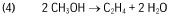Muriatic Acid in the Synthesis of Polyvinyl Chloride (PVC)
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PVC Synthesis Background
Polyvinyl chloride (PVC) has been a cornerstone of the plastics industry since its commercial introduction in the 1930s. The synthesis of PVC involves a complex process that has evolved significantly over the decades, with muriatic acid playing a crucial role in its production. Understanding the background of PVC synthesis is essential for appreciating the technological advancements and challenges in this field.
The journey of PVC began in 1872 when Eugen Baumann first discovered the polymer, although it remained a laboratory curiosity for many years. It wasn't until the 1920s that researchers began to explore its commercial potential. The breakthrough came in 1926 when Waldo Semon of B.F. Goodrich developed a method to plasticize PVC, making it more flexible and easier to process.
The basic raw materials for PVC production are salt (sodium chloride) and ethylene, derived from petroleum or natural gas. The salt is electrolyzed to produce chlorine, which is then combined with ethylene to form ethylene dichloride (EDC). EDC is further processed to create vinyl chloride monomer (VCM), the immediate precursor to PVC.
Muriatic acid, also known as hydrochloric acid, enters the picture at various stages of this process. It is a byproduct of the chlorination of ethylene to EDC and is also used in the purification of VCM. The efficient utilization and management of muriatic acid in PVC synthesis have been ongoing challenges and areas of innovation in the industry.
The polymerization of VCM to PVC can be achieved through several methods, including suspension, emulsion, and bulk polymerization. Each method has its advantages and is suited to different end-product requirements. The choice of polymerization technique significantly influences the properties of the final PVC product.
Environmental and health concerns have been significant drivers in the evolution of PVC synthesis. The industry has made substantial efforts to reduce emissions of VCM, a known carcinogen, and to develop more sustainable production methods. These efforts have led to improvements in reactor design, polymerization techniques, and waste management practices.
The global demand for PVC has grown steadily since its introduction, driven by its versatility, durability, and cost-effectiveness. This demand has spurred continuous research into improving synthesis methods, enhancing product quality, and reducing environmental impact. The use of muriatic acid in PVC synthesis remains an area of active research, with efforts focused on optimizing its utilization and exploring alternatives.
The journey of PVC began in 1872 when Eugen Baumann first discovered the polymer, although it remained a laboratory curiosity for many years. It wasn't until the 1920s that researchers began to explore its commercial potential. The breakthrough came in 1926 when Waldo Semon of B.F. Goodrich developed a method to plasticize PVC, making it more flexible and easier to process.
The basic raw materials for PVC production are salt (sodium chloride) and ethylene, derived from petroleum or natural gas. The salt is electrolyzed to produce chlorine, which is then combined with ethylene to form ethylene dichloride (EDC). EDC is further processed to create vinyl chloride monomer (VCM), the immediate precursor to PVC.
Muriatic acid, also known as hydrochloric acid, enters the picture at various stages of this process. It is a byproduct of the chlorination of ethylene to EDC and is also used in the purification of VCM. The efficient utilization and management of muriatic acid in PVC synthesis have been ongoing challenges and areas of innovation in the industry.
The polymerization of VCM to PVC can be achieved through several methods, including suspension, emulsion, and bulk polymerization. Each method has its advantages and is suited to different end-product requirements. The choice of polymerization technique significantly influences the properties of the final PVC product.
Environmental and health concerns have been significant drivers in the evolution of PVC synthesis. The industry has made substantial efforts to reduce emissions of VCM, a known carcinogen, and to develop more sustainable production methods. These efforts have led to improvements in reactor design, polymerization techniques, and waste management practices.
The global demand for PVC has grown steadily since its introduction, driven by its versatility, durability, and cost-effectiveness. This demand has spurred continuous research into improving synthesis methods, enhancing product quality, and reducing environmental impact. The use of muriatic acid in PVC synthesis remains an area of active research, with efforts focused on optimizing its utilization and exploring alternatives.
