazardous chemicals, including those used in chlorine and sodium hydroxide production.
At the state level, regulations can vary, but most states have their own environmental protection agencies that work in conjunction with federal regulations. These state-level agencies often impose additional requirements or stricter standards on chemical manufacturers, particularly in areas with higher population densities or sensitive ecosystems.
Internationally, the production of chlorine and sodium hydroxide is subject to various treaties and agreements. The Stockholm Convention on Persistent Organic Pollutants, for instance, impacts chlorine production due to its potential to generate harmful byproducts. The Montreal Protocol, while primarily focused on ozone-depleting substances, also has implications for certain chlorine-based compounds.
Compliance with these regulations requires extensive documentation, regular inspections, and adherence to strict safety protocols. Manufacturers must implement robust environmental management systems, conduct regular risk assessments, and maintain detailed records of their production processes and chemical handling procedures.
The regulatory landscape is not static; it continually evolves in response to new scientific findings, technological advancements, and changing environmental concerns. For instance, recent years have seen an increased focus on the circular economy and sustainable chemistry principles, which are beginning to influence regulations in the chemical manufacturing sector.
Manufacturers must also navigate product-specific regulations. For example, the use of chlorine in water treatment is subject to the Safe Drinking Water Act, which sets standards for chlorine levels in public water systems. Similarly, the use of sodium hydroxide in various consumer products falls under the purview of consumer safety regulations enforced by agencies like the Consumer Product Safety Commission.
In conclusion, the regulatory framework for chemical manufacturing, especially in the context of using muriatic acid for chlorine and sodium hydroxide production, is a comprehensive system that spans multiple levels of government and international agreements. It requires constant vigilance and adaptation from manufacturers to ensure compliance and maintain safe, environmentally responsible operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!