Market Analysis for PVC
The global market for Polyvinyl Chloride (PVC) has shown robust growth in recent years, driven by increasing demand across various industries. PVC, being one of the most widely used plastics worldwide, finds applications in construction, automotive, healthcare, and consumer goods sectors. The construction industry remains the largest consumer of PVC, accounting for a significant portion of the market share due to its use in pipes, fittings, windows, and flooring materials.
The PVC market has witnessed steady expansion, with a compound annual growth rate (CAGR) exceeding the average growth rate of the overall plastics industry. This growth is attributed to PVC's versatility, durability, and cost-effectiveness compared to alternative materials. Developing economies, particularly in Asia-Pacific and Latin America, have emerged as key growth drivers for the PVC market, fueled by rapid urbanization, infrastructure development, and increasing disposable incomes.
China stands out as the largest producer and consumer of PVC globally, followed by North America and Europe. The Asia-Pacific region is expected to maintain its dominance in the PVC market, with India and Southeast Asian countries showing promising growth potential. However, mature markets like North America and Europe are experiencing slower growth rates due to market saturation and environmental concerns.
Environmental regulations and sustainability issues pose challenges to the PVC industry. Concerns over the use of chlorine in PVC production and the disposal of PVC products have led to increased scrutiny and the development of eco-friendly alternatives. This has prompted industry players to invest in research and development of bio-based PVC and recycling technologies to address environmental concerns and maintain market competitiveness.
The automotive sector represents a growing market for PVC, particularly in interior components, wire harnesses, and underbody coatings. The healthcare industry also contributes significantly to PVC demand, with applications in medical devices, packaging, and hospital infrastructure. The packaging industry, while not the largest consumer, shows potential for growth in flexible PVC applications.
Market dynamics are influenced by raw material prices, particularly ethylene and chlorine. Fluctuations in these input costs can impact PVC pricing and profitability for manufacturers. The industry has also seen consolidation through mergers and acquisitions, as companies seek to strengthen their market position and achieve economies of scale.
Looking ahead, the PVC market is expected to continue its growth trajectory, albeit at a more moderate pace. Innovations in PVC formulations, such as lead-free stabilizers and plasticizers, are likely to open new opportunities and address regulatory challenges. The development of sustainable PVC products and recycling technologies will be crucial for long-term market growth and environmental compliance.
The PVC market has witnessed steady expansion, with a compound annual growth rate (CAGR) exceeding the average growth rate of the overall plastics industry. This growth is attributed to PVC's versatility, durability, and cost-effectiveness compared to alternative materials. Developing economies, particularly in Asia-Pacific and Latin America, have emerged as key growth drivers for the PVC market, fueled by rapid urbanization, infrastructure development, and increasing disposable incomes.
China stands out as the largest producer and consumer of PVC globally, followed by North America and Europe. The Asia-Pacific region is expected to maintain its dominance in the PVC market, with India and Southeast Asian countries showing promising growth potential. However, mature markets like North America and Europe are experiencing slower growth rates due to market saturation and environmental concerns.
Environmental regulations and sustainability issues pose challenges to the PVC industry. Concerns over the use of chlorine in PVC production and the disposal of PVC products have led to increased scrutiny and the development of eco-friendly alternatives. This has prompted industry players to invest in research and development of bio-based PVC and recycling technologies to address environmental concerns and maintain market competitiveness.
The automotive sector represents a growing market for PVC, particularly in interior components, wire harnesses, and underbody coatings. The healthcare industry also contributes significantly to PVC demand, with applications in medical devices, packaging, and hospital infrastructure. The packaging industry, while not the largest consumer, shows potential for growth in flexible PVC applications.
Market dynamics are influenced by raw material prices, particularly ethylene and chlorine. Fluctuations in these input costs can impact PVC pricing and profitability for manufacturers. The industry has also seen consolidation through mergers and acquisitions, as companies seek to strengthen their market position and achieve economies of scale.
Looking ahead, the PVC market is expected to continue its growth trajectory, albeit at a more moderate pace. Innovations in PVC formulations, such as lead-free stabilizers and plasticizers, are likely to open new opportunities and address regulatory challenges. The development of sustainable PVC products and recycling technologies will be crucial for long-term market growth and environmental compliance.
Muriatic Acid in PVC
Muriatic acid, also known as hydrochloric acid, plays a crucial role in the synthesis of polyvinyl chloride (PVC). This strong mineral acid is primarily used in the production of vinyl chloride monomer (VCM), the precursor to PVC. The process involves the oxychlorination of ethylene, where ethylene reacts with hydrogen chloride and oxygen to form VCM.
The use of muriatic acid in PVC synthesis dates back to the 1930s when industrial production of PVC began. Over the years, the process has been refined and optimized to improve efficiency and reduce environmental impact. Today, the balanced equation method is widely used, which combines direct chlorination and oxychlorination processes to maximize VCM yield and minimize waste.
In the direct chlorination process, ethylene reacts with chlorine to form 1,2-dichloroethane (EDC). This EDC is then thermally cracked to produce VCM and hydrogen chloride. The hydrogen chloride byproduct is not wasted but instead used in the oxychlorination process, where it reacts with ethylene and oxygen to produce more EDC. This integrated approach ensures efficient use of raw materials and reduces the overall environmental footprint of PVC production.
Muriatic acid's role extends beyond VCM production. It is also used in the purification of EDC and VCM, removing impurities that could affect the quality of the final PVC product. Additionally, it plays a part in catalyst regeneration and equipment cleaning in PVC manufacturing plants.
The concentration of muriatic acid used in PVC synthesis typically ranges from 30% to 37%, depending on the specific process requirements. Higher concentrations are generally preferred for more efficient reactions, but safety considerations often limit the maximum concentration used in industrial settings.
Recent technological advancements have focused on improving the sustainability of PVC production, including more efficient use of muriatic acid. Innovations in reactor design, catalysts, and process control have led to reduced acid consumption and improved product quality. Furthermore, research is ongoing to develop alternative methods that could potentially reduce or eliminate the need for muriatic acid in PVC synthesis, such as bio-based routes or electrochemical processes.
The use of muriatic acid in PVC synthesis dates back to the 1930s when industrial production of PVC began. Over the years, the process has been refined and optimized to improve efficiency and reduce environmental impact. Today, the balanced equation method is widely used, which combines direct chlorination and oxychlorination processes to maximize VCM yield and minimize waste.
In the direct chlorination process, ethylene reacts with chlorine to form 1,2-dichloroethane (EDC). This EDC is then thermally cracked to produce VCM and hydrogen chloride. The hydrogen chloride byproduct is not wasted but instead used in the oxychlorination process, where it reacts with ethylene and oxygen to produce more EDC. This integrated approach ensures efficient use of raw materials and reduces the overall environmental footprint of PVC production.
Muriatic acid's role extends beyond VCM production. It is also used in the purification of EDC and VCM, removing impurities that could affect the quality of the final PVC product. Additionally, it plays a part in catalyst regeneration and equipment cleaning in PVC manufacturing plants.
The concentration of muriatic acid used in PVC synthesis typically ranges from 30% to 37%, depending on the specific process requirements. Higher concentrations are generally preferred for more efficient reactions, but safety considerations often limit the maximum concentration used in industrial settings.
Recent technological advancements have focused on improving the sustainability of PVC production, including more efficient use of muriatic acid. Innovations in reactor design, catalysts, and process control have led to reduced acid consumption and improved product quality. Furthermore, research is ongoing to develop alternative methods that could potentially reduce or eliminate the need for muriatic acid in PVC synthesis, such as bio-based routes or electrochemical processes.
Current PVC Synthesis
01 Chemical properties and applications
Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and household applications. It is commonly used for cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature makes it effective for removing rust, scale, and other deposits.- Chemical properties and applications of muriatic acid: Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and household applications. It is commonly used for cleaning, pH adjustment, and as a chemical reagent in manufacturing processes. Its corrosive nature makes it effective for removing rust, scale, and other deposits.
- Use in water treatment and purification: Muriatic acid plays a crucial role in water treatment and purification processes. It is used to adjust pH levels in swimming pools, industrial water systems, and municipal water supplies. The acid helps to maintain proper water chemistry and prevent the growth of harmful microorganisms.
- Applications in metal processing and surface treatment: In the metal industry, muriatic acid is utilized for pickling and descaling metal surfaces, particularly steel. It effectively removes rust, scale, and other impurities from metal surfaces, preparing them for further processing or coating. The acid is also used in electroplating and metal etching processes.
- Production and handling of muriatic acid: The production of muriatic acid involves various chemical processes, including the reaction of sodium chloride with sulfuric acid. Specialized equipment and safety measures are required for its manufacture, storage, and transportation due to its corrosive nature. Proper handling and disposal procedures are essential to prevent environmental contamination and ensure worker safety.
- Environmental and safety considerations: The use of muriatic acid requires careful attention to environmental and safety regulations. Proper containment, neutralization, and disposal methods are necessary to prevent pollution and protect ecosystems. Safety measures, including personal protective equipment and proper ventilation, are crucial when handling this corrosive substance to prevent accidents and injuries.
02 Production and manufacturing processes
Various methods are employed to produce muriatic acid, including the reaction of sodium chloride with sulfuric acid, and as a byproduct in chlorine production. Manufacturing processes often involve specialized equipment and safety measures due to the acid's corrosive nature.Expand Specific Solutions03 Use in water treatment and purification
Muriatic acid plays a crucial role in water treatment and purification processes. It is used to adjust pH levels, remove alkalinity, and clean water treatment equipment. The acid's ability to dissolve mineral deposits makes it effective in maintaining water systems and preventing scale buildup.Expand Specific Solutions04 Applications in metal processing and surface treatment
In metal processing, muriatic acid is used for pickling, etching, and cleaning metal surfaces. It effectively removes rust, scale, and other impurities from metals, preparing them for further treatment or coating. The acid's reactivity with metals makes it valuable in various industrial processes.Expand Specific Solutions05 Safety considerations and handling precautions
Due to its corrosive nature, proper safety measures are essential when handling muriatic acid. This includes using appropriate personal protective equipment, ensuring proper ventilation, and following specific storage and disposal guidelines. Neutralization techniques and spill response procedures are also important aspects of safe muriatic acid handling.Expand Specific Solutions
Key PVC Manufacturers
The research on the use of Muriatic Acid in PVC synthesis is in a mature stage, with a well-established market and significant industry players. The global PVC market size is substantial, driven by demand in construction, automotive, and packaging sectors. Key companies like LG Chem, Dow Global Technologies, and BASF are at the forefront of technological advancements. The technology's maturity is evident from the involvement of diverse players, including specialty chemical manufacturers like Arkema and Eastman Chemical, as well as academic institutions such as Zhejiang University and Sichuan University, indicating ongoing research and development efforts to optimize processes and improve efficiency.
Inovyn Europe Ltd.
Technical Solution: Inovyn Europe Ltd. has developed an innovative approach to PVC synthesis using muriatic acid. Their process involves a two-stage reaction where muriatic acid is first converted to vinyl chloride monomer (VCM) through oxychlorination of ethylene. The VCM is then polymerized to produce PVC. This method allows for efficient use of muriatic acid, a byproduct of chlorine production, reducing waste and improving overall process economics[1]. Inovyn's technology also incorporates advanced catalysts that enhance reaction selectivity and reduce energy consumption during the synthesis process[3]. The company has implemented strict quality control measures to ensure consistent PVC properties, including molecular weight distribution and thermal stability[5].
Strengths: Efficient use of byproduct muriatic acid, reduced waste, improved process economics. Weaknesses: Potential for corrosion issues due to acid handling, may require specialized equipment.
LG Chem Ltd.
Technical Solution: LG Chem Ltd. has pioneered a novel approach to PVC synthesis utilizing muriatic acid. Their process involves a direct chlorination method where muriatic acid is used as a chlorine source in the presence of a proprietary catalyst system. This approach allows for the production of high-quality PVC with improved thermal stability and reduced residual monomer content[2]. LG Chem's technology also incorporates a closed-loop recycling system for unreacted muriatic acid, significantly reducing waste and improving overall process efficiency[4]. The company has implemented advanced process control systems to optimize reaction conditions, resulting in consistent product quality and reduced energy consumption[6].
Strengths: High-quality PVC production, improved thermal stability, efficient acid recycling. Weaknesses: May require specialized catalyst systems, potential for increased production costs.
Muriatic Acid Innovations
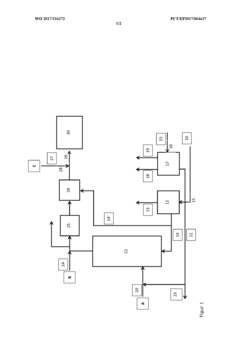
Method and plant for providing sustainable polyvinyl chloride (PVC)
PatentWO2017216272A1
Innovation
- A process that utilizes renewable electricity and inexpensive starting materials to produce PVC, minimizing water and air emissions by integrating chlor-alkali and water electrolysis to generate chlorine and hydrogen, followed by catalytic conversion of CO2 to methanol and subsequent dehydration to ethylene, which is then chlorinated to form vinyl chloride monomer, allowing for continuous production and recycling of by-products.
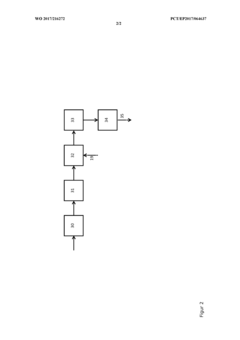
Process for the production of PVC in aqueous suspension using a mixture of initiators and an activity control agent
PatentWO2024132693A1
Innovation
- A process involving the formation of a mixture of diacyl peroxide and dialkyl peroxydicarbonate initiators, which are produced sequentially in a reactor without the need for purification, allowing for controlled reactivity and simplified storage and use, with the option to separate phases for cooling and storage, and the use of an alkali metal halide as an activity control agent to effectively terminate radicals.
Environmental Impact
The use of muriatic acid (hydrochloric acid) in the synthesis of polyvinyl chloride (PVC) has significant environmental implications that warrant careful consideration. The production process involves the reaction of ethylene with chlorine to form ethylene dichloride, which is then converted to vinyl chloride monomer (VCM) using hydrochloric acid. This process, while efficient, raises several environmental concerns.
One of the primary environmental impacts is the potential for air pollution. The production of PVC releases various volatile organic compounds (VOCs) and hazardous air pollutants (HAPs) into the atmosphere. These emissions can contribute to smog formation, ozone depletion, and pose health risks to workers and nearby communities. Additionally, the chlorine-based processes involved in PVC production can lead to the formation of dioxins, which are persistent organic pollutants known for their toxicity and bioaccumulation in the environment.
Water pollution is another significant concern associated with PVC production using muriatic acid. Wastewater from the manufacturing process may contain high levels of chlorinated compounds, heavy metals, and other toxic substances. If not properly treated, these contaminants can harm aquatic ecosystems and potentially enter the food chain. The risk of accidental spills or leaks of hydrochloric acid during transportation or storage also poses a threat to soil and water resources.
The production of PVC consumes substantial amounts of energy, contributing to greenhouse gas emissions and climate change. The energy-intensive nature of the chlor-alkali process used to produce chlorine and the subsequent vinyl chloride synthesis adds to the carbon footprint of PVC manufacturing. Furthermore, the disposal of PVC products at the end of their lifecycle presents additional environmental challenges, as PVC is not easily biodegradable and can release harmful substances when incinerated.
To mitigate these environmental impacts, the industry has been exploring alternative production methods and implementing more stringent pollution control measures. Closed-loop systems for recycling hydrochloric acid within the production process have been developed to reduce waste and emissions. Advanced air pollution control technologies, such as scrubbers and thermal oxidizers, are being employed to minimize the release of harmful substances into the atmosphere.
Efforts are also being made to improve the overall sustainability of PVC production. This includes increasing the use of recycled PVC in manufacturing, developing bio-based alternatives to traditional PVC, and implementing more efficient production processes that reduce energy consumption and waste generation. Additionally, research is ongoing to find less environmentally harmful substitutes for muriatic acid in the PVC synthesis process.
In conclusion, while the use of muriatic acid in PVC synthesis has enabled the widespread production of this versatile material, it comes with significant environmental challenges. Addressing these impacts requires a multifaceted approach, combining technological innovations, regulatory measures, and industry-wide commitment to sustainable practices. As environmental concerns continue to shape industrial processes, the PVC industry must adapt to ensure its long-term viability and minimize its ecological footprint.
One of the primary environmental impacts is the potential for air pollution. The production of PVC releases various volatile organic compounds (VOCs) and hazardous air pollutants (HAPs) into the atmosphere. These emissions can contribute to smog formation, ozone depletion, and pose health risks to workers and nearby communities. Additionally, the chlorine-based processes involved in PVC production can lead to the formation of dioxins, which are persistent organic pollutants known for their toxicity and bioaccumulation in the environment.
Water pollution is another significant concern associated with PVC production using muriatic acid. Wastewater from the manufacturing process may contain high levels of chlorinated compounds, heavy metals, and other toxic substances. If not properly treated, these contaminants can harm aquatic ecosystems and potentially enter the food chain. The risk of accidental spills or leaks of hydrochloric acid during transportation or storage also poses a threat to soil and water resources.
The production of PVC consumes substantial amounts of energy, contributing to greenhouse gas emissions and climate change. The energy-intensive nature of the chlor-alkali process used to produce chlorine and the subsequent vinyl chloride synthesis adds to the carbon footprint of PVC manufacturing. Furthermore, the disposal of PVC products at the end of their lifecycle presents additional environmental challenges, as PVC is not easily biodegradable and can release harmful substances when incinerated.
To mitigate these environmental impacts, the industry has been exploring alternative production methods and implementing more stringent pollution control measures. Closed-loop systems for recycling hydrochloric acid within the production process have been developed to reduce waste and emissions. Advanced air pollution control technologies, such as scrubbers and thermal oxidizers, are being employed to minimize the release of harmful substances into the atmosphere.
Efforts are also being made to improve the overall sustainability of PVC production. This includes increasing the use of recycled PVC in manufacturing, developing bio-based alternatives to traditional PVC, and implementing more efficient production processes that reduce energy consumption and waste generation. Additionally, research is ongoing to find less environmentally harmful substitutes for muriatic acid in the PVC synthesis process.
In conclusion, while the use of muriatic acid in PVC synthesis has enabled the widespread production of this versatile material, it comes with significant environmental challenges. Addressing these impacts requires a multifaceted approach, combining technological innovations, regulatory measures, and industry-wide commitment to sustainable practices. As environmental concerns continue to shape industrial processes, the PVC industry must adapt to ensure its long-term viability and minimize its ecological footprint.
Safety Regulations
The synthesis of Polyvinyl Chloride (PVC) using muriatic acid, also known as hydrochloric acid, requires strict adherence to safety regulations due to the hazardous nature of the chemicals involved. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have established comprehensive guidelines for handling and storing muriatic acid in industrial settings.
These regulations mandate the use of appropriate personal protective equipment (PPE) for workers, including chemical-resistant gloves, goggles, face shields, and protective clothing. Proper ventilation systems must be installed in production areas to prevent the accumulation of toxic fumes. Emergency eyewash stations and safety showers are required to be readily accessible in case of accidental exposure.
Storage of muriatic acid must comply with specific containment protocols. Acid-resistant containers with proper labeling are essential, and secondary containment measures are often required to prevent spills from spreading. Segregation from incompatible materials, such as strong bases and oxidizing agents, is crucial to prevent dangerous reactions.
Workplace safety plans must include detailed procedures for handling spills and leaks. This involves training employees in proper cleanup techniques and providing appropriate spill control equipment. Regular safety drills and emergency response training are mandated to ensure workers can react swiftly and effectively in case of accidents.
Environmental regulations also play a significant role in PVC synthesis using muriatic acid. Strict emission controls are necessary to prevent the release of harmful vapors into the atmosphere. Wastewater treatment systems must be in place to neutralize and properly dispose of acid-containing effluents, adhering to local and national environmental protection standards.
Transportation of muriatic acid is subject to stringent regulations outlined by agencies such as the Department of Transportation (DOT) in the US and the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) in Europe. These regulations specify requirements for packaging, labeling, and documentation during transport.
Regular safety audits and inspections are required to ensure ongoing compliance with these regulations. Companies must maintain detailed records of chemical inventories, safety training, and incident reports. Failure to comply with these safety regulations can result in severe penalties, including fines and potential shutdown of operations.
As research in PVC synthesis progresses, safety regulations continue to evolve. Ongoing efforts focus on developing safer alternatives and improving existing processes to minimize risks associated with muriatic acid use. This includes exploring catalytic methods that reduce acid concentrations and investigating novel polymerization techniques that may eliminate the need for strong acids altogether.
These regulations mandate the use of appropriate personal protective equipment (PPE) for workers, including chemical-resistant gloves, goggles, face shields, and protective clothing. Proper ventilation systems must be installed in production areas to prevent the accumulation of toxic fumes. Emergency eyewash stations and safety showers are required to be readily accessible in case of accidental exposure.
Storage of muriatic acid must comply with specific containment protocols. Acid-resistant containers with proper labeling are essential, and secondary containment measures are often required to prevent spills from spreading. Segregation from incompatible materials, such as strong bases and oxidizing agents, is crucial to prevent dangerous reactions.
Workplace safety plans must include detailed procedures for handling spills and leaks. This involves training employees in proper cleanup techniques and providing appropriate spill control equipment. Regular safety drills and emergency response training are mandated to ensure workers can react swiftly and effectively in case of accidents.
Environmental regulations also play a significant role in PVC synthesis using muriatic acid. Strict emission controls are necessary to prevent the release of harmful vapors into the atmosphere. Wastewater treatment systems must be in place to neutralize and properly dispose of acid-containing effluents, adhering to local and national environmental protection standards.
Transportation of muriatic acid is subject to stringent regulations outlined by agencies such as the Department of Transportation (DOT) in the US and the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) in Europe. These regulations specify requirements for packaging, labeling, and documentation during transport.
Regular safety audits and inspections are required to ensure ongoing compliance with these regulations. Companies must maintain detailed records of chemical inventories, safety training, and incident reports. Failure to comply with these safety regulations can result in severe penalties, including fines and potential shutdown of operations.
As research in PVC synthesis progresses, safety regulations continue to evolve. Ongoing efforts focus on developing safer alternatives and improving existing processes to minimize risks associated with muriatic acid use. This includes exploring catalytic methods that reduce acid concentrations and investigating novel polymerization techniques that may eliminate the need for strong acids altogether.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!